Is a Non-Caloric Sweetener-Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial
Abstract
:1. Background
2. Material and Methods
2.1. Trial Design
2.2. Ethics Approval and Consent to Participate
2.3. Eligibility Criteria for Participants
2.4. Interventions
2.5. Outcomes
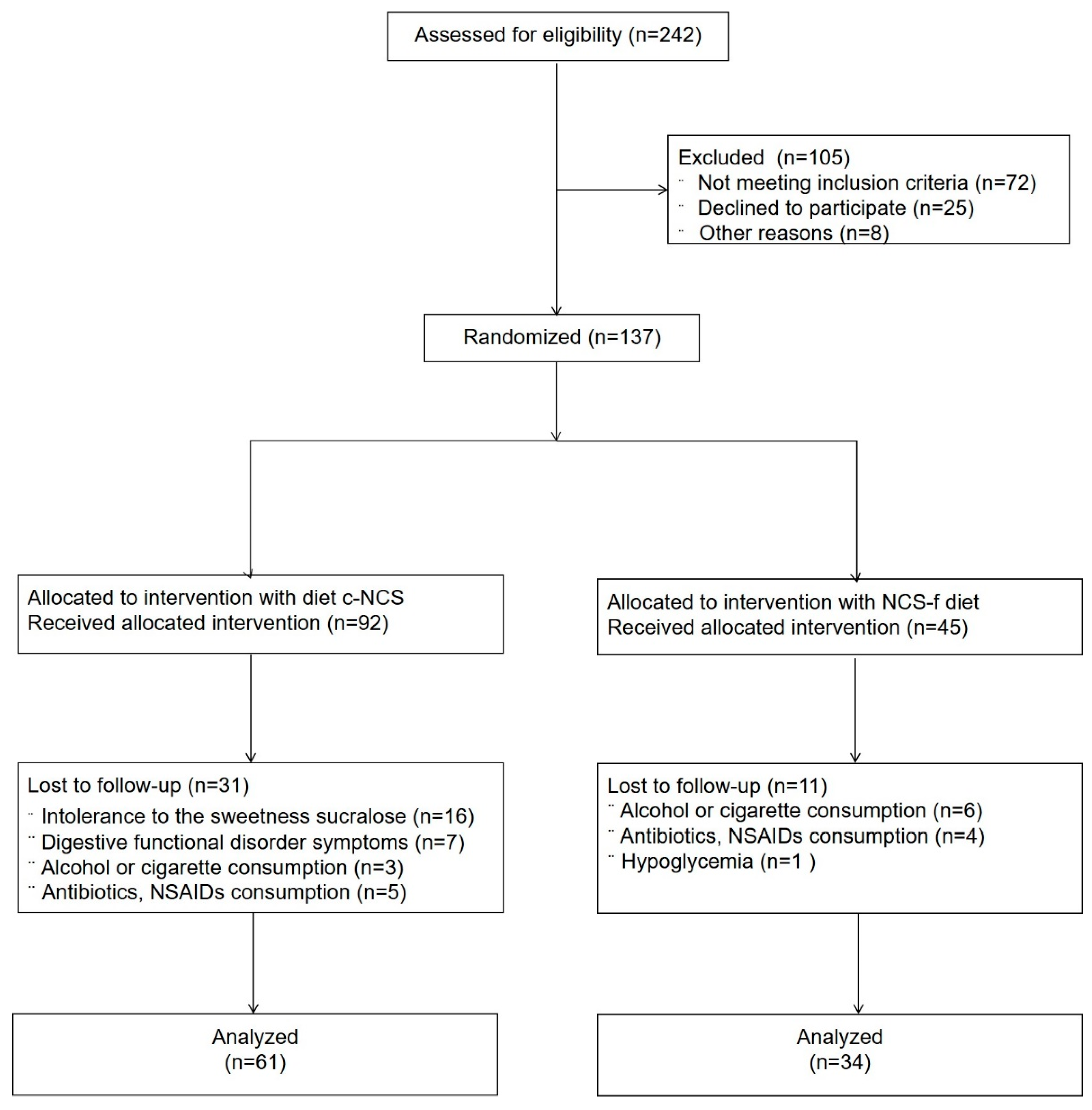
2.6. Sample Size and Randomization
2.7. Statistics
3. Results
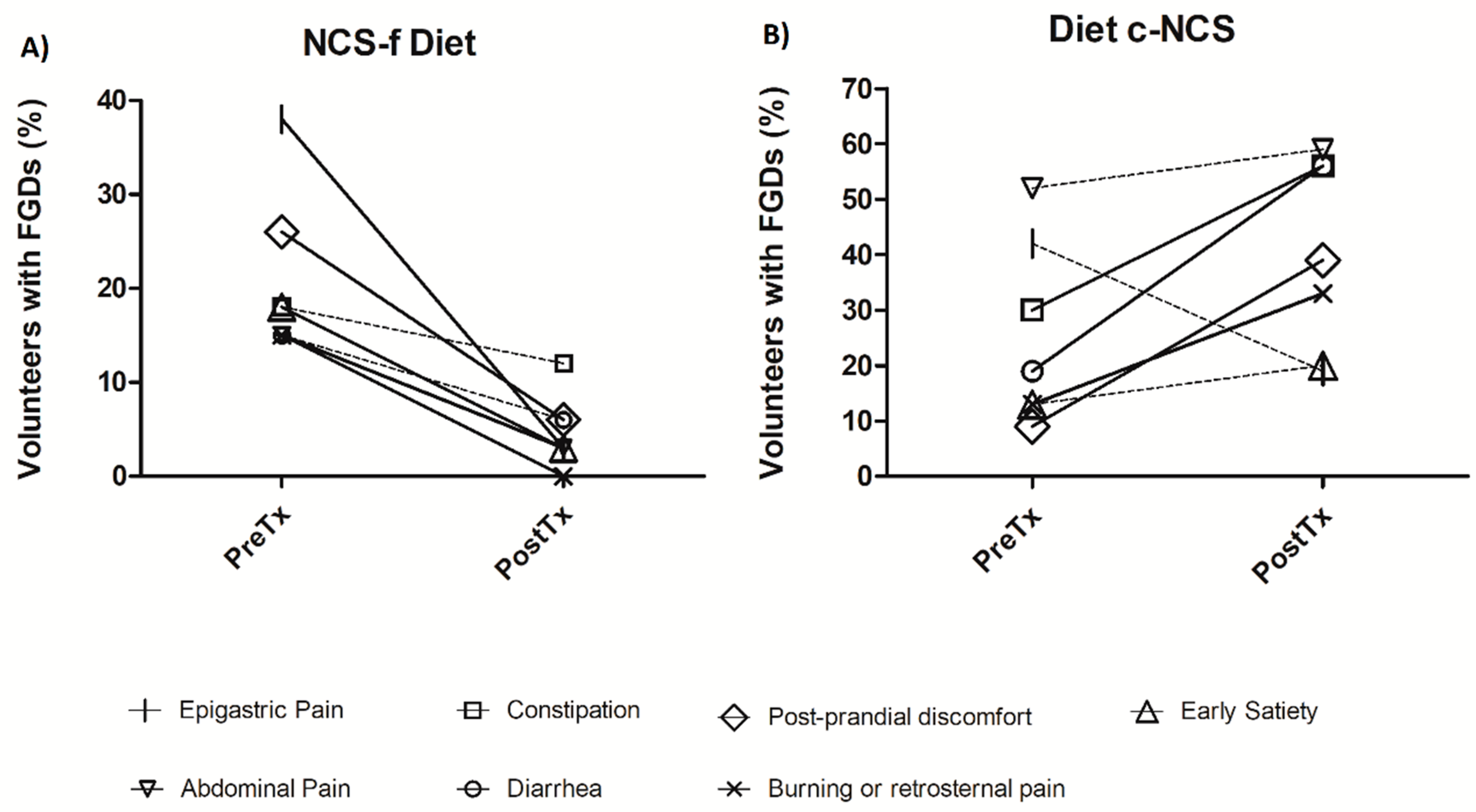
3.1. Gastrointestinal Symptoms
3.2. Dietary Effects
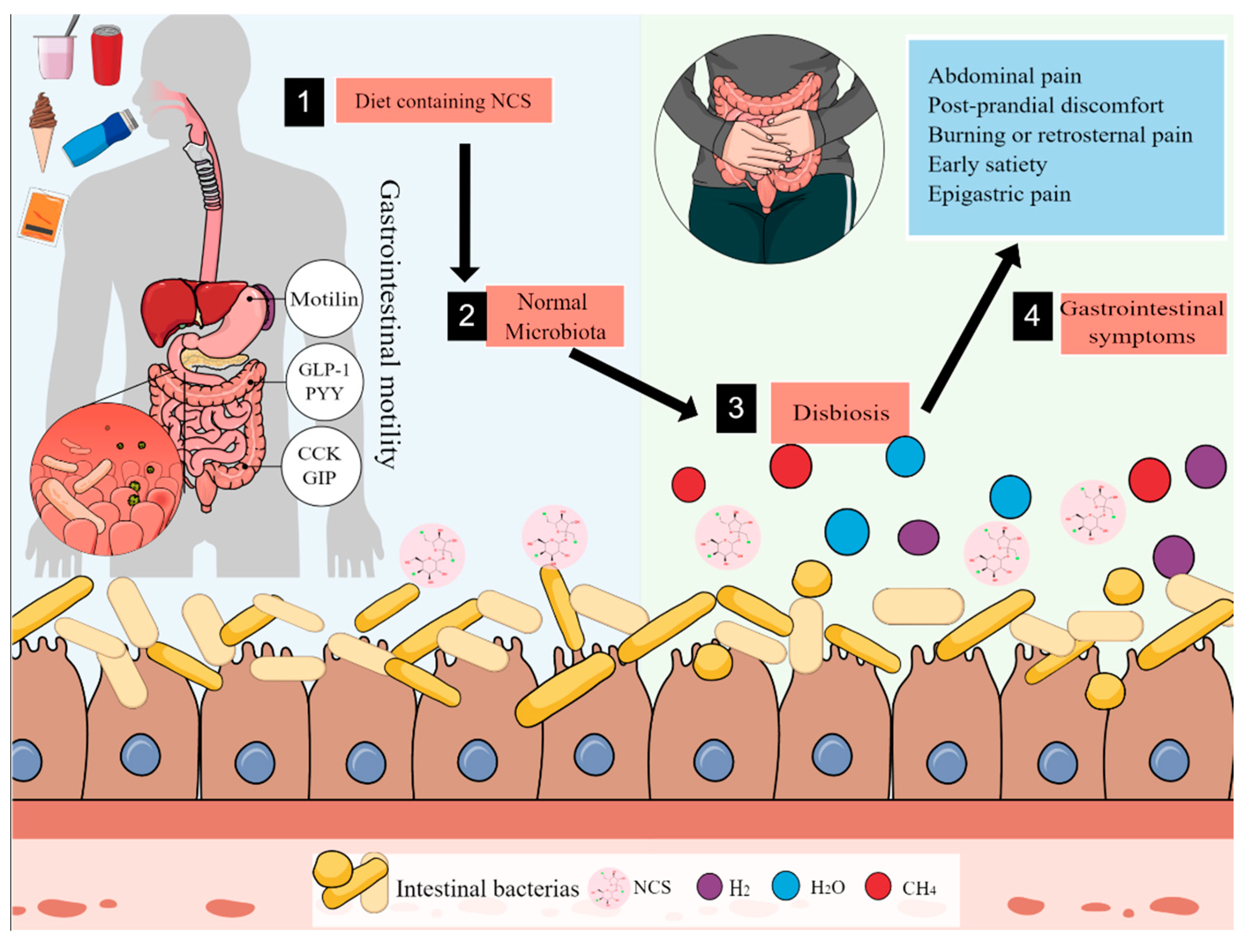
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CCK | cholecystokinin |
| CONSORT | Consolidated Standards of Reporting Trials |
| FGDs | gastrointestinal disorder symptoms |
| GERD | gastroesophageal reflux disease |
| GLP-1 | glucagon-like peptide 1 |
| GIP | gastric inhibitory polypeptide |
| IBS | irritable bowel syndrome |
| NCS | non-caloric sweeteners |
| NCS-f | non-caloric-sweetener-free diet |
| c-NCS | diet containing non-caloric sweeteners |
| PYY | peptide YY |
| PreTx | pretreatment |
| PostTx | post-treatment |
| SD | standard deviation |
| SE | standard error |
| UPFDs | ultra-processed food and drinks |
| WHR | waist–hip ratio |
References
- Rogers, P.J. The role of low-calorie sweeteners in the prevention and management of overweight and obesity: Evidence v. conjecture. Proc. Nutr. Soc. 2018, 77, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walbolt, J.; Koh, Y. Non-nutritive Sweeteners and Their Associations with Obesity and Type 2 Diabetes. J. Obes. Metab. Syndr. 2020, 29, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: Implications for host-microbe interactions contributing to obesity. Obes. Rev. 2012, 13, 799–809. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Almeda-Valdés, P.; Brito-Córdova, G.X.; Gómez-Pérez, F.J. Prevalence of non-nutritive sweeteners consumption in a population of patients with diabetes in Mexico. Gac. Med. Mex. 2017, 153, 61–74. [Google Scholar] [PubMed]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Yaqiong Tian, T.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Hernández, N.; Vázquez-Frías, R.; Abreu, Y.A.A.T.; Almeda-Valdés, P.; Barajas-Nava, L.A.; Carmona-Sánchez, R.I.; Chávez-Sáenz, J.; Consuelo-Sánchez, A.; Espinosa-Flores, A.J.; Hernández-Rosiles, V.; et al. Review of the scientific evidence and technical opinion on noncaloric sweetener consumption in gastrointestinal diseases. Rev. Gastroenterol. Mex. (Engl. Ed.) 2019, 84, 492–510. [Google Scholar] [CrossRef]
- Bueno-Hernández, N.; Jiménez-Cruz, B.L.; Zavala-Solares, M.R.; Meléndez-Mier, G. Association of Natural and Artificial Nonnutritive Sweeteners on Gastrointestinal Disorders: A Narrative Review. J. Nutr. Food Sci. 2018, 8, 2. [Google Scholar]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Drug, V.L. The role of diet in the overlap between gastroesophageal reflux disease and functional dyspepsia. Turk. J. Gastroenterol. 2016, 27, 73–80. [Google Scholar] [CrossRef]
- Shivaji, U.N.; Ford, A.C. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterol. 2014, 5, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Huerta-Iga, F.; Bielsa-Fernández, M.V.; Remes-Troche, J.M.; Valdovinos-Díaz, M.A.; Tamayo-de la Cuesta, J.L. Diagnosis and treatment of gastroesophageal reflux disease: Recommendations of the Asociación Mexicana de Gastroenterología. Rev. Gastroenterol. Mex. 2016, 81, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kille, J.W.; Tesh, J.M.; McAnulty, P.A.; Ross, F.W.; Willoughby, C.R.; Bailey, G.P.; Wilby, O.K.; Tesh, S.A. Sucralose: Assessment of teratogenic potential in the rat and the rabbit. Food Chem. Toxicol. 2000, 38 (Suppl. 2), S43–S52. [Google Scholar] [CrossRef]
- Spencer, M.; Gupta, A.; Dam, L.V.; Shannon, C.; Menees, S.; Chey, W.D. Artificial Sweeteners: A Systematic Review and Primer for Gastroenterologists. J. Neurogastroenterol. Motil. 2016, 22, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denova-Gutiérrez, E.; Ramírez-Silva, I.; Rodríguez-Ramírez, S.; Jiménez-Aguilar, A.; Shamah-Levy, T.; Rivera-Dommarco, J.A. Validity of a food frequency questionnaire to assess food intake in Mexican adolescent and adult population. Salud. Publica Mex. 2016, 58, 617–628. [Google Scholar] [CrossRef]
- Bueno-Hernández, N.; Alcántara-Suarez, R.; Pérez-Castañeda, M.; Hernández-León, Y.A.; Ruiz-Barranco, A.; Escobedo, G.; Islas-Andrade, S.; Melendez, G. Content Validity and Reliability of a Food Frequency Questionnaire with Intense Sweeteners (FFQIS) in a Hispanic Population. J. Nutr. Food Sci. 2018, 8, 2. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Aziz, Q.; Fass, R.; Gyawali, C.P.; Miwa, H.; Pandolfino, J.E.; Zerbib, F. Functional Esophageal Disorders. Gastroenterology 2016, 130, 1459–1465. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Alexandratos, N. The Mediterranean diet in a world context. Public Health Nutr. 2006, 9, 111–117. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives Meeting; World Health Organization. Safety Evaluation of Certain Contaminants in Food; prepared by the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448. [Google Scholar] [CrossRef]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, 1191-e315. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M.; Näslund, E.; Edholm, T.; Schmidt, P.T.; Kristensen, J.; Theodorsson, E.; Holst, J.J.; Efendic, S. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2008, 20, 649–659. [Google Scholar] [CrossRef]
- Meyer-Gerspach, A.C.; Biesiekierski, J.R.; Deloose, E.; Clevers, E.; Rotondo, A.; Rehfeld, J.F.; Depoortere, I.; Van Oudenhove, L.; Tack, J. Effects of caloric and noncaloric sweeteners on antroduodenal motility, gastrointestinal hormone secretion and appetite-related sensations in healthy subjects. Am. J. Clin. Nutr. 2018, 107, 707–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolino, S.; Pacini, G.; Schenone, C.; Patanè, M.; Sulli, A.; Sukkar, S.G.; Lercara, A.; Pizzorni, C.; Gotelli, E.; Cattelan, F.; et al. Nutritional Status and Bone Microarchitecture in a Cohort of Systemic Sclerosis Patients. Nutrients 2020, 12, 1632. [Google Scholar] [CrossRef]
- Gharagozlian, S.; Mala, T.; Brekke, H.K.; Kolbjørnsen, L.C.; Ullerud, Å.A.; Johnson, E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer—A cross-sectional pilot study. Clin. Nutr. ESPEN 2020, 37, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.B.; Hashemi, Z.; Subhan, F.B. The impact of low and no-caloric sweeteners on glucose absorption, incretin secretion, and glucose tolerance. Appl. Physiol. Nutr. Metab. 2017, 42, 793–801. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164, 488–493. [Google Scholar] [CrossRef]
- Lertrit, A.; Srimachai, S.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.O.; Areevut, C.; Katekao, P.; Ongphiphadhanakul, B.; Sriphrapradang, C. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutrition 2018, 55–56, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Prévost, G.; Ducrotté, P.; Cailleux, A.; Khalfi, K.; Basuyau, J.P.; Lefebvre, H.; Kuhn, J.M. Glucose-induced incretin hormone release and insulin sensitivity are impaired in patients with idiopathic gastroparesis: Results from a pilot descriptive study. Neurogastroenterol. Motil. 2013, 25, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F.; Knop, F.K.; Asmar, M. Gastrin secretion in normal subjects and diabetes patients is inhibited by glucagon-like peptide 1: A role in the gastric side effects of GLP-1-derived drugs? Scand. J. Gastroenterol. 2019, 54, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, L.A.; Bueno-Hernández, N.; Cid-Soto, M.A.; De León, K.L.; Mendoza-Martínez, V.M.; Espinosa-Flores, A.J.; Carrero-Aguirre, M.; Esquivel-Velázquez, M.; León-Hernández, M.; Viurcos-Sanabria, R.; et al. Ten-Week Sucralose Consumption Induces Gut Dysbiosis and Altered Glucose and Insulin Levels in Healthy Young Adults. Microorganisms 2022, 10, 434. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zilberman-Schapira, G.; Segal, E.; Elinav, E. Non-caloric artificial sweeteners and the microbiome: Findings and challenges. Gut Microbes 2015, 6, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- Mujagic, Z.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Kruimel, J.W.; Lenaerts, K.; Hanssen, N.M.; Conchillo, J.M.; Jonkers, D.M.; Masclee, A.A. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment. Pharmacol. Ther. 2014, 40, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J. Toxicol. Environ. Health A 2008, 71, 1415–1429. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef]
- Guandalini, S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011, 45, S149–S153. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
| NCS-F N = 34 | C-NCS N = 61 | p * | |
|---|---|---|---|
| Age, years (m ± SD) | 22 ± 3.2 | 22 ± 3.1 | 0.74 |
| Men, n (%) | 14 (41) | 23 (38) | 0.73 |
| Women, n (%) | 20 (59) | 38 (62) | 0.91 |
| Weight, kg (m ± SD) | 64.9 ± 12.7 | 64.9 ± 12.7 | 0.98 |
| WHR, (m ± SD) | 0.81 ± 0.67 | 0.81 ± 0.65 | 0.52 |
| BMI, kg/m2 (m ± SD) | 24.16 ± 3.8 | 23.9 ± 3.1 | 0.75 |
| Fat; % (m ± SD) | 35.5 ± 7.2 | 35.5 ± 5.7 | 0.85 |
| Fat-free mass, % (m ± SD) | 64.5 ± 7.2 | 64.1 ± 5.7 | 0.85 |
| Total body water, % (m ± SD) | 46.2 ± 5.4 | 45.9 ± 4.5 | 0.79 |
| Carbohydrates, g/day ( ± SD) | 289.4 ± 118 | 246.6 ± 104 | 0.08 |
| Protein, g/day (m ± SD) | 103.9 ± 74 | 97.6 ± 52 | 0.66 |
| Lipid, g/day (m ± SD) | 71.5 ± 59 | 68.4 ± 43 | 0.78 |
| Kilocalorie, kcal/day (m ± SD) | 2264.7 ± 1247 | 2036.1 ± 837 | 0.34 |
| Consumption of NCS, % (n) | 23 (67) | 41 (67) | 0.96 |
| Consumption amount of NCS, mg/day (m ± SE) | 42.5 (20) | 50.0 (16) | 0.77 |
| Adherence to treatment, % | 85 | 90 | 0.26 |
| Presence of gastrointestinal symptoms, % (n) ** | 20 (58) | 33 (54) | 0.65 |
| NCS-f (n = 34) | Mean Difference between Pre/Post (95% CI for MD) | p * | c-NCS (n = 61) | Mean Difference between Pre/Post (95% CI for MD) | p * | Mean Difference between Diet Groups (95% CI for MD) | p * | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PreTx | PostTx | PreTx | PostTx | |||||||
| Weight, kg (m ± SD) | 64.9 ± 12.1 | 64.67 ± 12.73 | 0.24 (−0.1; 0.6) | 0.25 | 64.9 ± 12.7 | 64.9 ± 12.7 | 0.05 (−0.5; 0.7) | 0.85 | 0.19 (−0.7; 1.1) | 0.69 |
| WHR, (m ± SD) | 0.81 ± 0.06 | 0.81 ± 0.05 | 0.006 (−0.01; 0.02) | 0.50 | 0.8 ± 0.6 | 0.8 ± 0.06 | 0.003 (−0.006; 0.013) | 0.50 | 0.01 (−0.0; 0.0) | 0.33 |
| BMI, kg/m2 (m ± SD) | 24.1 ± 3.8 | 24 ± 3.9 | 0.08 (−0.08; 0.24) | 0.33 | 23.9 ± 3.1 | 23.9 ± 3.2 | 0.01 (−0.2; 0.2) | 0.87 | 0.06 (−0.2; 0.3) | 0.69 |
| Fat, % (m ± SD) | 35.5 ± 7.2 | 34.3 ± 6.9 | 0.87 (0.34; 1.41) | <0.01 | 35.5 ± 5.7 | 34.6 ± 6.3 | 1.1 (0.47; 1.86) | <0.01 | 0.56 (−6.6; 7.7) | 0.87 |
| Fat-free mass, % (m ± SD) | 64.5 ± 7.2 | 65.4 ± 6.9 | −0.87 (−1.41; −0.34) | <0.01 | 64.1 ± 5.7 | 65.4 ± 6.3 | −1.1 (−1.86; −0.47) | <0.01 | 0.09 (−8.3; 8.4) | 0.98 |
| Total body water, % (m ± SD) | 46.2 ± 5.4 | 47.6 ± 5.4 | −1.04 (−1.6; −0.47) | <0.01 | 45.9 ± 4.5 | 47.3 ± 4.9 | −1.2 (−1.87; −0.67) | <0.01 | 0.85 (−8.3; 10.0) | 0.85 |
| Consumption of NCS, mg/day (m ± SD) | 42.5 ± 20.9 | 2.6 ± 1.7 | 39.86 (−3.1; 82.82) | 0.06 | 50.03 ± 16 | 74.2 ± 3.3 | −24 (16.83; 57.9) | 0.15 | 64.16 (9.4; 118.8) | 0.02 |
| Carbohydrates, g/day (m ± SD) | 289.4 ± 119 | 219.4 ± 77 | 70 (32; 107) | <0.01 | 246.5 ± 108 | 260.3 ± 108 | −13.8 (−42.5; 14.9) | 0.33 | 71.09 (18.0; 124.1) | <0.05 |
| Protein, g/day (m ± SD) | 104.7 ± 77 | 83.8 ± 67 | 20.8 (1.5; 40.1) | 0.03 | 94.3 ± 51 | 87.8 ± 41 | 6.5 (−8.3; 21.3) | 0.38 | 6.54 (−19.8; 32.9) | 0.62 |
| Lipid, g/day (m ± SD) | 73.2 ± 61 | 63 ± 51 | 10.1 (−6.7; 27) | 0.22 | 67.5 ± 42 | 69.1 ± 42 | −1.6 (−14.7; 11.5) | 0.80 | 6.18 (−15.6; 27.9) | 0.57 |
| Kilocalorie, kcal/day (m ± SD) | 2281 ± 1282 | 1828 ± 1025 | 453 (132; 774) | <0.01 | 2014 ± 832 | 2058 ± 832 | 551 (−420; 195) | 0.71 | 363 (−82.3; 810.0) | 0.10 |
| Presence of FGDs, % (n) | 20 (58) | 8 (23) | N.A. | 0.02 ** | 31 (50) | 39 (63) | N.A. | 0.07 | N.A. | <0.05 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Martínez, V.M.; Zavala-Solares, M.R.; Espinosa-Flores, A.J.; León-Barrera, K.L.; Alcántara-Suárez, R.; Carrillo-Ruíz, J.D.; Escobedo, G.; Roldan-Valadez, E.; Esquivel-Velázquez, M.; Meléndez-Mier, G.; et al. Is a Non-Caloric Sweetener-Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial. Nutrients 2022, 14, 1095. https://doi.org/10.3390/nu14051095
Mendoza-Martínez VM, Zavala-Solares MR, Espinosa-Flores AJ, León-Barrera KL, Alcántara-Suárez R, Carrillo-Ruíz JD, Escobedo G, Roldan-Valadez E, Esquivel-Velázquez M, Meléndez-Mier G, et al. Is a Non-Caloric Sweetener-Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial. Nutrients. 2022; 14(5):1095. https://doi.org/10.3390/nu14051095
Chicago/Turabian StyleMendoza-Martínez, Viridiana Montsserrat, Mónica Rocío Zavala-Solares, Aranza Jhosadara Espinosa-Flores, Karen Lorena León-Barrera, Raúl Alcántara-Suárez, José Damián Carrillo-Ruíz, Galileo Escobedo, Ernesto Roldan-Valadez, Marcela Esquivel-Velázquez, Guillermo Meléndez-Mier, and et al. 2022. "Is a Non-Caloric Sweetener-Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial" Nutrients 14, no. 5: 1095. https://doi.org/10.3390/nu14051095
APA StyleMendoza-Martínez, V. M., Zavala-Solares, M. R., Espinosa-Flores, A. J., León-Barrera, K. L., Alcántara-Suárez, R., Carrillo-Ruíz, J. D., Escobedo, G., Roldan-Valadez, E., Esquivel-Velázquez, M., Meléndez-Mier, G., & Bueno-Hernández, N. (2022). Is a Non-Caloric Sweetener-Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial. Nutrients, 14(5), 1095. https://doi.org/10.3390/nu14051095








