Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Design
2.3. Preparation and Examination of Samoles
2.4. Histopathological Analysis
2.5. Immunohistochemical (IHC) Determination of Collagen I
2.6. Determination of Levels of Inflammatory Cytokines, Oxidative Stress and PF Markers
2.7. Western Blotting
2.8. qRT-PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Compositions of TRF and Total Mixed-Carotene Complex 20% Oil Concentrate (Carotene)
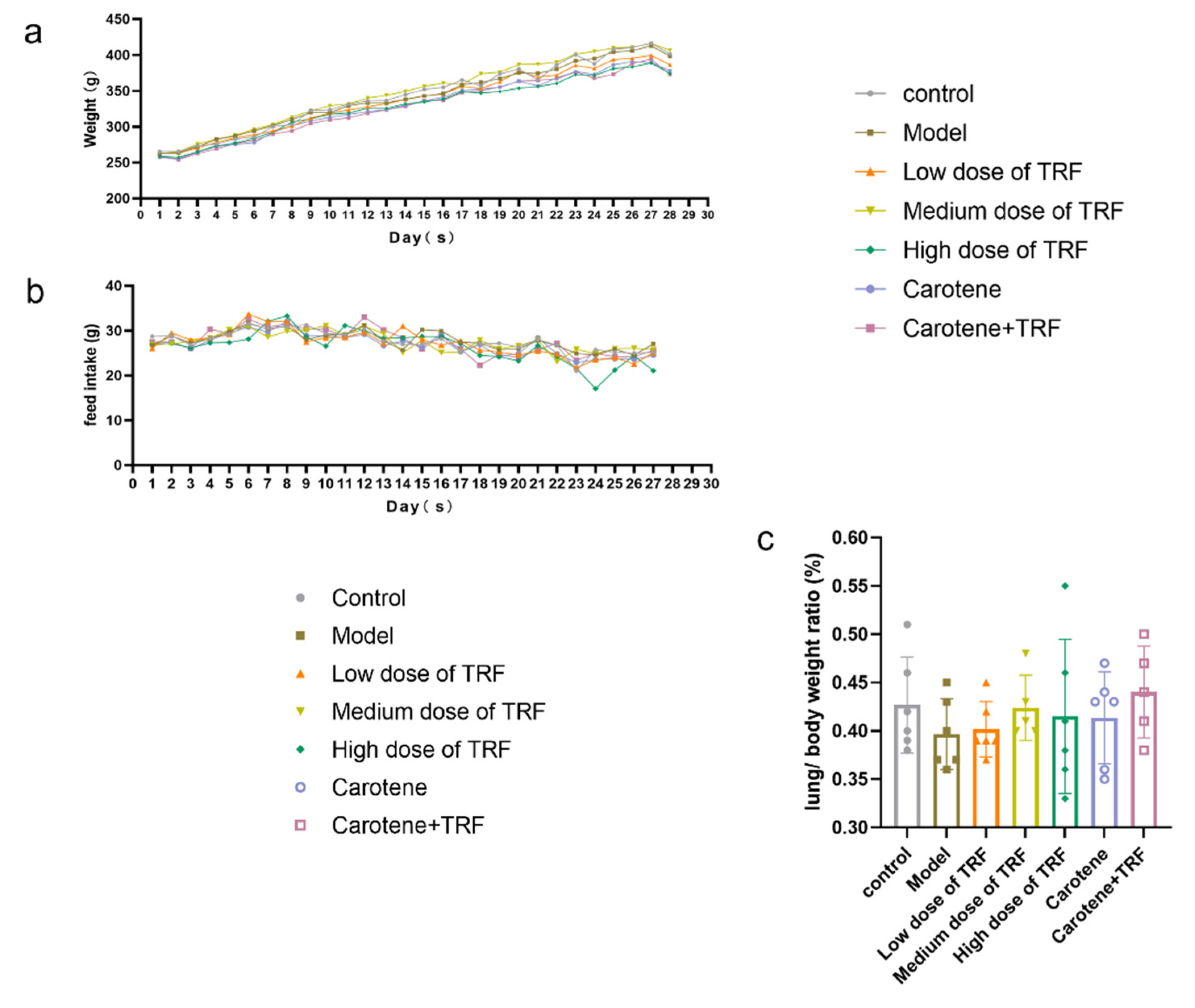
3.2. Effects of TRF and Carotene on Weight (g), Feed Intake (g) and Lung/Body Weight Ratio (%)
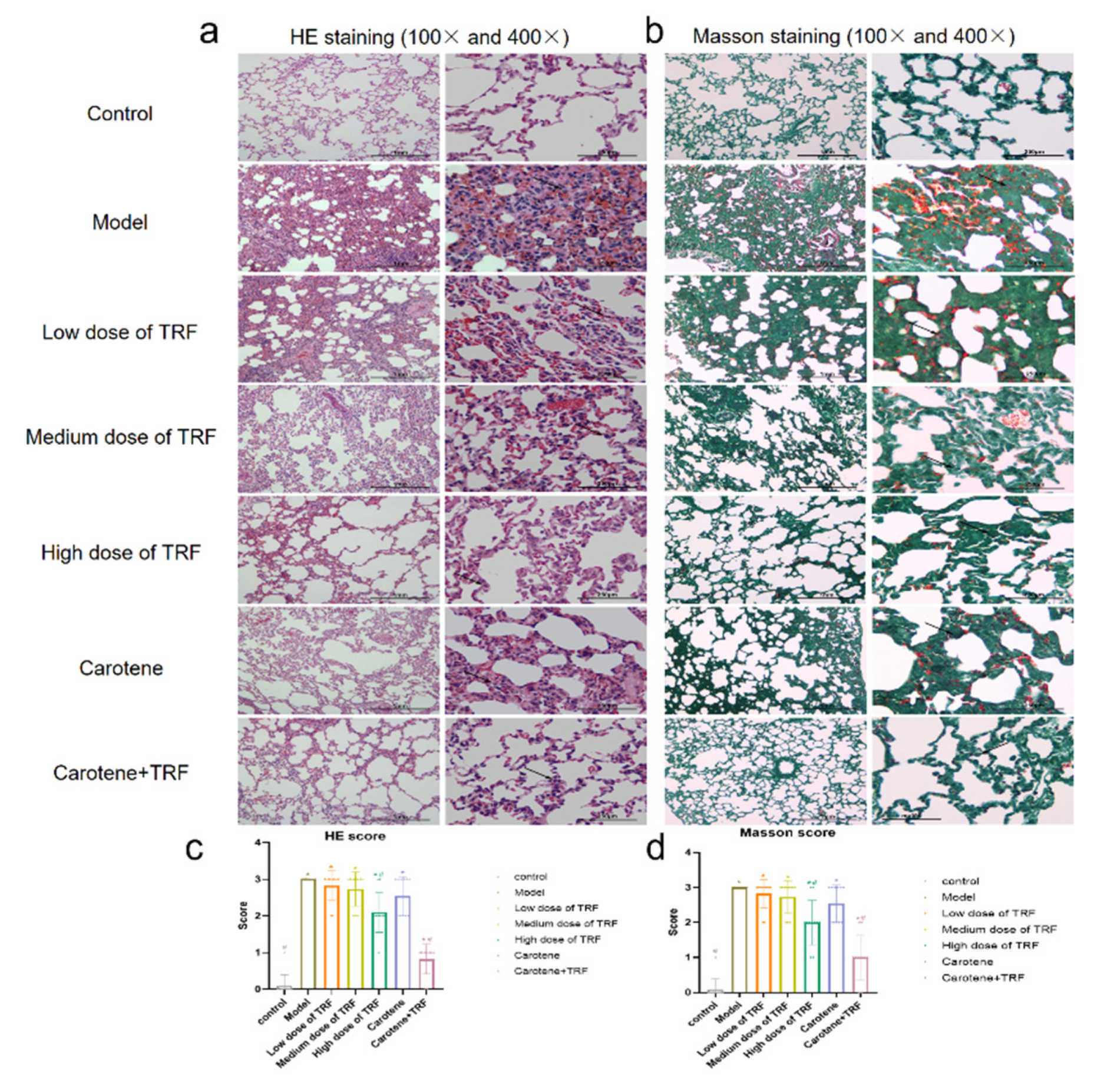
3.3. Effects of TRF and Carotene on Histological Evaluation
3.4. Immunohistochemical Determination of Collagen I
3.5. Effects of TRF and Carotene on Inflammatory Markers and Antioxidant Enzymes
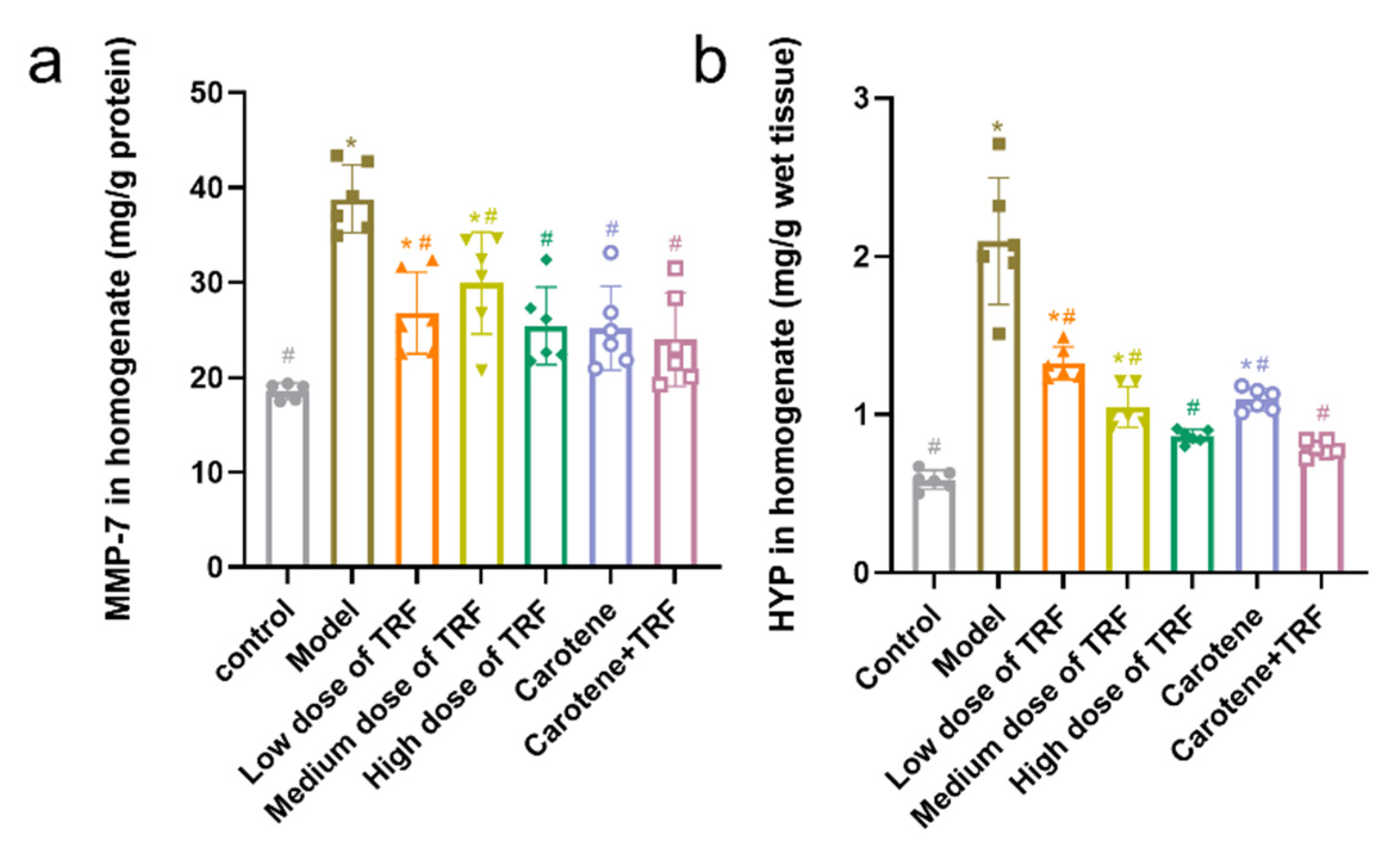
3.6. Effects of TRF and Carotene on HYP and MMP-7 Levels
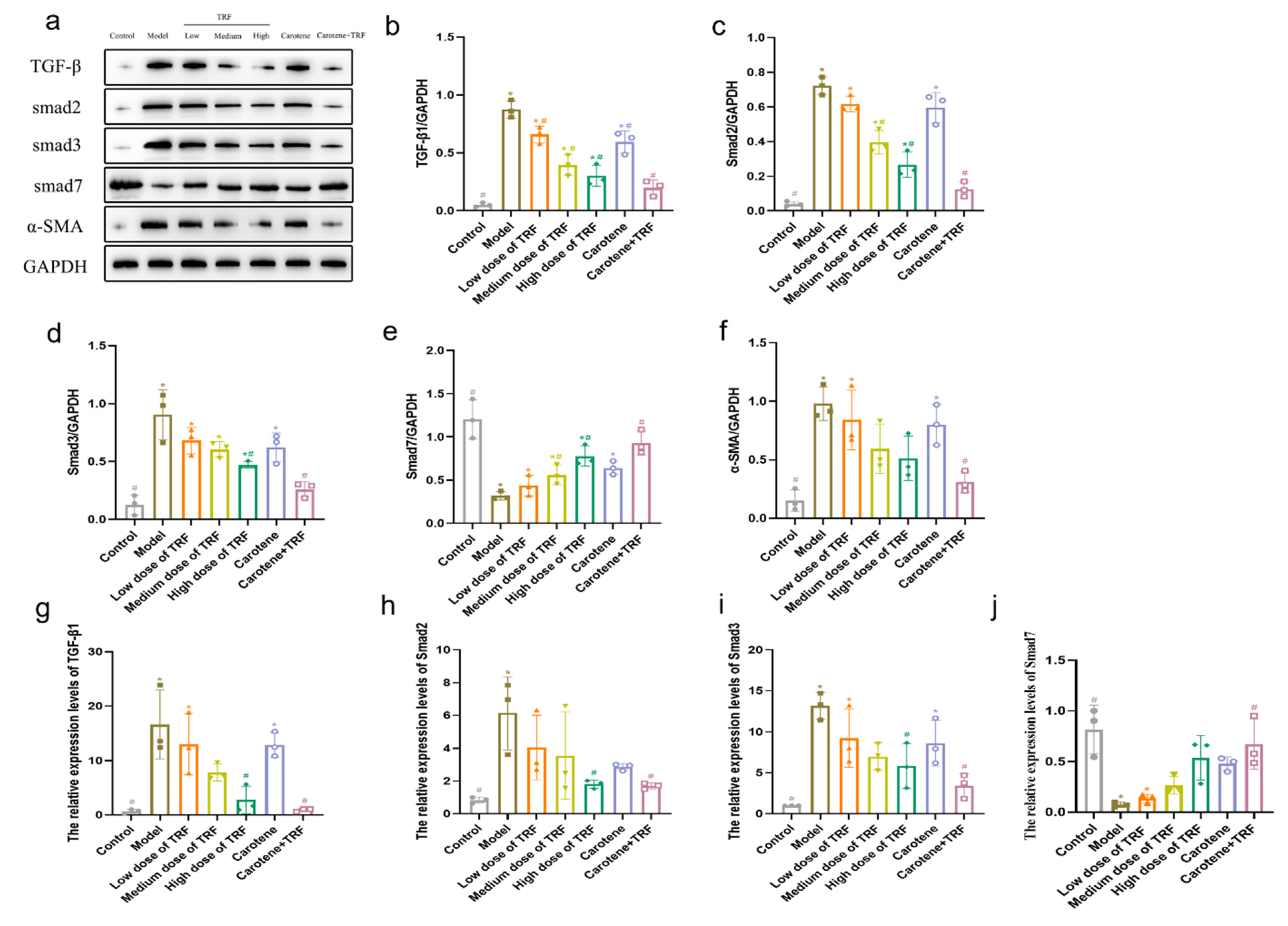
3.7. TRF and Carotene Prevent PF by Suppressing TGF-β/Smad Signaling Pathway
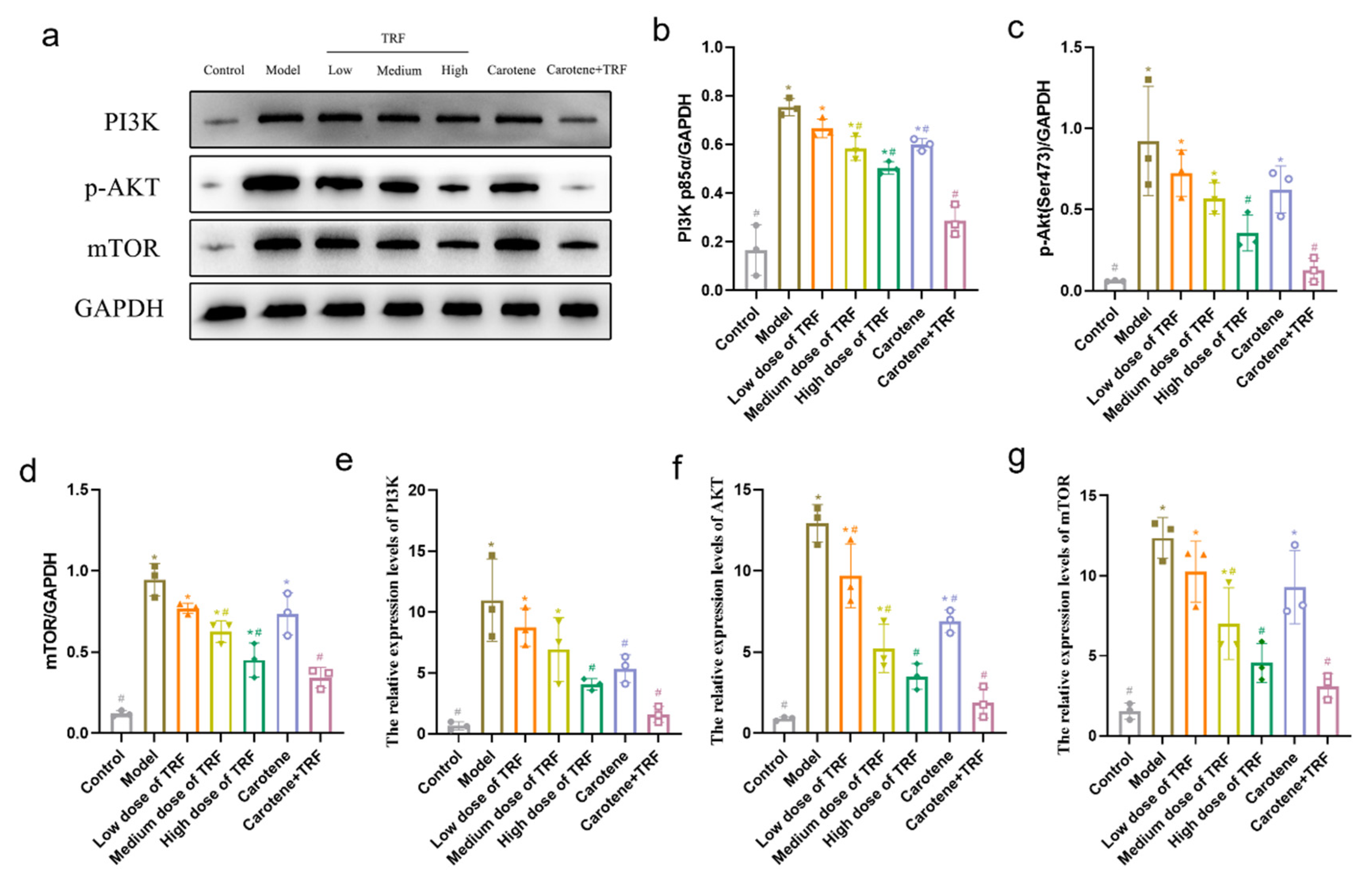
3.8. TRF and Carotene Inhibit Pulmonary Fibrosis by Suppressing PI3K/Akt/mTOR Signaling Pathway
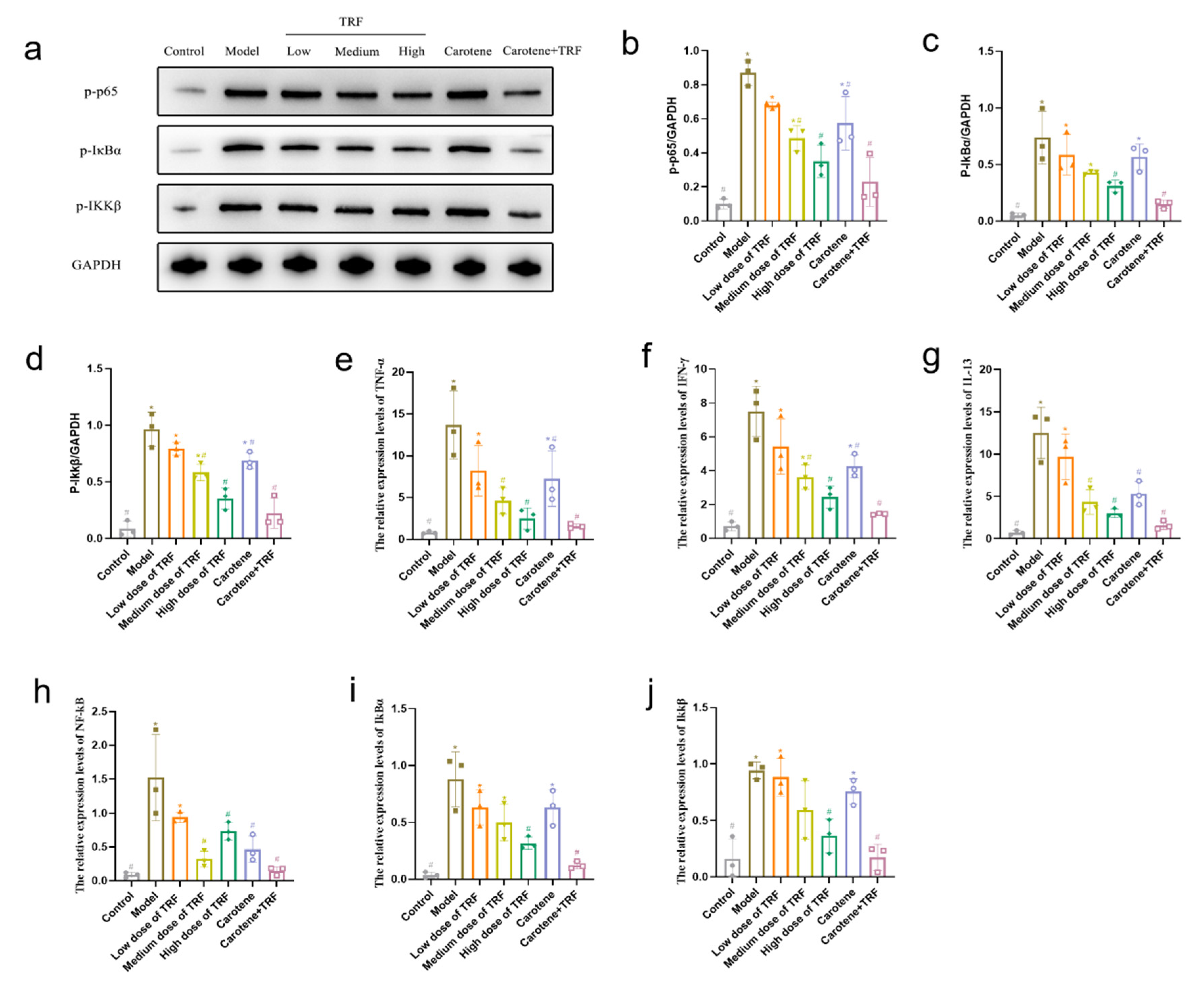
3.9. TRF and Carotene Inhibit Pulmonary Fibrosis by Suppressing NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRF | Tocotrienol-rich fraction |
| PF | Pulmonary fibrosis |
| SARS | Severe acute respiratory syndrome |
| BLM | Bleomycin |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| WHO | World Health Organization |
| YesARDS | Acute respiratory distress syndrome |
| EMT | Epithelial–mesenchymal transition |
| MAPK | Mitogen-activated protein kinase |
| AMPK | Adenylate-activated protein kinase |
| DLD | Diffuse lung disease |
| VE | Vitamin E |
| MPOB | Malaysian Palm Oil Board |
| IL-1β | Interleukin-1β |
| YeesIL-6 | Interleukin-6 |
| IL-13 | Interleukin-13 |
| NF-κB | Nuclear factor kappa-B |
| IkBα | NF-kappa-B inhibitor alpha |
| Ikkβ | Inhibitor of nuclear factor kappa-B kinase |
| MPO | Myeloperoxidase |
| TGF-β1 | Transforming growth factor beta 1 |
| TNF-α | Tumor necrosis factor-alpha |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | (protein kinase B, PKB); |
| mTOR | Mammalian target of rapamycin |
| IFN-γ | Interferon-γ |
| CAT | Catalase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| SOD | Superoxide dismutase |
| PVDF | Polyvinylidene fluoride |
| BCA | Bicinchoninic acid |
| SD | Sprague-Dawley |
| SPF | Specific-pathogen free |
| SLAC | Shanghai Laboratory Animals Center |
| CMC-Na | Sodium carboxymethyl cellulose |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemical |
| PBS | Phosphate-buffered saline |
| DAB | diaminobenzidine |
| HYP | Hydroxyproline |
| ECL | Chemiluminescence |
| HPLC | High-performance liquid chromatography |
| FDA | Food and Drug Administration. |
References
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Kayarat, B.; Khanna, P.; Sarkar, S. Pulmonary Fibrosis in COVID-19 Recovered Patients: Problem and Potential Management. Indian J. Crit. Care Med. 2021, 25, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Esteban, A.; Fernandez-Segoviano, P.; Rodriguez, J.M.; Aramburu, J.A.; Vargas-Errazuriz, P.; Martin-Pellicer, A.; Lorente, J.A.; Frutos-Vivar, F. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: A prospective cohort study of clinical autopsies. Lancet Respir. Med. 2013, 1, 395–401. [Google Scholar] [CrossRef]
- Grillo, F.; Barisione, E.; Ball, L.; Mastracci, L.; Fiocca, R. Lung fibrosis: An undervalued finding in COVID-19 pathological series. Lancet Infect. Dis. 2020, 21, E72. [Google Scholar] [CrossRef]
- Mo, X.N.; Jian, W.H.; Su, Z.Q.; Chen, M.; Peng, H.; Peng, P.; Lei, C.L.; Chen, R.C.; Zhong, N.S.; Li, S.Y. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Zahedipour, F.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pulmonary fibrosis: Therapeutic and mechanistic insights into the role of phytochemicals. Biofactors 2021, 47, 250–269. [Google Scholar] [CrossRef]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Vancheri, C.; Kreuter, M.; Richeldi, L.; Ryerson, C.J.; Valeyre, D.; Grutters, J.C.; Wiebe, S.; Stansen, W.; Quaresma, M.; Stowasser, S.; et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis. Results of the INJOURNEY Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 356–363. [Google Scholar] [CrossRef]
- Newton, C.A.; Zhang, D.; Oldham, J.M.; Kozlitina, J.; Ma, S.F.; Martinez, F.J.; Raghu, G.; Noth, I.; Garcia, C.K. Telomere Length and Use of Immunosuppressive Medications in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 336–347. [Google Scholar] [CrossRef]
- John, H.; Shahriar, S.; Geoffrey, L. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991, 65, 81–94. [Google Scholar]
- Imadaya, A.; Terasawa, K.; Tosa, H.; Mitsuma, T.; Sudou, S.; Kumagai, A. Case of mixed connective tissue disease with severe pulmonary fibrosis treated with herbal drugs. Ryumachi 1983, 23, 354–361. [Google Scholar] [PubMed]
- Wang, L.; Li, S.; Yao, Y.; Yin, W.; Ye, T. The role of natural products in the prevention and treatment of pulmonary fibrosis: A review. Food Funct. 2021, 12, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Chitra, P.; Saiprasad, G.; Manikandan, R.; Sudhandiran, G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J. Mol. Med. 2015, 93, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-kappaB, Muc5ac, TNF-alpha and IL-1beta. Chem. Biol. Interact. 2015, 237, 151–165. [Google Scholar] [CrossRef]
- Nikbakht, J.; Hemmati, A.A.; Arzi, A.; Mansouri, M.T.; Rezaie, A.; Ghafourian, M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharm. Rep 2015, 67, 1061–1067. [Google Scholar] [CrossRef]
- Tao, L.; Yang, J.; Cao, F.; Xie, H.; Zhang, M.; Gong, Y.; Zhang, C. Mogroside IIIE, a Novel Anti-Fibrotic Compound, Reduces Pulmonary Fibrosis through Toll-Like Receptor 4 Pathways. J. Pharm. Exp. 2017, 361, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Liu, Y.J.; Mao, Y.F.; Dong, W.W.; Zhu, X.Y.; Jiang, L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clin. Nutr. 2015, 34, 752–760. [Google Scholar] [CrossRef]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Pasquali, M.A.; Gelain, D.P.; de Oliveira, M.R.; Behr, G.A.; da Motta, L.L.; da Rocha, R.F.; Klamt, F.; Moreira, J.C. Vitamin A supplementation for different periods alters oxidative parameters in lungs of rats. J. Med. Food 2009, 12, 1375–1380. [Google Scholar] [CrossRef]
- Pasquali, M.A.; Schnorr, C.E.; Feistauer, L.B.; Gelain, D.P.; Moreira, J.C. Vitamin A supplementation to pregnant and breastfeeding female rats induces oxidative stress in the neonatal lung. Reprod. Toxicol. 2010, 30, 452–456. [Google Scholar] [CrossRef]
- Cantin, A.M.; White, T.B.; Cross, C.E.; Forman, H.J.; Sokol, R.J.; Borowitz, D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic. Biol. Med. 2007, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharm. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharm. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. The location and function of vitamin E in membranes (review). Mol. Membr. Biol. 2000, 17, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Mahipal, A.; Klapman, J.; Vignesh, S.; Yang, C.S.; Neuger, A.; Chen, D.T.; Malafa, M.P. Pharmacokinetics and safety of vitamin E delta-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites. Cancer Chemother Pharm. 2016, 78, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Gregory, M.S.; Kazim, H.; Anthony, N.; Barbara, C.; Dung-Tsa, C.; Tai, Z.H.; Richard, M.L.; Saïd, S.; Mokenge, P.M. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 11, 2352–3964. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Yokozawa, T.; Terasawa, K.; Nakanishi, K. Therapeutic usefulness of Keishi-bukuryo-gan for diabetic nephropathy. J. Pharm. Pharm. 2003, 55, 219–227. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Ahmad Ansari, M.; Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats. Biomed. Pharm. 2018, 108, 224–231. [Google Scholar] [CrossRef]
- Rong, Y.; Cao, B.; Liu, B.; Li, W.; Chen, Y.; Chen, H.; Liu, Y.; Liu, T. A novel Gallic acid derivative attenuates BLM-induced pulmonary fibrosis in mice. Int. Immunopharmacol. 2018, 64, 183–191. [Google Scholar] [CrossRef]
- Liu, M.-w.; Su, M.-x.; Tang, D.-y.; Hao, L.; Xun, X.-H.; Huang, Y.-q. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing miR-193a expression. BMC Pulm. Med. 2019, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Sem, H.P.; James, V.; David, S. Rat Lung Fibroblast Collagen Metabolism in Bleomycin-induced Pulmonary Fibrosis. J. Clin. Investig. 1985, 76, 241–247. [Google Scholar]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem.-Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, J.T.; Zhang, R.; Li, Z.; Yin, Y.; Fang, X.M.; Ding, X.P.; Cai, Y.; Yang, S.D.; Mu, H.J.; Zong, D.; et al. Nuclear Smad6 promotes gliomagenesis by negatively regulating PIAS3-mediated STAT3 inhibition. Nat. Commun. 2018, 9, 2504. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Miyazaki, H.; Hara, T.; Furuya, T.; Imamura, T.; Watabe, T.; Miyazono, K. Roles for the MH2 domain of Smad7 in the specific inhibition of transforming growth factor-beta superfamily signaling. J. Biol. Chem. 2004, 279, 31568–31574. [Google Scholar] [CrossRef] [Green Version]
- Moustakas, A.; Heldin, C.H. The regulation of TGF beta signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [Green Version]
- Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat. Rev. 2014, 40, 862–871. [Google Scholar] [CrossRef]
- Roncolato, F.; Lindemann, K.; Wilson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, H.; Chen, B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp. Eye Res. 2019, 189, 107830. [Google Scholar] [CrossRef]
- Hsu, H.-S.; Liu, C.-C.; Lin, J.-H.; Hsu, T.-W.; Hsu, J.-W.; Su, K.; Hung, S.-C. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci. Rep. 2017, 7, 14272. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L. Depleting microRNA-146a-3p attenuates lipopolysaccharide-induced acute lung injury via up-regulating SIRT1 and mediating NF-kappa B pathway. J. Drug Target. 2021, 29, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, A.; Ali, J.; Ullah, H.; Khan, A.; Ali, H.; Irshad, N.; Khan, S. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol. 2020, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Morbini, P.; Inghilleri, S.; Campo, I.; Oggionni, T.; Zorzetto, M.; Luisetti, M. Incomplete expression of epithelial-mesenchymal transition markers in idiopathic pulmonary fibrosis. Pathol. Res. Pract. 2011, 207, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Lee, S.-B.; Karna, A.; Kim, H.-J.; Shen, A.; Pandit, A.; Lee, S.; Yang, S.-H.; So, H.-S. Increased Cellular NAD(+) Level through NQO1 Enzymatic Action Has Protective Effects on Bleomycin-Induced Lung Fibrosis in Mice. Tuberc. Respir. Dis. 2016, 79, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Cuartin, G.; Molina-Molina, M. Assessing quality of life of idiopathic pulmonary fibrosis patients: The INSTAGE study. Breathe 2019, 15, 144–146. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Q.; Zhou, C.-C.; Yu, L.-Y.; Wang, L.; Deng, J.-L.; Tao, Y.-L.; Zhang, F.; Chen, W.-S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef] [PubMed]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-Induced Interstitial Pulmonary-Disease in The Nude, Athymic Mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar]
- Wu, X.; Lin, L.; Wu, H. Ferulic acid alleviates lipopolysaccharide-induced acute lung injury through inhibiting TLR4/NF-kappa B signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22664. [Google Scholar] [CrossRef]
- Beddard, G.S.; Davidson, R.S.; Trethewey, K.R. Quenching of Chlorophyll Fluorescence by Beta-Carotene. Nature 1977, 267, 373–374. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Beta-Carotene-An Unusual Type of Lipid Antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Dede, S.; Mert, H.; Mert, N.; Yur, F.; Ertekin, A.; Deger, Y. Effects of alpha-tocopherol on serum trace and major elements in rats with bleomycin-induced pulmonary fibrosis. Biol. Trace Elem. Res. 2006, 114, 175–184. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Nazari, Z.; Samei, M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm. Pharm. 2008, 21, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, A.; Deger, Y.; Mert, H.; Mert, N.; Yur, F.; Dede, S.; Demir, H. Investigation of the effects of alpha-tocopherol on the levels of Fe, Cu, Zn, Mn, and carbonic anhydrase in rats with bleomycin-induced pulmonary fibrosis. Biol. Trace Elem. Res. 2007, 116, 289–300. [Google Scholar] [CrossRef] [PubMed]


| mRNA | Forward Primers | Reverse Primers |
|---|---|---|
| TGF-β1 | TCGCCCTTTCATTTCAGAT | TTTGCCGATGCTTTCTTG |
| Smad2 | AGGTGTCTCATCGGAAAG | CTCTGGTAGTGGTAAGGGT |
| Smad3 | AGCTTACAAGGCGGCACA | TGGGAGACTGGACGAAAA |
| Smad7 | CTTCCTCCGATGAAACCG | TCGAGTCTTCTCCTCCCAGTA |
| PI3K | GAAACCCAGTCACCTAGGGC | GGTGGGCAGTACGAACTCAA |
| AKT | GAGGAGCGGGAAGAGTG | GTGCCCTTGCCCAGTAG |
| mTOR | GGTGGACGAGCTCTTTGTC | AGGAGCCCTAACACTCGGAT |
| TNF-α | TGAGCACAGAAAGCATGATC | CATCTGCTGGTACCACCAGTT |
| IFN-γ | TTGCAGCTCTGCCTCAT | TTCGTGTTACCGTCCTT |
| IL-13 | CTCGCTTGCCTTGGTGG | TGATGTTGCTCAGCTCCTC |
| NF-κB | CTGTTTCCCCTCATCTTTCC | GTGCGTCTTAGTGGTATCTGTG |
| IkBα | CCAACTACAACGGCCACA | CAACAGGAGCGAGACCAG |
| Ikkβ | CATTGTTGTTAGCGAGGAC | CCCTTTGCCGAGGTTGC |
| GAPDH | AAGAAGG TGGTGAAGCAGGC | TCCACCACCCT GTTGCTGTA |
| Compositions | Values (mg/g) |
|---|---|
| α-Carotene | 65 ± 1.15 |
| β-Carotene | 135 ± 2.31 |
| γ-Carotene | 0.5 ± 0.06 |
| Lycopene | 0.1 ± 0.01 |
| Total mixed-carotene complex | 200.6 |
| Compositions | Value (wt/wt) |
|---|---|
| α-Tocopherol | 12.5 ± 0.17 |
| α-Tocotrienol | 12.8 ± 0.06 |
| β-Tocotrienol | 2.0 ± 0.12 |
| γ-Tocotrienol | 19.5 ± 0.35 |
| δ-Tocotrienol | 5.5 ± 0.06 |
| Total mixed tocotrienols | 39.8 |
| Tocotrienol/tocopherol complex | 52.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, Y.; Pan, Z.; Yang, C.; Chen, L.; Wang, Y.; Xu, D.; Xia, H.; Wang, S.; Chen, S.; et al. Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways. Nutrients 2022, 14, 1094. https://doi.org/10.3390/nu14051094
Lu Y, Zhang Y, Pan Z, Yang C, Chen L, Wang Y, Xu D, Xia H, Wang S, Chen S, et al. Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways. Nutrients. 2022; 14(5):1094. https://doi.org/10.3390/nu14051094
Chicago/Turabian StyleLu, Yifei, Yihan Zhang, Zhenyu Pan, Chao Yang, Lin Chen, Yuanyuan Wang, Dengfeng Xu, Hui Xia, Shaokang Wang, Shiqing Chen, and et al. 2022. "Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways" Nutrients 14, no. 5: 1094. https://doi.org/10.3390/nu14051094
APA StyleLu, Y., Zhang, Y., Pan, Z., Yang, C., Chen, L., Wang, Y., Xu, D., Xia, H., Wang, S., Chen, S., Hao, Y. J., & Sun, G. (2022). Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways. Nutrients, 14(5), 1094. https://doi.org/10.3390/nu14051094








