Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy
Abstract
1. Introduction
2. Molecular and Cellular Interactions in Diabetic Retinopathy
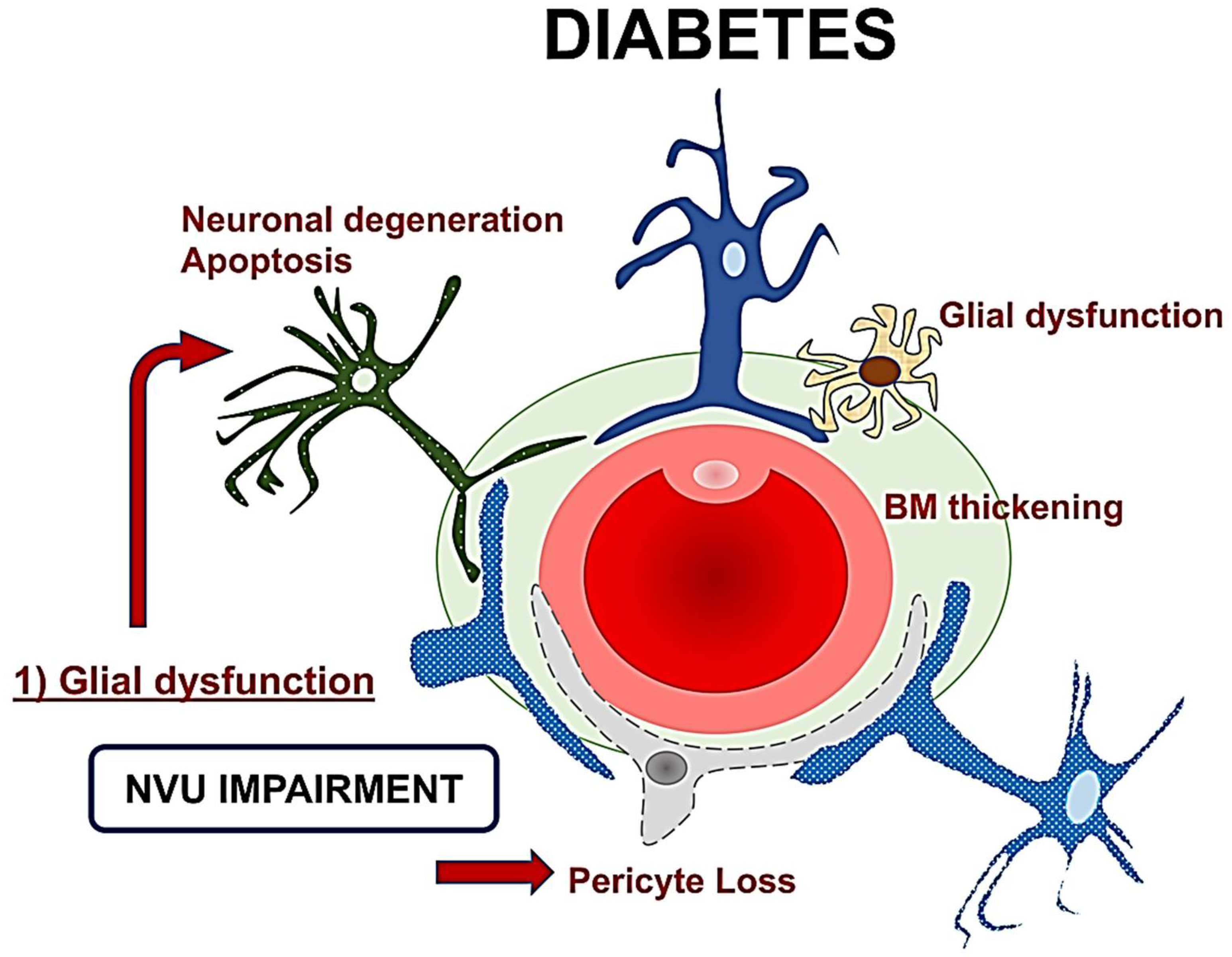
2.1. Neurovascular Unit (NVU)
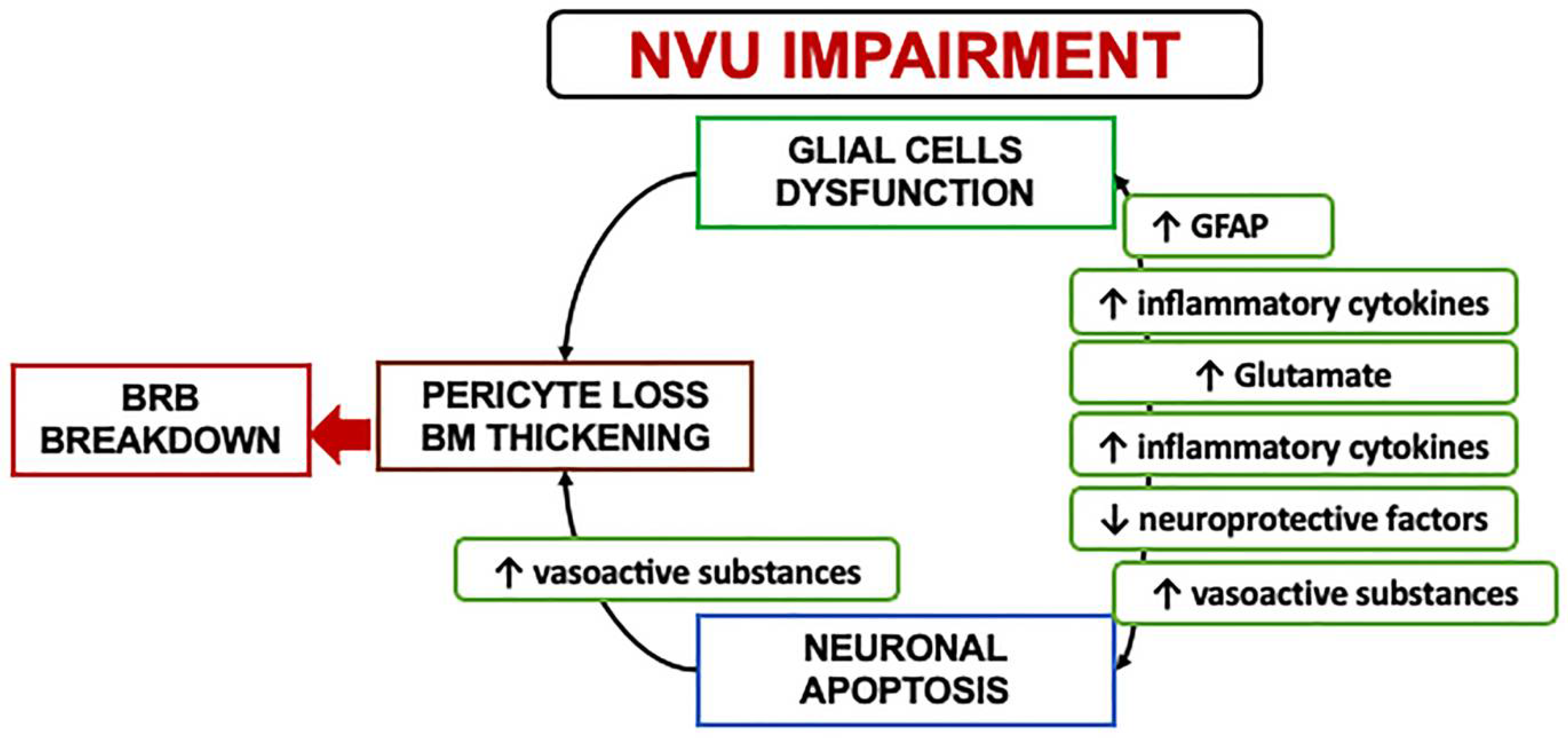
2.2. Neurovascular Unit Impairment in Diabetes
2.3. Mechanisms and Clinical Implications of Neurodegeneration
3. Clinical Hallmarks of Neurodegeneration in Diabetes
3.1. Functional Assessment
3.2. Structural Features on Retinal Imaging
4. Clinical Trials and In Vivo Evidence Targeting Neurodegeneration
4.1. The Effect of Topical Neuroprotective Agents
4.1.1. Microvascular Changes Induced by Brimonidine and Somatostatin
4.1.2. Serum Biomarkers and Retinal Neurodysfunction
4.2. Topical Citicoline
The Effect of Citicoline on Retinal Function
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end-products |
| BRB | blood-retinal barrier |
| DR | diabetic retinopathy |
| FDT | frequency doubling technology perimetry |
| GCL | ganglion cell layer |
| INL | inner nuclear layer |
| I | inner plexiform layer |
| PL | hyperreflective foci |
| HRF | implicit time |
| IT | multifocal electroretinogram |
| mfERG | optical coherence tomography |
| OCT | optical coherence tomography angiography |
| OCTA | outer plexiform layer |
| OPL | neurovascular unit |
| NVU | retinal ganglion cell layer |
| RGCs | retinal fiber layer |
References
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Cheng, C.Y.; Wong, T.Y.; Sabanayagam, C. Do we have enough ophthalmologists to manage vision-threatening diabetic retinopathy? A global perspective. Eye 2020, 34, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 2020, 127, S99–S119. [Google Scholar] [CrossRef]
- Rolev, K.D.; Shu, X.S.; Ying, Y. Targeted pharmacotherapy against neurodegeneration and neuroinflammation in early diabetic retinopathy. Neuropharmacology 2021, 187, 108498. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Rungger-Brandle, E.; Dosso, A.A.; Leuenberger, P.M. Glial reactivity, an early feature of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investg. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Verbraak, F.D.; Stehouwer, M.; Kok, P.H.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vis. Res. 2011, 51, 224–228. [Google Scholar] [CrossRef]
- Bearse, M.A., Jr.; Adams, A.J.; Han, Y.; Schneck, M.E.; Ng, J.; Bronson-Castain, K.; Barez, S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog. Retin Eye Res. 2006, 25, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.S.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Adams, A.J. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Investg. Ophthalmol. Vis. Sci. 2008, 49, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Jacobsen, C.H.; Adams, A.J. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Investg. Ophthalmol. Vis. Sci. 2004, 45, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.J.; Lipton, J.; Scase, M.O.; Foster, D.H.; Scarpello, J.H. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br. J. Ophthalmol. 1992, 76, 461–464. [Google Scholar] [CrossRef]
- Dosso, A.A.; Bonvin, E.R.; Morel, Y.; Golay, A.; Assal, J.P.; Leuenberger, P.M. Risk factors associated with contrast sensitivity loss in diabetic patients. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 300–305. [Google Scholar] [CrossRef]
- Di Leo, M.A.; Caputo, S.; Falsini, B.; Porciatti, V.; Minnella, A.; Greco, A.V.; Ghirlanda, G. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care 1992, 15, 620–625. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Kok, P.H.; Garvin, M.; Sonka, M.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; Verbraak, F.D.; Abramoff, M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3404–3409. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef]
- Gundogan, F.C.; Akay, F.; Uzun, S.; Yolcu, U.; Cagiltay, E.; Toyran, S. Early Neurodegeneration of the Inner Retinal Layers in Type 1 Diabetes Mellitus. Ophthalmologica 2016, 235, 125–132. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Z.; Bao, T.; Wang, S.; Jiang, T.; Zhang, Y.; Li, Q.; Zhu, X. Evaluation of Early Retinal Nerve Injury in Type 2 Diabetes Patients Without Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 475672. [Google Scholar] [CrossRef]
- Hernandez, C.; dal Monte, M.; Simo, R.; Casini, G. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J. Diabetes Res. 2016, 2016, 9508541. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T. Diabetic retinopathy: Neurovascular disease requiring neuroprotective and regenerative therapies. Neural Regen. Res. 2022, 17, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Taylor, W.R.; Kuhlbrodt, K.; Wiessner, M. High-affinity glutamate transporters in the rat retina: A major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell Tissue Res. 1998, 291, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Hosoi, N.; Tachibana, M. Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons. J. Neurosci. 1999, 19, 6755–6766. [Google Scholar] [CrossRef]
- Derouiche, A.; Rauen, T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: Evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res. 1995, 42, 131–143. [Google Scholar] [CrossRef]
- Barbour, B.; Brew, H.; Attwell, D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature 1988, 335, 433–435. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Watanabe, M.; Inoue, Y.; Sakagawa, T.; Nakayama, N.; Sasaki, S.; Okuyama, S.; Watase, K.; Wada, K.; et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc. Natl. Acad. Sci. USA 1998, 95, 4663–4666. [Google Scholar] [CrossRef]
- Biedermann, B.; Bringmann, A.; Reichenbach, A. High-affinity GABA uptake in retinal glial (Muller) cells of the guinea pig: Electrophysiological characterization, immunohistochemical localization, and modeling of efficiency. Glia 2002, 39, 217–228. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Muller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004, 36, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Horio, Y.; Inanobe, A.; Fujita, A.; Haug, F.M.; Nielsen, S.; Kurachi, Y.; Ottersen, O.P. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 1999, 26, 47–54. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Veruki, M.L.; Torp, R.; Haug, F.M.; Laake, J.H.; Nielsen, S.; Agre, P.; Ottersen, O.P. Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in Muller cells and fibrous astrocytes. J. Neurosci. 1998, 18, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Iandiev, I.; Pannicke, T.; Reichel, M.B.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci. Lett. 2005, 388, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Taurone, S.; Spoletini, M.; Biro, Z.; Varsanyi, B.; Scuderi, G.; Orlando, M.P.; Turchetta, R.; Micera, A.; Artico, M. Ultrastructure of neurovascular changes in human diabetic retinopathy. Int. J. Immunopathol. Pharmacol. 2018, 31, 394632017748841. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Li, Q.; Puro, D.G. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Investg. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Kowluru, R.A.; Engerman, R.L.; Case, G.L.; Kern, T.S. Retinal glutamate in diabetes and effect of antioxidants. Neurochem. Int. 2001, 38, 385–390. [Google Scholar] [CrossRef]
- Lieth, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef]
- Ghaseminejad, F.; Kaplan, L.; Pfaller, A.M.; Hauck, S.M.; Grosche, A. The role of Muller cell glucocorticoid signaling in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 221–230. [Google Scholar] [CrossRef]
- Eichler, W.; Kuhrt, H.; Hoffmann, S.; Wiedemann, P.; Reichenbach, A. VEGF release by retinal glia depends on both oxygen and glucose supply. Neuroreport 2000, 11, 3533–3537. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ramirez, M.; Hernandez, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; Garcia-Arumi, J.; Simo, R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Hernandez, C.; Miralles, A.; Huguet, P.; Farres, J.; Simo, R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investg. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Mathur, A.; Kakkar, P. Emerging role of Unfolded Protein Response (UPR) mediated proteotoxic apoptosis in diabetes. Life Sci. 2019, 216, 246–258. [Google Scholar] [CrossRef]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the pathogenesis of diabetic retinopathy: Molecular mechanisms and therapeutic implications. Curr. Med. Chem. 2013, 20, 3241–3250. [Google Scholar] [CrossRef]
- Jackson, G.R.; Scott, I.U.; Quillen, D.A.; Walter, L.E.; Gardner, T.W. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012, 96, 699–703. [Google Scholar] [CrossRef]
- Greenstein, V.C.; Thomas, S.R.; Blaustein, H.; Koenig, K.; Carr, R.E. Effects of early diabetic retinopathy on rod system sensitivity. Optom. Vis. Sci. 1993, 70, 18–23. [Google Scholar] [CrossRef]
- Parravano, M.; Oddone, F.; Mineo, D.; Centofanti, M.; Borboni, P.; Lauro, R.; Tanga, L.; Manni, G. The role of Humphrey Matrix testing in the early diagnosis of retinopathy in type 1 diabetes. Br. J. Ophthalmol. 2008, 92, 1656–1660. [Google Scholar] [CrossRef]
- Montesano, G.; Ometto, G.; Higgins, B.E.; Das, R.; Graham, K.W.; Chakravarthy, U.; McGuiness, B.; Young, I.S.; Kee, F.; Wright, D.M.; et al. Evidence for Structural and Functional Damage of the Inner Retina in Diabetes with No Diabetic Retinopathy. Investg. Ophthalmol. Vis. Sci. 2021, 62, 35. [Google Scholar] [CrossRef]
- Gualtieri, M.; Bandeira, M.; Hamer, R.D.; Damico, F.M.; Moura, A.L.; Ventura, D.F. Contrast sensitivity mediated by inferred magno- and parvocellular pathways in type 2 diabetics with and without nonproliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Ribeiro, L.; Bandello, F.; Lattanzio, R.; Egan, C.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; Gibson, J.; Grauslund, J.; Harding, S.P.; et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes 2017, 66, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Schneck, M.E.; Bearse, M.A., Jr.; Barez, S.; Jacobsen, C.H.; Jewell, N.P.; Adams, A.J. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Investg. Ophthalmol. Vis. Sci. 2004, 45, 4106–4112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, W.W.; Bearse, M.A., Jr.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investg. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef]
- McAnany, J.J.; Persidina, O.S.; Park, J.C. Clinical electroretinography in diabetic retinopathy: A review. Surv. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Huang, S.; Wu, D. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc. Ophthalmol. 2008, 117, 129–135. [Google Scholar] [CrossRef]
- McFarlane, M.; Wright, T.; Stephens, D.; Nilsson, J.; Westall, C.A. Blue flash ERG PhNR changes associated with poor long-term glycemic control in adolescents with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 741–748. [Google Scholar] [CrossRef]
- Gerendas, B.S.; Hatz, K.; Kaider, A.; Zulewski, H.; Lehmann, R.; Montuoro, A.; Schmidt-Erfurth, U.; Pruente, C. Ganglion cell layer thickening in well-controlled patients with type 1 diabetes: An early sign for diabetic retinopathy? Acta Ophthalmol. 2020, 98, e292–e300. [Google Scholar] [CrossRef]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence from Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, I.T.; Park, C.K. Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coherence tomography. Br. J. Ophthalmol. 2011, 95, 1223–1228. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pritchard, N.; Sampson, G.P.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Retinal tissue thickness in type 1 and type 2 diabetes. Clin. Exp. Optom. 2016, 99, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Pritchard, N.; Sampson, G.P.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Retinal thickness profile of individuals with diabetes. Ophthalmic Physiol. Opt. 2016, 36, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wanek, J.; Blair, N.P.; Chau, F.Y.; Lim, J.I.; Leiderman, Y.I.; Shahidi, M. Alterations in Retinal Layer Thickness and Reflectance at Different Stages of Diabetic Retinopathy by En Face Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT341-347. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef] [PubMed]
- Carpineto, P.; Toto, L.; Aloia, R.; Ciciarelli, V.; Borrelli, E.; Vitacolonna, E.; di Nicola, M.; di Antonio, L.; Mastropasqua, R. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye 2016, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal Nerve Fiber Layer and Ganglion Cell Complex Thicknesses Are Reduced in Children with Type 1 Diabetes with No Evidence of Vascular Retinopathy. Investg. Ophthalmol. Vis. Sci. 2016, 57, 5355–5360. [Google Scholar] [CrossRef]
- Scuderi, G.; Fragiotta, S.; Scuderi, L.; Iodice, C.M.; Perdicchi, A. Ganglion Cell Complex Analysis in Glaucoma Patients: What Can It Tell Us? Eye Brain 2020, 12, 33–44. [Google Scholar] [CrossRef]
- Scarinci, F.; Picconi, F.; Virgili, G.; Giorno, P.; di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Single Retinal Layer Evaluation in Patients with Type 1 Diabetes with No or Early Signs of Diabetic Retinopathy: The First Hint of Neurovascular Crosstalk Damage between Neurons and Capillaries? Ophthalmologica 2017, 237, 223–231. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yan, Y.; Shen, X. Diabetic macular morphology changes may occur in the early stage of diabetes. BMC Ophthalmol. 2016, 16, 12. [Google Scholar] [CrossRef]
- Montesano, G.; Bryan, S.R.; Crabb, D.P.; Fogagnolo, P.; Oddone, F.; McKendrick, A.M.; Turpin, A.; Lanzetta, P.; Perdicchi, A.; Johnson, C.A.; et al. A Comparison between the Compass Fundus Perimeter and the Humphrey Field Analyzer. Ophthalmology 2019, 126, 242–251. [Google Scholar] [CrossRef]
- Fragiotta, S.; Carnevale, C.; Cutini, A.; Rigoni, E.; Grenga, P.L.; Vingolo, E.M. Factors Influencing Fixation Stability Area: A Comparison of Two Methods of Recording. Optom. Vis. Sci. 2018, 95, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, H.; Choi, Y.A.; Kim, H.C.; Chung, H. Association Between Soluble CD14 in the Aqueous Humor and Hyperreflective Foci on Optical Coherence Tomography in Patients with Diabetic Macular Edema. Investg. Ophthalmol. Vis. Sci. 2018, 59, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Bini, S.; Torresin, T.; Berton, M.; Midena, G.; Parrozzani, R.; Martini, F.; Pucci, P.; Daniele, A.R.; Cavarzeran, F.; et al. Hyperreflective retinal spots in normal and diabetic eyes: B-Scan and En Face Spectral Domain Optical Coherence Tomography Evaluation. Retina 2017, 37, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Frydkjaer-Olsen, U.; Soegaard Hansen, R.; Simó, R.; Cunha-Vaz, J.; Peto, T.; Grauslund, J. Correlation between Retinal Vessel Calibre and Neurodegeneration in Patients with Type 2 Diabetes Mellitus in the European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Ophthalmic Res. 2016, 56, 10–16. [Google Scholar] [CrossRef]
- Hernandez, C.; Garcia-Ramirez, M.; Corraliza, L.; Fernandez-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Simo, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Saylor, M.; McLoon, L.K.; Harrison, A.R.; Lee, M.S. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: An evidence-based review. Arch. Ophthalmol. 2009, 127, 402–406. [Google Scholar] [CrossRef]
- Grauslund, J.; Frydkjaer-Olsen, U.; Peto, T.; Fernández-Carneado, J.; Ponsati, B.; Hernández, C.; Cunha-Vaz, J.; Simó, R. Topical Treatment with Brimonidine and Somatostatin Causes Retinal Vascular Dilation in Patients with Early Diabetic Retinopathy From the EUROCONDOR. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2257–2262. [Google Scholar] [CrossRef]
- Knudtson, M.D.; Lee, K.E.; Hubbard, L.D.; Wong, T.Y.; Klein, R.; Klein, B.E. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 2003, 27, 143–149. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Moss, S.E.; Wong, T.Y. Retinal Vessel Caliber and Microvascular and Macrovascular Disease in Type 2 Diabetes. Ophthalmology 2007, 114, 1884–1892. [Google Scholar] [CrossRef]
- Wong, T.Y.; Islam, F.M.A.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Castro, C.; Sharrett, A.R.; Shahar, E. Retinal Vascular Caliber, Cardiovascular Risk Factors, and Inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA). Investig. Opthalmol. Vis. Sci. 2006, 47, 2341. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons. Arch. Intern. Med. 2006, 166, 2388. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Wong, T.Y.; Tsai, M.Y. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch. Ophthalmol. 2006, 124, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Mangino, G.; Iuliano, M.; Potenza, C.; Bernardini, N.; Skroza, N.; Vingolo, E.M.; Romeo, G. Role of CD 20(+) T cells and related cytokines in mediating retinal microvascular changes and ocular complications in chronic-plaque type psoriasis. Cytokine 2020, 136, 155253. [Google Scholar] [CrossRef]
- Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; et al. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. J. Clin. Med. 2020, 9, 1233. [Google Scholar] [CrossRef]
- Oddone, F.; Rossetti, L.; Parravano, M.; Sbardella, D.; Coletta, M.; Ziccardi, L.; Roberti, G.; Carnevale, C.; Romano, D.; Manni, G.; et al. Citicoline in Ophthalmological Neurodegenerative Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 281. [Google Scholar] [CrossRef]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5’-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Barbano, L.; Giorno, P.; Varano, M.; Parravano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 36-Month Pilot Study Evaluating Macular Electrophysiological Changes. Adv. Ther. 2021, 38, 3924–3936. [Google Scholar] [CrossRef]
- Parravano, M.; Scarinci, F.; Parisi, V.; Giorno, P.; Giannini, D.; Oddone, F.; Varano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 3-year Pilot Study Evaluating Morpho-Functional Retinal Changes. Adv. Ther. 2020, 37, 1646–1663. [Google Scholar] [CrossRef]
- Miller, D.J.; Fort, P.E. Heat Shock Proteins Regulatory Role in Neurodevelopment. Front. Neurosci. 2018, 12, 821. [Google Scholar] [CrossRef]
- Battaglia Parodi, M.; Brunoro, A.; Tomasso, L.; Scuderi, G. Benefits of micronutrient supplementation for reducing the risk of wet age-related macular disease and diabetic retinopathy: An update. Eur. J. Ophthalmol. 2020, 30, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Vingolo, E.M.; Fragiotta, S.; Mafrici, M.; Cutini, A.; Marinelli, C.; Concistre, A.; Iannucci, G.; Petramala, L.; Letizia, C. Vitreous and plasma changes of endothelin-1, adrenomedullin and vascular endothelium growth factor in patients with proliferative diabetic retinopathy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 662–668. [Google Scholar] [PubMed]
- Sanz-Gonzalez, S.M.; Garcia-Medina, J.J.; Zanon-Moreno, V.; Lopez-Galvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Valero-Vello, M.; Ramirez, A.I.; Arevalo, J.F.; Pinazo-Duran, M.D.; et al. Clinical and Molecular-Genetic Insights into the Role of Oxidative Stress in Diabetic Retinopathy: Antioxidant Strategies and Future Avenues. Antioxidants 2020, 9, 1101. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef]
- Roig-Revert, M.J.; Lleo-Perez, A.; Zanon-Moreno, V.; Vivar-Llopis, B.; Marin-Montiel, J.; Dolz-Marco, R.; Alonso-Munoz, L.; Albert-Fort, M.; Lopez-Galvez, M.I.; Galarreta-Mira, D.; et al. Enhanced Oxidative Stress and Other Potential Biomarkers for Retinopathy in Type 2 Diabetics: Beneficial Effects of the Nutraceutic Supplements. Biomed. Res. Int. 2015, 2015, 408180. [Google Scholar] [CrossRef]
- Rodriguez-Carrizalez, A.D.; Castellanos-Gonzalez, J.A.; Martinez-Romero, E.C.; Miller-Arrevillaga, G.; Pacheco-Moises, F.P.; Roman-Pintos, L.M.; Miranda-Diaz, A.G. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: A phase IIa, randomized, double-blind, and placebo-controlled study. Redox Rep. 2016, 21, 155–163. [Google Scholar] [CrossRef]
- Rodriguez-Carrizalez, A.D.; Castellanos-Gonzalez, J.A.; Martinez-Romero, E.C.; Miller-Arrevillaga, G.; Villa-Hernandez, D.; Hernandez-Godinez, P.P.; Ortiz, G.G.; Pacheco-Moises, F.P.; Cardona-Munoz, E.G.; Miranda-Diaz, A.G. Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J. Diabetes 2014, 6, 167–175. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragiotta, S.; Pinazo-Durán, M.D.; Scuderi, G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients 2022, 14, 792. https://doi.org/10.3390/nu14040792
Fragiotta S, Pinazo-Durán MD, Scuderi G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients. 2022; 14(4):792. https://doi.org/10.3390/nu14040792
Chicago/Turabian StyleFragiotta, Serena, Maria D. Pinazo-Durán, and Gianluca Scuderi. 2022. "Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy" Nutrients 14, no. 4: 792. https://doi.org/10.3390/nu14040792
APA StyleFragiotta, S., Pinazo-Durán, M. D., & Scuderi, G. (2022). Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients, 14(4), 792. https://doi.org/10.3390/nu14040792





