Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units
Abstract
:1. Introduction
2. Methods
2.1. Population and Study Design
2.2. Sarcopenia Determination
2.3. Nutritional Assessment
2.4. Other Parameters Investigated
2.5. Statistical Analyses
3. Results
3.1. Patients Characteristics
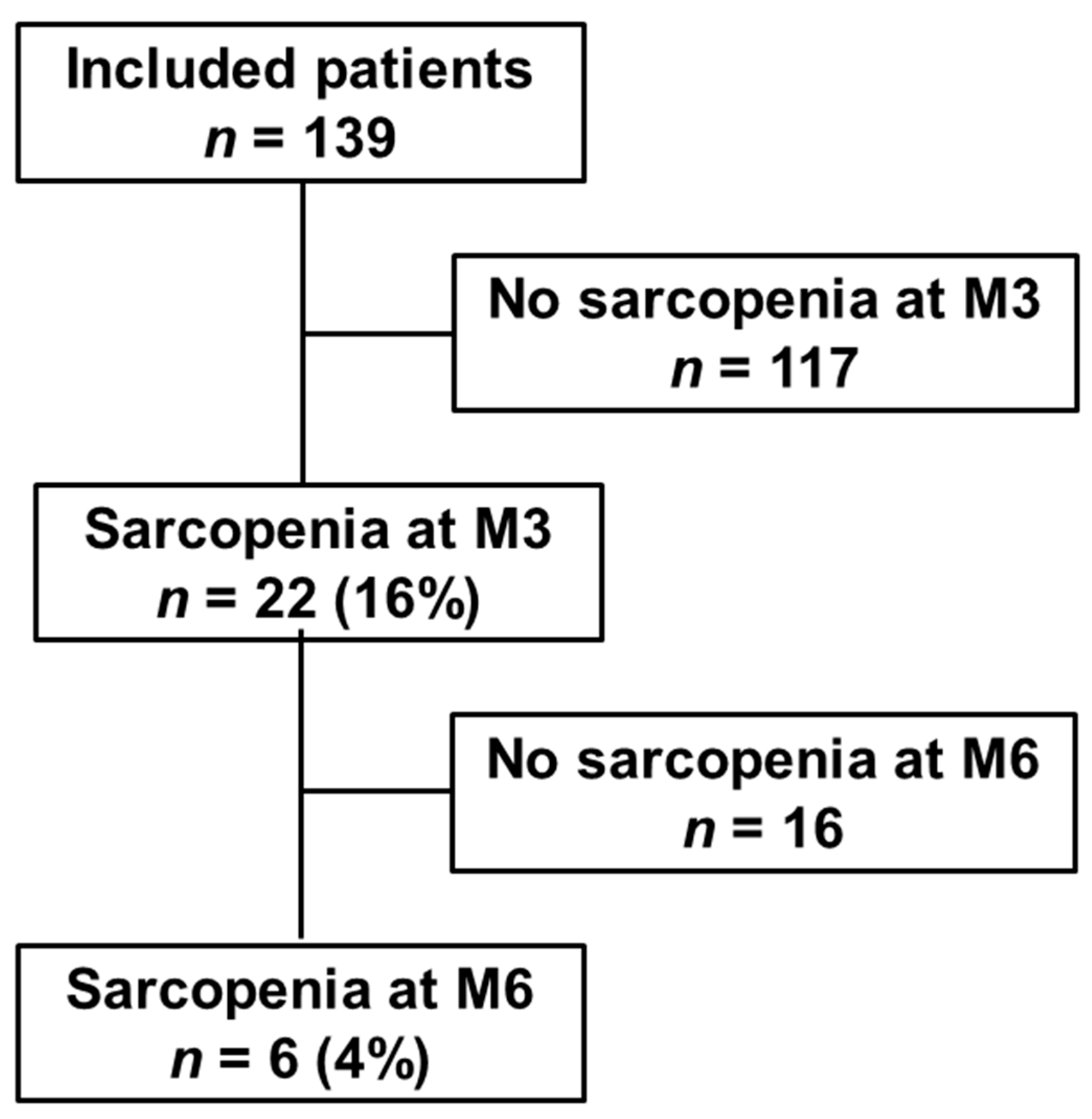
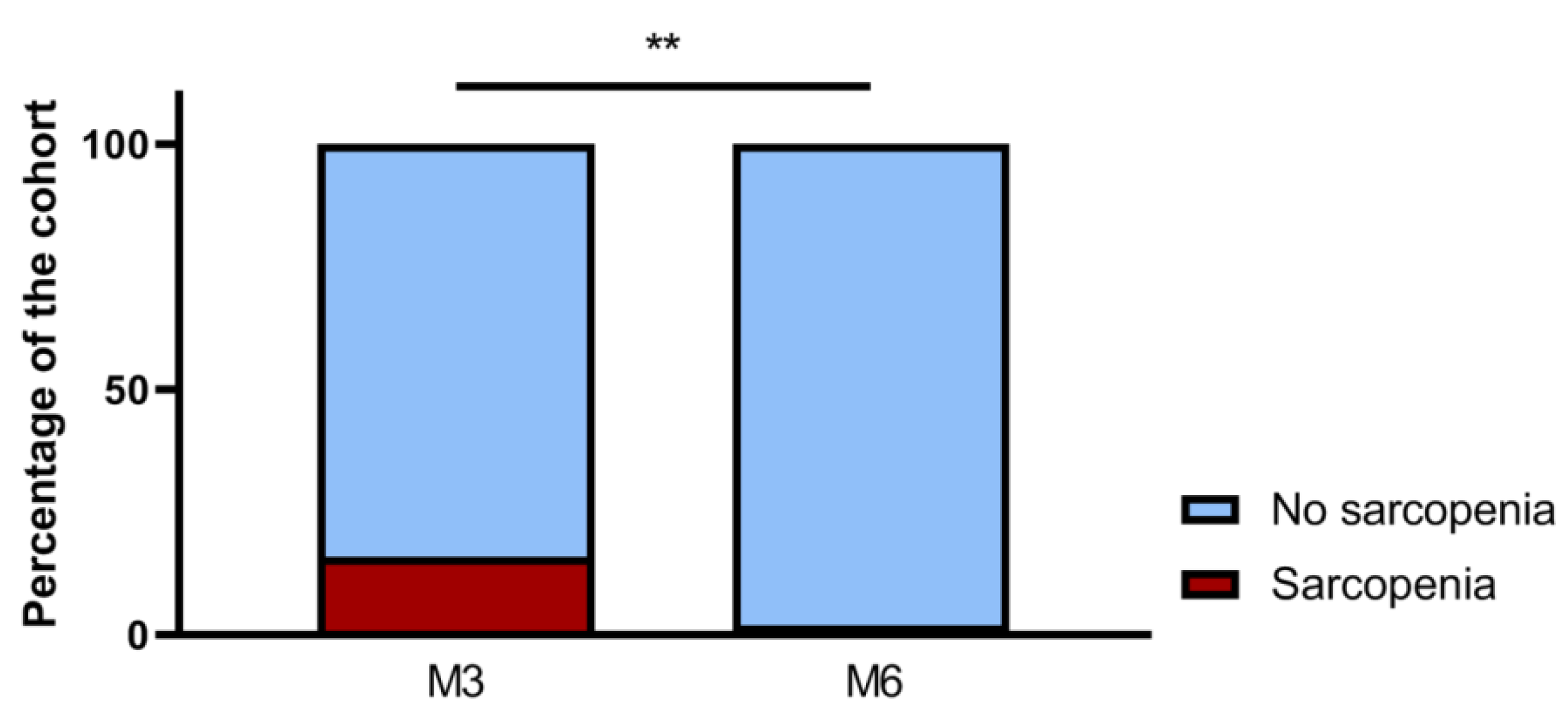
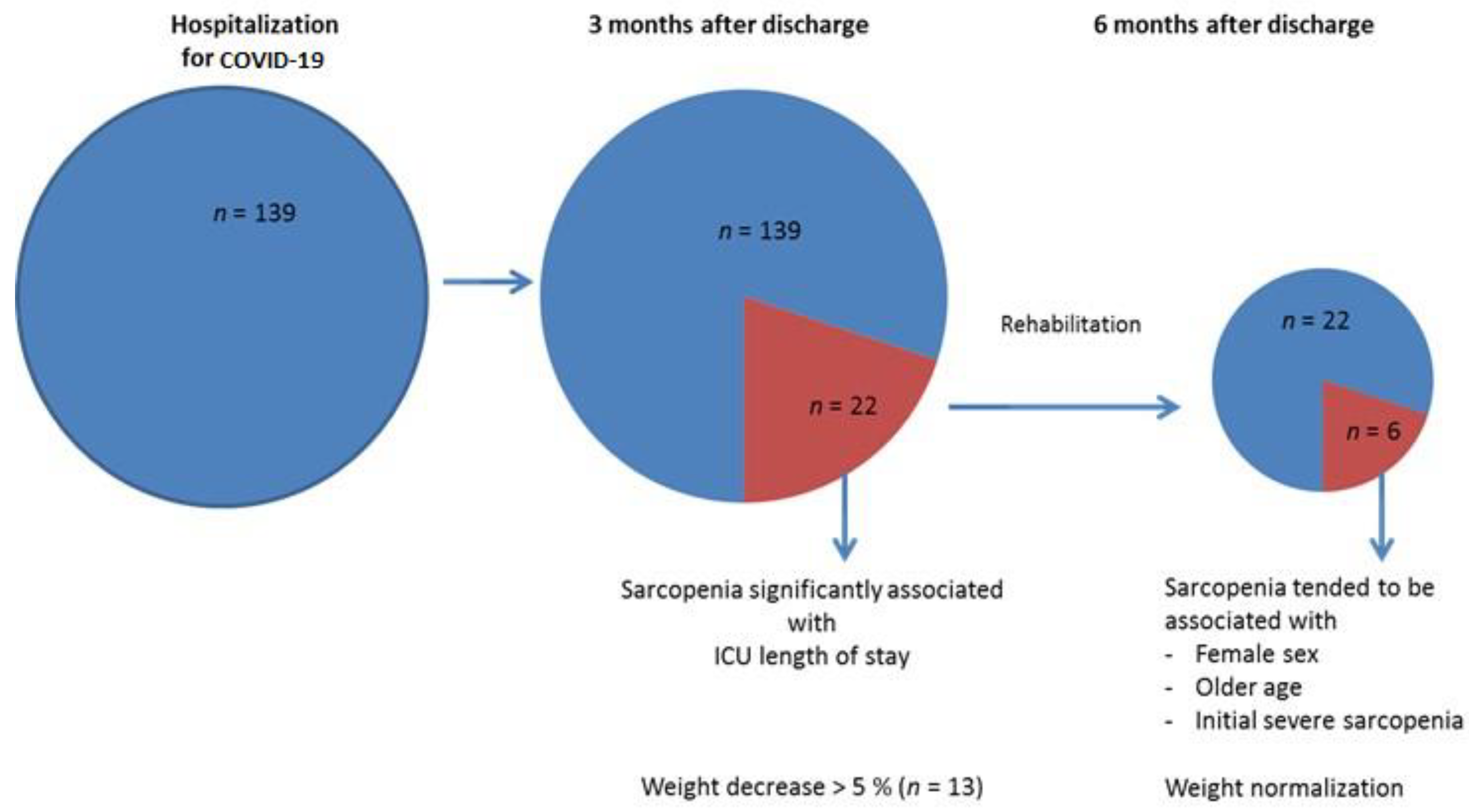
3.2. Sarcopenia Evolution 3 and 6 months after Hospitalization for COVID-19
3.3. Weight Evolutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-Reported Impact of the COVID-19 Pandemic on Nutrition and Physical Activity Behaviour in Dutch Older Adults Living Independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, J.; Colicchio, B.; Andrès, E.; Geny, B.; Dieterlen, A. Lockdown Effect on Elderly Nutritional Health. J. Clin. Med. 2021, 10, 5052. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated Factors of Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Clinical Management: Living Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 15 December 2021).
- Gérard, M.; Mahmutovic, M.; Malgras, A.; Michot, N.; Scheyer, N.; Jaussaud, R.; Nguyen-Thi, P.L.; Quilliot, D. Long-Term Evolution of Malnutrition and Loss of Muscle Strength after COVID-19: A Major and Neglected Component of Long COVID-19. Nutrients. 2021, 13, 3964. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damanti, S.; Cristel, G.; Ramirez, G.A.; Bozzolo, E.P.; Da Prat, V.; Gobbi, A.; Centurioni, C.; Di Gaeta, E.; Del Prete, A.; Calabrò, M.G.; et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Riesgo, H.; Castro, A.; Del Amo, S.; San Ceferino, M.J.; Izaola, O.; Primo, D.; Gómez Hoyos, E.; López Gómez, J.J.; de Luis, D.A. Prevalence of Risk of Malnutrition and Risk of Sarcopenia in a Reference Hospital for COVID-19: Relationship with Mortality. Ann. Nutr. Metab. 2021, 77, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Jacob Filho, W.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira Júnior, G.N.; Swinton, P.; Gualano, B.; et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: A prospective observational study. J. Cachexia Sarcopenia Muscle 2021, 12, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachexia Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef]
- Meyer, A.; Zoll, J.; Charles, A.L.; Charloux, A.; de Blay, F.; Diemunsch, P.; Sibilia, J.; Piquard, F.; Geny, B. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: Central actor and therapeutic target. Exp. Physiol. 2013, 98, 1063–1078. [Google Scholar] [CrossRef]
- Manzano, G.S.; Woods, J.K.; Amato, A.A. COVID-19-Associated Myopathy Caused by Type I Interferonopathy. N. Engl. J. Med. 2020, 383, 2389–2390. [Google Scholar] [CrossRef]
- Kiekens, C.; Boldrini, P.; Andreoli, A.; Avesani, R.; Gamna, F.; Grandi, M.; Lombardi, F.; Lusuardi, M.; Molteni, F.; Perboni, A.; et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 323–326. [Google Scholar] [CrossRef]
- Kress, J.P.; Hall, J.B. ICU-Acquired Weakness and Recovery from Critical Illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Qaisar, R.; Karim, A.; Muhammad, T.; Shah, I.; Iqbal, M.S. The coupling between sarcopenia and COVID-19 is the real problem. Eur. J. Intern. Med. 2021, 93, 105–106. [Google Scholar] [CrossRef]
- HAS. Diagnostic de la Dénutrition de l’Enfant et de l’Adulte. Saint-Denis La Plaine. Available online: https://www.hassante.fr/upload/docs/application/pdf/2019-11/reco277_recommandations_rbp_denutrition_cd_2019_11_13_v0.pdf (accessed on 20 July 2021).
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef]
- Rodrigues, M.; Costa, A.J.; Santos, R.; Diogo, P.; Gonçalves, E.; Barroso, D.; Almeida, M.P.; Vaz, I.M.; Lima, A. Inpatient rehabilitation can improve functional outcomes of post-intensive care unit COVID-19 patients-a prospective study. Disabil. Rehabil. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Quilliot, D.; Gérard, M.; Bonsack, O.; Malgras, A.; Vaillant, M.F.; Di Patrizio, P.; Jaussaud, R.; Ziegler, O.; Nguyen-Thi, P.L. Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open. 2021, 14, e048948. [Google Scholar] [CrossRef]
- Gobbi, M.; Bezzoli, E.; Ismelli, F.; Trotti, G.; Cortellezzi, S.; Meneguzzo, F.; Arreghini, M.; Seitanidis, I.; Brunani, A.; Aspesi, V.; et al. Skeletal Muscle Mass, Sarcopenia and Rehabilitation Outcomes in Post-Acute COVID-19 Patients. J. Clin. Med. 2021, 10, 5623. [Google Scholar] [CrossRef]
- Rengo, J.L.; Khadanga, S.; Savage, P.D.; Ades, P.A. Response to Exercise Training During Cardiac Rehabilitation Differs by Sex. J. Cardiopulm. Rehabil. Prev. 2020, 40, 319–324. [Google Scholar] [CrossRef]
- Paolucci, S.; Bragoni, M.; Coiro, P.; De Angelis, D.; Fusco, F.R.; Morelli, D.; Venturiero, V.; Pratesi, L. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke 2006, 37, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Recommandations sur les Alimentations en Établissements de Santé. Available online: https://www.sfncm.org/1186-recommandations-sur-les-alimentations-en-etablissements-de-sante (accessed on 24 January 2022).
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Hosein, E.; Harvey, M.; Nguyen, T.; Givan, A.; Hamilton, M.; Turner, K.; Kretzmer, R.; Rock, M.; Swartz, M.C.; et al. Impact of COVID-19 Infection and Persistent Lingering Symptoms on Patient Reported Indicators of Nutritional Risk and Malnutrition. Nutrients 2022, 14, 642. [Google Scholar] [CrossRef]

| Global Distribution (n = 139) | Sarcopenic Patients (n = 22) | Non-Sarcopenic Patients (n = 117) | Uni-Variate p-Value | Multi-Variate p-Value | |
|---|---|---|---|---|---|
| Age, years (range) | 62 (29–82) | 65.5 (37–82) | 62 (29–81) | 0.07 | — |
| Female, n (%) | 44 (32) | 5 (23) | 39 (33) | 0.45 | — |
| Weigh, kg | 86 (55–133) | 79.5 (63–104) | 89 (55–133) | 0.008 | — |
| Height, cm | 172 (148–196) | 170.5 (154–185) | 172 (148–196) | 0.71 | — |
| BMI, kg/m2 | 29 (21–44) | 26.6 (21.8–32) | 29.4 (21–44) | 0.004 | — |
| Obesity, n (%) | 60 (43) | 5 (23) | 55 (47) | 0.03 | — |
| COMORBIDITIES, n (%) | 97 (70) | 18 (82) | 79 (68) | 0.21 | |
| Hypertension, n (%) | 80 (58) | 16 (73) | 64 (55) | 0.12 | — |
| Diabetes, n (%) | 44 (32) | 9 (41) | 35 (30) | 0.31 | — |
| Smoker, n (%) | 28 (20) | 6 (27) | 22 (19) | 0.36 | — |
| Asthma, n (%) | 4 (3) | 1 (5) | 3 (3) | 0.50 | — |
| COPD, n (%) | 5 (4) | 2 (9) | 3 (3) | 0.18 | — |
| Asthma or COPD, n (%) | 9 (6) | 3 (14) | 6 (5) | 0.15 | — |
| Chronic cardiac failure, n (%) | 4 (3) | 0 | 4 (3) | 1 | — |
| Chronic renal failure, n (%) | 7 (5) | 1 (5) | 6 (5) | 1 | — |
| HOSPITALIZATION DATA | |||||
| COVID-19 severity, moderate/severe/critical, n (%) | 18 (13)/17 (12)/104 (75) | 0/0/22 (100) | 18 (15)/17 (15)/82 (70) | 0.01 | 0.89 |
| Total hospital length of stay, day | 21 (2–156) | 41.5 (6–156) | 20 (2–120) | 0.01 | — |
| ICU admission, n (%) | 99 (71) | 18 (82) | 81 (69) | 0.23 | — |
| ICU length of stay, day (range) | 7 (0–115) | 24 (0–115) | 7 (0–71) | 0.01 | 0.01 |
| Tracheostomy, n (%) | 19 (14) | 7 (32) | 12 (10) | 0.01 | — |
| Rehabilitation after discharge, n (%) | 93 (68) | 20 (91) | 67 (61) | 0.007 | 0.16 |
| Duration of rehabilitation, day (range) | 14 (0–70) | 30 (0–62) | 0 (0–70) | 0.002 | — |
| MUSCLE EXPLORATIONS | |||||
| Dynapenia, n (%) | 45 (33) | 22 (100) | 23 (20) | <0.001 | — |
| Maximal right strength, kg(range) | 28 (4–56) | 18 (4–26) | 30 (8–56) | <0.001 | — |
| Maximal left strength, kg (range) | 26 (3–56) | 16.5 (3–25) | 28 (3–56) | <0.001 | — |
| Low muscle mass (DXA), n (%) | 29 (22) | 22 (100) | 7 (6) | <0.001 | — |
| Appendicular muscle mass, kg/m2 (range) | 7.26 (4.24–13.3) | 6.26 (4.92–6.98) | 7.62 (4.24–13.3) | <0.001 | — |
| Sarcopenia, n (%) | 22 (16) | ||||
| Severe Sarcopenia, n (%) | 5/139 (4) | 5 (23) | |||
| PULMONARY FUNCTION TESTS | |||||
| FEV, ml (range) | 2705 (1120–4720) | 2450 (1120–3940) | 2710 (1350–4720) | 0.75 | — |
| FEV, % | 97 (13–147) | 96.5 (36–124) | 97 (13–147) | 0.68 | — |
| FVC, ml (range) | 3480 (1420–5430) | 3470 (1420–5430) | 3490 (1580–5290) | 0.64 | — |
| FVC, % (range) | 100 (35–155) | 99 (35–129) | 101 (38–155) | 0.44 | — |
| FEV/FVC (range) | 80 (40–100) | 79 (57–91) | 80 (40–100) | 0.91 | — |
| DLCO, % (range) | 72 (21–115) | 63 (40–88) | 74 (21–115) | 0.11 | — |
| MMEF, % (range) | 108 (41–175) | 100 (46–175) | 108 (41–175) | 0.97 | — |
| RV, % (range) | 85 (37–148) | 81 (48–135) | 87 (37–148) | 0.70 | — |
| CARDIOLOGIC EXPLORATIONS | |||||
| LVEF, % (range) | 62 (35–75) | 62 (43–74) | 62 (35–75) | 0.48 | — |
| Patients with Persistent Sarcopenia at M6 (n = 6) | Patients Recovering from Sarcopenia at M6 (n = 16) | p-Value | |
|---|---|---|---|
| Age, years (range) | 70.0 (64–80) | 63.5 (37–82) | 0.14 |
| Female, n (%) | 3 (50) | 2 (13) | 0.10 |
| Weigh, kg | 72.5 (70–82) | 81 (63–104) | 0.05 |
| Height, cm | 165 (154–180) | 172 (160–185) | 0.09 |
| BMI, kg/m2 | 21.3 (23.5–30) | 26.7 (21.8–32.0) | 0.83 |
| Obesity, n (%) | 1 (17) | 4 (25) | 1.0 |
| Comorbidities, n (%) | 5 (83) | 13 (81) | 1.0 |
| Hypertension, n (%) | 5 (83) | 11 (69) | 0.63 |
| Diabetes, n (%) | 1 (17) | 8 (50) | 0.33 |
| Smoker, n (%) | 1 (17) | 5 (31) | 0.63 |
| Asthma, n (%) | 1 (17) | 0 | 0.27 |
| COPD, n (%) | 0 | 2 (13) | 1.0 |
| Asthma or COPD, n (%) | 1 (17) | 2 (13) | 1.0 |
| Chronic cardiac failure, n (%) | 0 | 0 | 1.0 |
| Chronic renal failure, n (%) | 1 (17) | 0 | 0.27 |
| HOSPITALIZATION DATA | |||
| COVID-19 severity, moderate/severe/critical, n (%) | 0/0/6 | 0/0/16 | 1.0 |
| Total hospital length of stay, day (range) | 48.5 (10–86) | 39 (6–156) | 0.48 |
| ICU admission, n (%) | 5 (83) | 13 (81) | 1.0 |
| ICU length of stay, day (range) | 38.5 (0–65) | 22.5 (0–115) | 0.55 |
| Tracheotomy, n (%) | 1 (17) | 6 (38) | 0.62 |
| Rehabilitation after discharge, n (%) | 5 (83) | 15 (94) | 0.48 |
| Duration of rehabilitation, day (range) | 43.5 (0–60) | 26 (0–62) | 0.67 |
| MUSCLE EXPLORATIONS | |||
| M3 Severe Sarcopenia, n (%)M6 Severe Sarcopenia, n (%) | 3 (60)3 (60) | 4 (25)0 | 0.281.0 |
| Right strength variation between M3 and M6, kg | +5 (−0.8; +18) | +11 (4.5; +24) | 0.30 |
| Left strength variation between M3 and M6, kg | +10 (+3, +15) | +16.5 (+9; +19) | 0.11 |
| ALM variation between M3 and M6, kg/m2 | +0.11 (−0.15; +0.89) | +0.63 (−0.42; +1.69) | 0.16 |
| PULMONARY FUNCTION TESTS | |||
| FEV, ml (range) | 2055 (1120–2250) | 2820 (1220–3940) | 0.03 |
| FEV, % | 72.8 (36–123) | 97.5 (59.5–124) | 0.25 |
| FVC, ml (range) | 2540 (1420–3360) | 3666 (1580–5430) | 0.07 |
| FVC, % (range) | 80.7 (35–121) | 99 (62.5–128.6) | 0.46 |
| FEV/FVC (range) | 81.5 (57–91) | 79 (68–86) | 0.61 |
| DLCO, % (range) | 60 (53-85.7) | 64.5 (38–89) | 0.67 |
| MMEF, % (range) | 83 (46–101) | 109 (55–175) | 0.14 |
| RV, % (range) | 102 (56–135) | 79 (48–109) | 0.33 |
| CARDIOLOGIC EXPLORATIONS | |||
| LVEF, % (range) | 58 (43–74) | 64.5 (51–74) | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, D.; Giannini, M.; Oulehri, W.; Riou, M.; Marcot, C.; Pizzimenti, M.; Debrut, L.; Charloux, A.; Geny, B.; Meyer, A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients 2022, 14, 912. https://doi.org/10.3390/nu14040912
Levy D, Giannini M, Oulehri W, Riou M, Marcot C, Pizzimenti M, Debrut L, Charloux A, Geny B, Meyer A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients. 2022; 14(4):912. https://doi.org/10.3390/nu14040912
Chicago/Turabian StyleLevy, Dan, Margherita Giannini, Walid Oulehri, Marianne Riou, Christophe Marcot, Megane Pizzimenti, Lea Debrut, Anne Charloux, Bernard Geny, and Alain Meyer. 2022. "Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units" Nutrients 14, no. 4: 912. https://doi.org/10.3390/nu14040912
APA StyleLevy, D., Giannini, M., Oulehri, W., Riou, M., Marcot, C., Pizzimenti, M., Debrut, L., Charloux, A., Geny, B., & Meyer, A. (2022). Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients, 14(4), 912. https://doi.org/10.3390/nu14040912





