The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of PE Fruit Polyphenol
2.3. Determination of Total Polyphenol Content (TPC)
2.4. Optimization of Polyphenol Extraction from PE Fruit
2.4.1. Single Factor Extraction Experiments
2.4.2. Experimental Design for RSM
2.5. Identification of Phenolic Compounds by UPLC-ESI-QTOF-MS
2.6. In Vitro Antioxidant Assays
2.6.1. DPPH Radical Scavenging Assay
2.6.2. ABTS·+ Radical Scavenging Assay
2.6.3. OH· Radical Scavenging Assay
2.6.4. FRAP Assay
2.7. Inhibition of Cholinesterase Activity Assay In Vitro
2.8. In Vivo Assays
2.8.1. C. elegans Strains and Maintenance
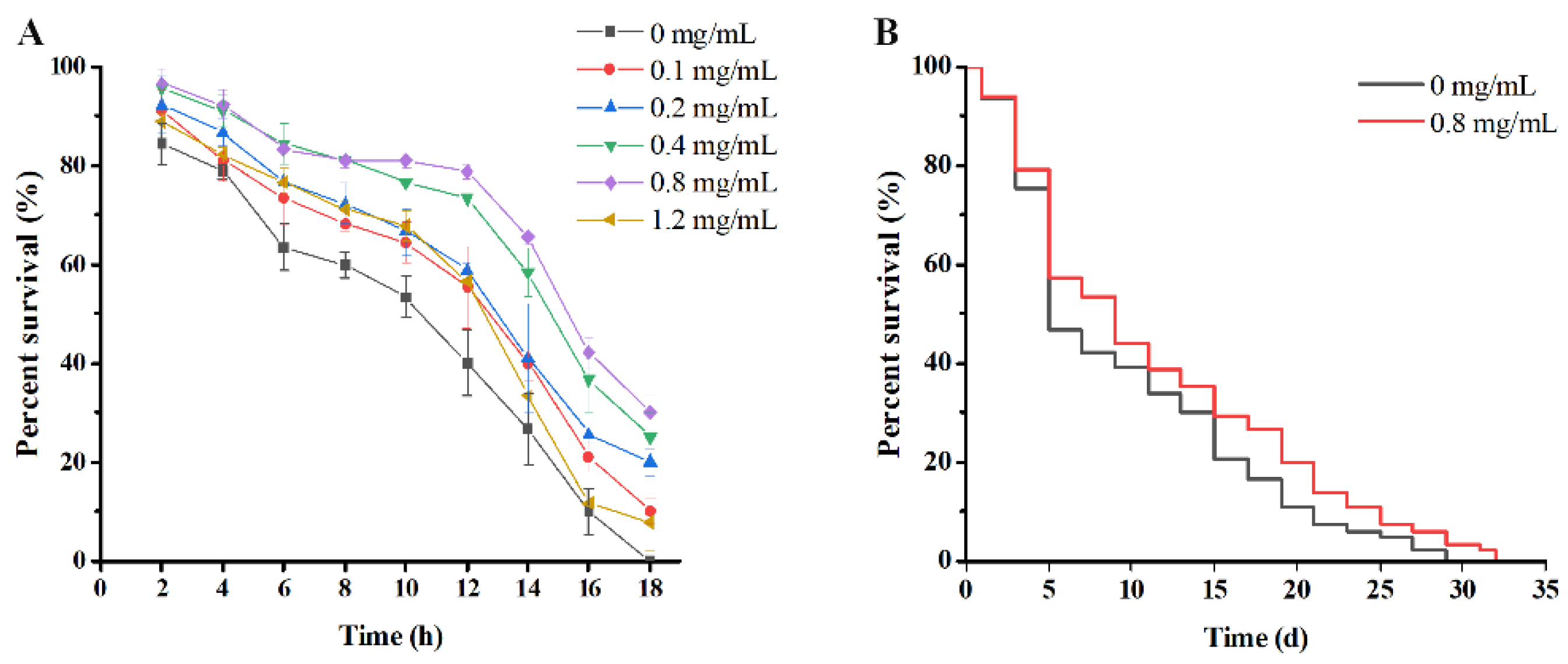
2.8.2. Thermal Stress Resistance Assay
2.8.3. Lifespan Assay
2.8.4. Determination of Cholinesterase Activities
2.8.5. Determination of Antioxidant Enzyme Activities and MDA Levels
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimizing the Extraction of PE Fruit Polyphenols
3.2. Phenolic Compounds of PE
3.3. In Vitro Antioxidant Activity of PE Fruit Polyphenols
3.4. In Vitro Anti-Aging Activity of PE Fruit Polyphenols
3.5. In Vivo Biological Activity Analysis
3.5.1. PE Fruit Polyphenols Increased Thermal Resistance in C. elegans
3.5.2. PE Fruit Polyphenols Prolonged Lifespan of C. elegans
3.5.3. PE Fruit Polyphenols Inhibited the Cholinesterase Activities in C. elegans
3.5.4. PE Fruit Polyphenols Enhanced Antioxidant Enzymes Activities and Reduced MDA Level in C. elegans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by d-Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Castelli, V.; Grassi, D.; Bocale, R.; d’Angelo, M.; Antonosante, A.; Cimini, A.; Ferri, C.; Desideri, G. Diet and Brain Health: Which Role for Polyphenols? Curr. Pharm. Des. 2018, 24, 227–238. [Google Scholar] [CrossRef]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; El Alfy, T.S.; Abdelrahman, E.H.; Obied, H.K. Biophenols of mints: Antioxidant, acetylcholinesterase, butyrylcholinesterase and histone deacetylase inhibition activities targeting Alzheimer’s disease treatment. J. Funct. Foods 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Lakra, A.K.; Ramatchandirane, M.; Kumar, S.; Suchiang, K.; Arul, V. Physico-chemical characterization and aging effects of fructan exopolysaccharide produced by Weissella cibaria MD2 on Caenorhabditis elegans. LWT-Food Sci. Technol. 2021, 143, 111100. [Google Scholar] [CrossRef]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2019, 173, 113719. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.E.; Hashem, F.A.; Shabana, M.H.; Hammam, A.M.; Madboli, A.N.A.; Al-Mahdy, D.A.; Farag, M.A. A biochemometric approach for the assessment of Phyllanthus emblica female fertility effects as determined via UPLC-ESI-qTOF-MS and GC-MS. Food Funct. 2019, 10, 4620–4635. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, R.; Li, Y.; Miao, J.; Liu, G.; Lan, Y.; Chen, Y.; Cao, Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J. Ethnopharmacol. 2020, 254, 112740. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Haung, X.-Y.; Chiu, C.-C.; Lin, M.-Y.; Lin, W.-H.; Chang, W.-T.; Tseng, C.-C.; Wang, H.-M.D. Inhibitions of melanogenesis via Phyllanthus emblica fruit extract powder in B16F10 cells. Food Biosci. 2019, 28, 177–182. [Google Scholar] [CrossRef]
- Lu, C.; Li, C.; Chen, B.; Shen, Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pedunculata Pall. Food Chem. 2018, 265, 111–119. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, S.; Shen, S.; Wang, H.; Yuan, M.; Liu, J.; Huang, Y.; Ding, C. The antioxidant activities effect of neutral and acidic polysaccharides from Epimedium acuminatum Franch. on Caenorhabditis elegans. Carbohydr. Polym. 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.N.; Wang, C.Y.; Chang, T.; Jiang, H.C. Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: Optimization study. Ultrason. Sonochemistry 2021, 78, 105739. [Google Scholar] [CrossRef]
- Sabir, S.M.; Hussain, R.; Shah, A.H. Total Phenolic and Ascorbic acid Contents and Antioxidant activities of Twelve Different Ecotypes of Phyllanthus emblica from Pakistan. Chiang Mai J. Sci. 2015, 42, 1–9. [Google Scholar]
- Sousa, A.D.; Maia, A.I.V.; Rodrigues, T.H.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Cassia Alves Pereira, R.; Vieira, R.F.; de Brito, E.S. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crops Prod. 2016, 79, 91–103. [Google Scholar] [CrossRef]
- Jhaumeer Laulloo, S.; Bhowon, M.G.; Chua, L.S.; Gaungoo, H. Phytochemical Screening and Antioxidant Properties of Phyllanthus emblica from Mauritius. Chem. Nat. Compd. 2018, 54, 50–55. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Veerappan, K.; Ranjan, A.; Kim, Y.J.; Chellappan, D.K.; Dua, K.; Lee, J.; Perumalsamy, H. Phyllanthus emblica fruit extract attenuates lipid metabolism in 3T3-L1 adipocytes via activating apoptosis mediated cell death. Phytomedicine 2020, 66, 153129. [Google Scholar] [CrossRef]
- Rose, K.; Wan, C.P.; Thomas, A.; Seeram, N.P.; Ma, H. Phenolic Compounds Isolated and Identified from Amla (Phyllanthus emblica) Juice Powder and their Antioxidant and Neuroprotective Activities. Nat. Prod. Commun. 2018, 13, 1309–1311. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Wang, W.; Li, L.; Cao, L.; Yang, C.; Liu, J.; Liang, Q.; Chen, J.; Wu, S.; et al. Characterization of phenolic compounds from Phyllanthus emblica fruits using HPLC-ESI-TOF-MS as affected by an optimized microwave-assisted extraction. Int. J. Food Prop. 2019, 22, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crops Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011, 126, 277–282. [Google Scholar] [CrossRef]
- Jiang, X.L.; Wang, L.; Wang, E.J.; Zhang, G.L.; Chen, B.; Wang, M.K.; Li, F. Flavonoid glycosides and alkaloids from the embryos of Nelumbo nucifera seeds and their antioxidant activity. Fitoterapia 2018, 125, 184–190. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Lu, Y.; Du, Y.; Qin, X.; Wu, H.; Huang, Y.; Cheng, Y.; Wei, Y. Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities. Ind. Crops Prod. 2019, 129, 242–252. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, H.Y.; Shen, L. Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants. Bioorganic Med. Chem. Lett. 2006, 16, 4095–4098. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdylo, A.; Turkiewicz, I.P.; Nowicka, P. Anti-diabetic, anti-cholinesterase, and antioxidant potential, chemical composition and sensory evaluation of novel sea buckthorn-based smoothies. Food Chem. 2021, 338, 128105. [Google Scholar] [CrossRef]
- Wojdylo, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhou, H.; Sun, X.; Chen, X.; Xu, N. The anti-aging effects of Gracilaria lemaneiformis polysaccharide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2019, 140, 600–604. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, A.; Sun, Y.; Zhang, W.; Zhang, T.; Gao, Y.; Shan, D.; Wang, S.; Li, G.; Zeng, K.; et al. Beneficial effects of sappanone A on lifespan and thermotolerance in Caenorhabditis elegans. Eur. J. Pharmacol. 2020, 888, 173558. [Google Scholar] [CrossRef]
- Gu, J.; Li, Q.; Liu, J.; Ye, Z.; Feng, T.; Wang, G.; Wang, W.; Zhang, Y. Ultrasonic-assisted extraction of polysaccharides from Auricularia auricula and effects of its acid hydrolysate on the biological function of Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 167, 423–433. [Google Scholar] [CrossRef]
- Grunz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar] [CrossRef]
- Duenas, M.; Surco-Laos, F.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Gomez-Orte, E.; Cabello, J.; Santos-Buelga, C. Deglycosylation is a key step in biotransformation and lifespan effects of quercetin-3-O-glucoside in Caenorhabditis elegans. Pharmacol. Res. 2013, 76, 41–48. [Google Scholar] [CrossRef]
- Xue, Y.L.; Ahiko, T.; Miyakawa, T.; Amino, H.; Hu, F.; Furihata, K.; Kita, K.; Shirasawa, T.; Sawano, Y.; Tanokura, M. Isolation and Caenorhabditis elegans lifespan assay of flavonoids from onion. J. Agric. Food Chem. 2011, 59, 5927–5934. [Google Scholar] [CrossRef]
- Gutierrez-Zetina, S.M.; Gonzalez-Manzano, S.; Ayuda-Duran, B.; Santos-Buelga, C.; Gonzalez-Paramas, A.M. Caffeic and Dihydrocaffeic Acids Promote Longevity and Increase Stress Resistance in Caenorhabditis elegans by Modulating Expression of Stress-Related Genes. Molecules 2021, 26, 1517. [Google Scholar] [CrossRef]
- Dilberger, B.; Weppler, S.; Eckert, G.P. Impact of Phenolic Acids on the ennergy metabolism and longevity in C. elegans. Biorxiv 2020. [Google Scholar] [CrossRef]
- Tota, S.; Awasthi, H.; Kamat, P.K.; Nath, C.; Hanif, K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav. Brain Res. 2010, 209, 73–79. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Katsikoudi, A.; Kontogianni, V.G.; Kellici, T.F.; Iatrou, G.; Lamari, F.N.; Tzakos, A.G.; Margarity, M. Rosemary tea consumption results to anxiolytic- and anti-depressant-like behavior of adult male mice and inhibits all cerebral area and liver cholinesterase activity; phytochemical investigation and in silico studies. Chem. Biol. Interact. 2015, 237, 47–57. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar] [CrossRef]
- Hui, H.; Xin, A.; Cui, H.; Jin, H.; Yang, X.; Liu, H.; Qin, B. Anti-aging effects on Caenorhabditis elegans of a polysaccharide, O-acetyl glucomannan, from roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 2020, 155, 846–852. [Google Scholar] [CrossRef]

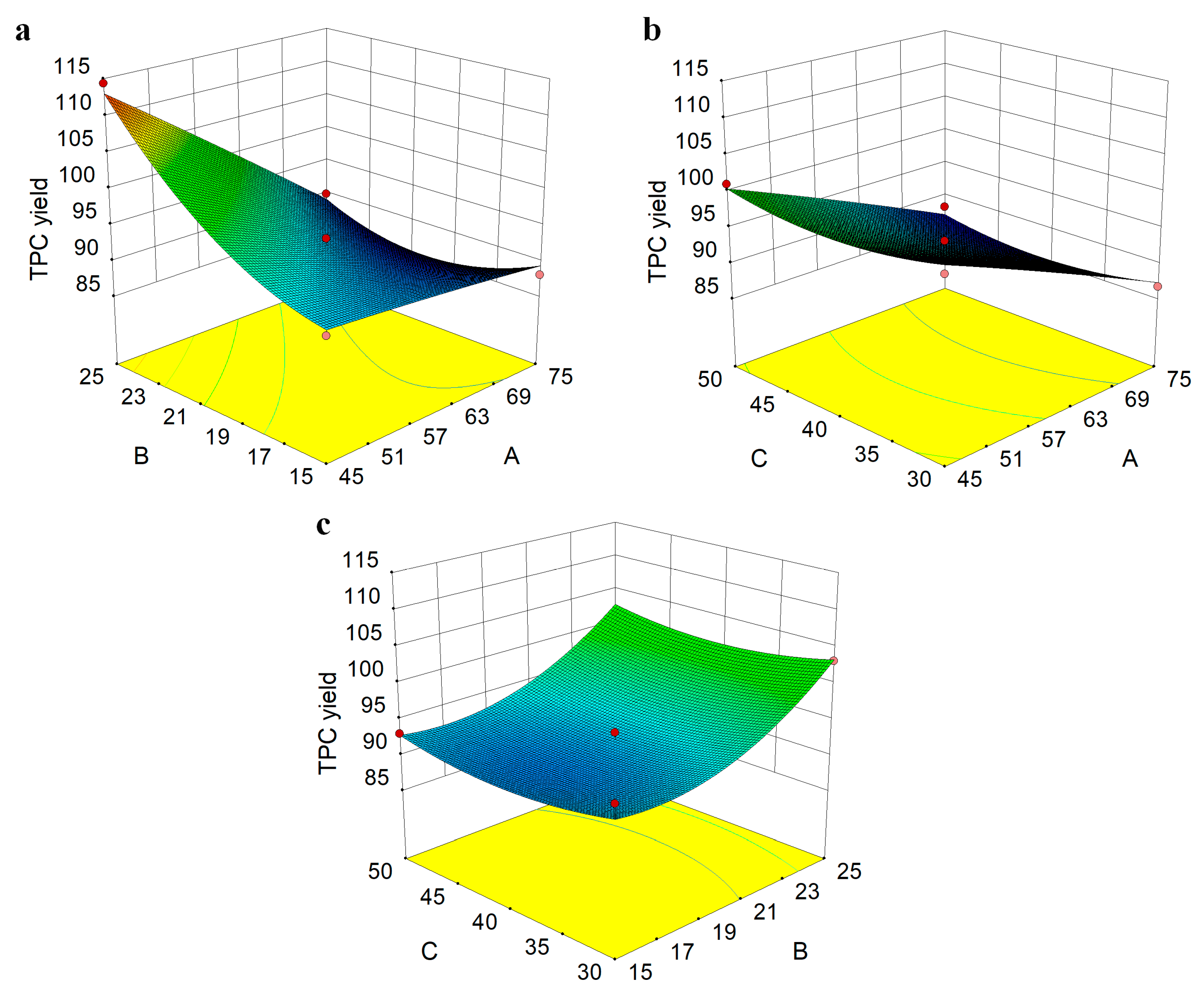


| Run | A: Ethanol Concentration (%) | B: Liquid-Solid Ratio (mL/g) | C: Extraction Temperature (°C) | Y: TPC Yield (mg GAE/g DW) |
|---|---|---|---|---|
| 1 | 60 | 20 | 40 | 93.20 |
| 2 | 45 | 20 | 50 | 101.10 |
| 3 | 60 | 20 | 40 | 93.20 |
| 4 | 75 | 15 | 40 | 88.03 |
| 5 | 60 | 20 | 40 | 90.88 |
| 6 | 75 | 25 | 40 | 89.77 |
| 7 | 60 | 25 | 30 | 103.14 |
| 8 | 75 | 20 | 30 | 86.69 |
| 9 | 60 | 15 | 30 | 95.00 |
| 10 | 60 | 20 | 40 | 91.81 |
| 11 | 45 | 20 | 30 | 99.71 |
| 12 | 60 | 20 | 40 | 92.27 |
| 13 | 60 | 15 | 50 | 93.03 |
| 14 | 75 | 20 | 50 | 88.09 |
| 15 | 45 | 15 | 40 | 91.69 |
| 16 | 45 | 25 | 40 | 114.35 |
| 17 | 60 | 25 | 50 | 100.81 |
| No | Rt (min) | (m/z) [M-H]−/+ | Tentative Identification | Proposed Formula | Molecular Weight |
|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||
| 1 | 2.415 | 153.0193 [M + H]− | Protocatechuic acid | C7H6O4 | 154.0266 |
| 2 | 2.337 | 169.0139 [M + H]− 171.0292 [M + H]+ | Gallic acid | C7H6O5 | 170.0215 |
| 3 | 3.642 | 183.0288 [M + H]− | 3-O-Methylgallate | C8H7O5− | 184.0372 |
| 4 | 5.232 | 197.0440 [M + H]− 199.0590 [M + H]+ | Syringic acid | C9H10O5 | 198.0528 |
| 5 | 2.533 | 243.0492 [M + H]− | 1-O-Galloylglycerol | C10H12O7 | 244.0583 |
| 6 | 2.990 | 185.0441 [M + H]+ | 4-O-Methylgallic acid | C8H8O5 | 184.0372 |
| 7 | 4.658 | 300.9981 [M + H]− 303.0145 [M + H]+ | Ellagic acid | C14H6O8 | 302.0063 |
| 8 | 1.527 | 331.0653 [M + H]− 333.0799 [M + H]+ | beta-Glucogallin | C13H16O10 | 332.0743 |
| 9 | 0.821 | 361.0410 [M-H]− 363.0567 [M + H]+ | 2-O-Galloylgalactaric acid | C13H14O12 | 362.0485 |
| 10 | 2.342 | 375.0575 [M-H]− 399.0526 [M + Na]+ | 1-Methyl 2-galloylgalactarate | C14H16O12 | 376.0642 |
| 11 | 2.839 | 379.0087 [M + Cl]− 345.0426 [M + H]+ | 2-O-Galloyl-1,4-galactarolactone | C13H12O11 | 344.038 |
| 12 | 2.525 | 483.0736 [M-H]− | 2,6-Digalloylglucose | C20H20O14 | 484.0853 |
| 13 | 3.429 | 483.0780 [M-H]− 507.0729 [M + Na]+ | 1-O,6-O-Digalloyl-beta-D-glucose | C20H20O14 | 484.0853 |
| 14 | 2.034 | 495.0754 [M-H]− 497.0912 [M + H]+ | 3,4-Di-O-galloylquinic acid | C21H20O14 | 496.0853 |
| 15 | 2.023 | 357.0462 [M + H]+ | Chebulic acid | C14H12O11 | 356.0380 |
| Hydroxycinnamic acids | |||||
| 16 | 4.557 | 177.0190 [M + H]− | Esculetin | C9H6O4 | 178.0266 |
| 17 | 3.526 | 179.0349 [M + H]− | Caffeic acid | C9H8O4 | 180.0423 |
| 18 | 2.439 | 311.0396 [M + H]− 313.0557 [M + H]+ | Caftaric acid | C13H12O9 | 312.0481 |
| 19 | 2.651 | 369.0436 [M-H]− 371.0620 [M + H]+ | 2-O-Caffeoylhydroxycitric acid | C15H14O11 | 370.0536 |
| 20 | 2.881 | 369.0790 [M-H]− | Fraxin | C16H18O10 | 370.0900 |
| 21 | 3.326 | 383.0608 [M-H]− 385.0747 [M + H]+ | 2-O-Feruloylhydroxycitric acid | C16H16O11 | 384.0693 |
| 22 | 1.759 | 391.0475 [M + Cl]− | Caffeic acid 3-O-glucuronide | C15H16O10 | 356.0743 |
| 23 | 1.800 | 297.0598 [M + H]+ | Caffeoylmalic acid | C13H12O8 | 296.0532 |
| 24 | 2.660 | 355.1001 [M + H]+ 377.0816 [M + Na]+ | Chlorogenic acid | C16H18O9 | 354.0951 |
| 25 | 4.796 | 373.0750 [M + H]+ | 2-O-Caffeoylglucarate | C15H16O11 | 372.0693 |
| Flavanones | |||||
| 26 | 6.525 | 271.0594 [M + H]− 273.0743 [M-H]+ | Naringenin | C15H12O5 | 272.0685 |
| 27 | 4.840 | 427.1794 [M-H]− | Heteroflavanone B | C24H28O7 | 428.1835 |
| 28 | 6.519 6.525 | 433.1108 [M-H]− 435.1303 [M + H]+ | Naringenin-7-O-glucoside | C21H22O10 | 434.1213 |
| 29 | 8.565 | 579.1503 [M-H]− | 6′′-p-Coumaroylprunin | C30H28O12 | 580.1581 |
| 30 | 11.954 | 405.1541 [M + H]+ | Citromitin | C21H24O8 | 404.1471 |
| 31 | 7.710 | 417.1533 [M + H]+ | 4′-Methylliquiritigenin 7-rhamnoside | C22H24O8 | 416.1471 |
| 32 | 5.803 | 465.1341 [M + H]+ | Hesperetin 5-O-glucoside | C22H24O11 | 464.1319 |
| 33 | 3.167 | 689.1165 [M + Cl]− | Hesperetin 5,7-O-diglucuronide | C28H30O18 | 654.1432 |
| Flavan-3-ols | |||||
| 34 | 4.909 | 481.0958 [M-H]− | (-)-Epigallocatechin 3′-glucuronide | C21H22O13 | 482.106 |
| 35 | 2.798 | 323.0729 [M + H]+ | Leucodelphidin | C15H14O8 | 322.0689 |
| 36 | 1.105 | 867.1327 [M-H]− | Theaflavin 3,3′-digallate | C43H32O20 | 868.1487 |
| 37 | 2.553 | 621.0720 [M + H]+ | Tannin | C26H20O18 | 620.0650 |
| Flavonols | |||||
| 38 | 2.627 | 411.0528 [M + Cl]− | Limocitrol | C18H16O9 | 376.0794 |
| 39 | 7.041 | 287.0553 [M-H]+ | Fisetin | C15H10O6·xH2O | 286.0477 |
| 40 | 7.042 | 431.0979 [M-H]− | Afzelin | C21H20O10 | 432.1056 |
| 41 | 6.316 | 447.0897 [M-H]− 449.1091 [M + H]+ | Quercitrin | C21H20O11 | 448.1006 |
| 42 | 6.316 | 303.0501 [M + H]+ | Quercetin | C15H10O7 | 302.0427 |
| 43 | 7.906 | 461.1060 [M-H]− | Kaempferide 7-glucoside | C22H22O11 | 462.1162 |
| 44 | 5.463 | 463.0863 [M-H]− | Isoquercetin | C21H20O12 | 464.0955 |
| 45 | 5.631 | 463.0863 [M-H]− | Spiraeoside | C21H20O12 | 464.0955 |
| 46 | 6.211 | 469.0483 [M + Cl]− | Quercetin 7-xyloside | C20H18O11 | 434.0849 |
| 47 | 5.458 | 319.0435 [M + H]+ | Myricetin | C15H10O8 | 318.0376 |
| 48 | 4.727 | 341.0328 [M + Na]+ | Gossypetin | C15H10O8 | 318.0376 |
| 49 | 4.796 | 507.1093 [M-H]− | Syringetin-3-O-galactoside | C23H24O13 | 508.1217 |
| 50 | 2.674 | 529.0789 [M + Cl]− | Laricitrin 3-glucoside | C22H22O13 | 494.106 |
| 51 | 2.714 | 609.1281 [M-H]− | 6′′-O-Caffeoylastragalin | C30H26O14 | 610.1323 |
| 52 | 5.581 | 625.1426 [M-H]− | Quercetin 4′,7-diglucoside | C27H30O17 | 626.1483 |
| 53 | 3.301 | 419.0990 [M + H]+ | Kaempferol 3-alpha-L-arabinopyranoside | C20H18O10 | 418.0900 |
| 54 | 4.183 | 667.0737 [M + Cl]− | Myricetin 7-(6′′-galloylglucoside) | C28H24O17 | 632.1013 |
| 55 | 2.541 | 675.1030 [M + Cl]− | Nelumboside | C27H28O18 | 640.1276 |
| 56 | 4.727 | 434.9980 [M + Na]+ | Quercetagetin 3-methyl ether 7-O-sulfate | C16H12O11S | 412.0100 |
| 57 | 0.910 | 723.2186 [M-H]− | Natsudaidain 3-(4-O-3-hydroxy-3-methylglutaroylglucoside) | C33H40O18 | 724.2215 |
| 58 | 5.232 | 451.0887 [M + H]+ | Myricetin 3-xyloside | C20H18O12 | 450.0798 |
| 59 | 6.525 | 463.0887 [M + H]+ | Kaempferol 3-glucuronide | C21H18O12 | 462.0798 |
| 60 | 5.794 | 473.0717 [M + Na]+ | Myricetin 3-arabinoside | C20H18O12 | 450.0798 |
| 61 | 1.827 | 837.1519 [M + Cl]− | Quercetin 7-glucuronoside 3-sophoroside | C33H38O23 | 802.1804 |
| 62 | 2.039 | 479.0858 [M + H]+ | Quercetin 3-O-glucuronide | C21H18O13 | 478.0747 |
| 63 | 5.582 | 481.0944 [M + H]+ | Myricetin 3-glucoside | C21H20O13 | 480.0904 |
| 64 | 5.458 | 487.0868 [M + Na]+ | Myricitrin | C21H20O12 | 464.0955 |
| 65 | 4.141 | 495.0767 [M + H]+ | Myricetin 3-glucuronide | C21H18O14 | 494.0697 |
| 66 | 3.660 | 507.1097 [M + H]+ | Quercetin 3-O-(6′′-acetyl-glucoside) | C23H22O13 | 506.1060 |
| Anthocyanidins | |||||
| 67 | 3.302 | 417.0806 [M-H]− | Cyanidin 3-arabinoside | C20H19O10 | 419.0978 |
| 68 | 5.463 | 451.0853 [M + Cl]− | Pelargonidin 3-rhamnoside | C21H21O9+ | 417.1186 |
| 69 | 0.672 | 603.1002 [M-H]− | Pelargonidin 3-O-3′′,6′′-O-dimalonylglucoside | C27H25O16+ | 604.1064 |
| 70 | 3.183 | 645.1292 [M + Cl]− | Cyanidin 3-galactoside-5-glucoside | C27H31O16+ | 610.1534 |
| 71 | 1.113 | 661.1228 [M + Cl]− | Delphinidin 3-sophoroside | C27H31O17 | 627.1561 |
| 72 | 12.700 | 610.1867 [M + H]+ | Peonidin 3-rhamnoside 5-glucoside | C28H33O15 | 609.1819 |
| Dihydroflavonols | |||||
| 73 | 5.689 | 449.1048 [M-H]− | Neoastilbin | C21H22O11 | 450.1162 |
| Flavones | |||||
| 74 | 6.519 | 461.0708 [M-H]− | Scutellarein 5-glucuronide | C21H18O12 | 462.0798 |
| 75 | 7.899 | 489.1036 [M-H]− | 2′′-O-Acetylisoorientin | C23H22O12 | 490.1111 |
| 76 | 2.541 | 499.0691 [M + Cl]− | 2′-Hydroxyisoorientin | C21H20O12 | 464.0955 |
| 77 | 0.752 | 409.0930 [M + Na]+ | Chrysin 5-xyloside | C20H18O8 | 386.1002 |
| 78 | 1.559 | 685.1240 [M + Cl]− | 6′′-Malonylapiin | C29H30O17 | 650.1483 |
| Isoflavones | |||||
| 79 | 4.636 | 465.0620 [M + Cl]− | Daidzein 4′-O-glucuronide | C21H18O10 | 430.0900 |
| 80 | 6.382 | 465.0974 [M + Cl]− | Ononin | C22H22O9 | 430.1264 |
| 81 | 0.657 | 517.1393 [M-H]− | Medicarpin 3-O-(6′-malonylglucoside) | C25H26O12 | 518.1424 |
| 82 | 4.846 | 405.1706 [M + H]+ | Osajin | C25H24O5 | 404.1624 |
| 83 | 5.223 | 419.0613 [M + H]+ | Shoyuflavone C | C19H14O11 | 418.0536 |
| 84 | 7.041 | 455.0916 [M + Na]+ | Genistin | C21H20O10 | 432.1056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients 2022, 14, 857. https://doi.org/10.3390/nu14040857
Wu M, Cai J, Fang Z, Li S, Huang Z, Tang Z, Luo Q, Chen H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients. 2022; 14(4):857. https://doi.org/10.3390/nu14040857
Chicago/Turabian StyleWu, Min, Jianhang Cai, Zhengfeng Fang, Shanshan Li, Zhiqing Huang, Zizhong Tang, Qingying Luo, and Hong Chen. 2022. "The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit" Nutrients 14, no. 4: 857. https://doi.org/10.3390/nu14040857
APA StyleWu, M., Cai, J., Fang, Z., Li, S., Huang, Z., Tang, Z., Luo, Q., & Chen, H. (2022). The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients, 14(4), 857. https://doi.org/10.3390/nu14040857








