A Qualitative Exploration of Nutrition Screening, Assessment and Oral Support Used in Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Context
2.3. Data Collection
2.4. Data Analysis
2.5. Rigour
3. Results
3.1. Sample Details
3.2. Themes
3.2.1. Nutritional Assessment
3.2.2. Nutritional Interventions
3.2.3. Barriers to Nutritional Care
3.2.4. Facilitators for Nutritional Care
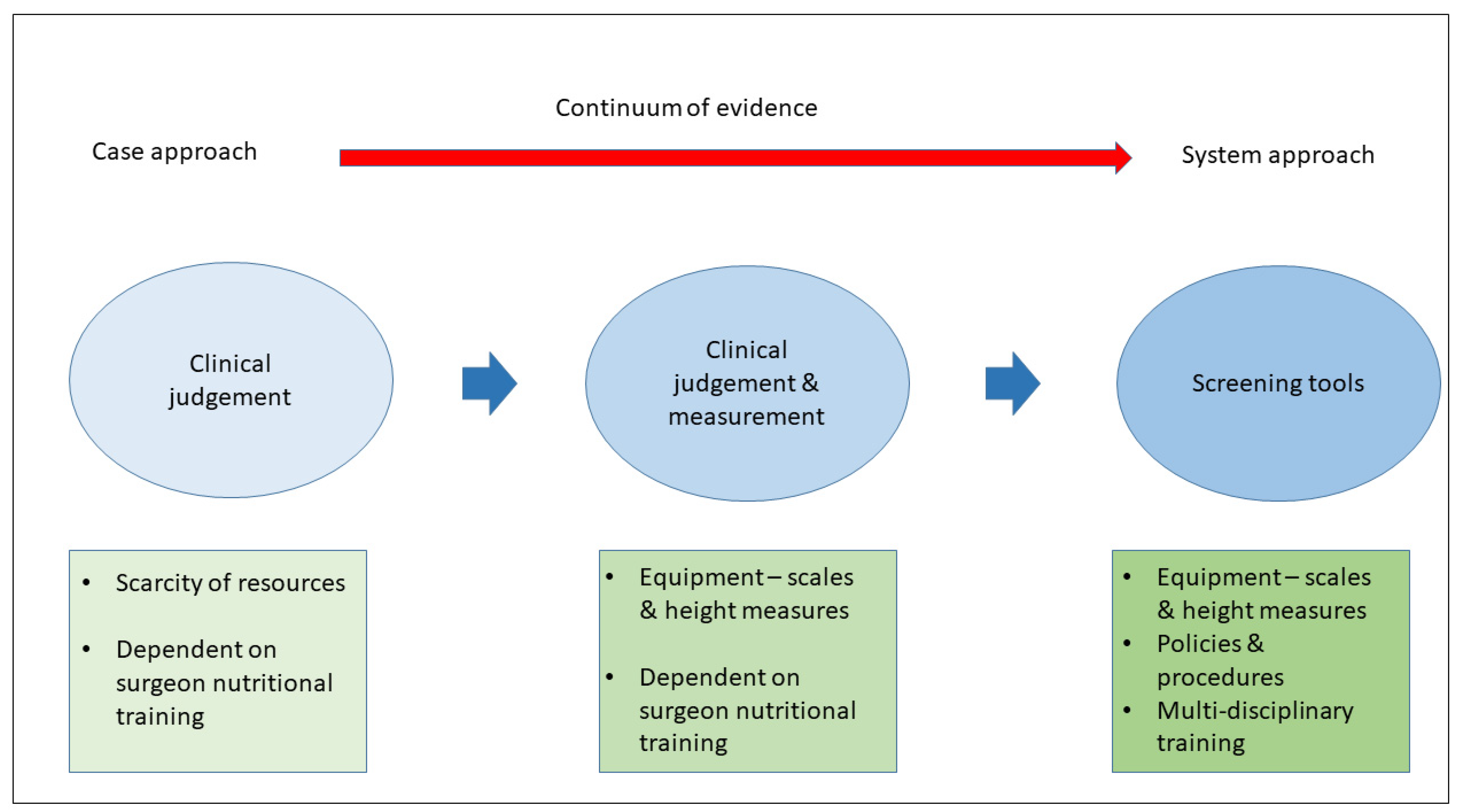
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meara, J.G.; Hagander, L.; Leather, A.J. Surgery and global health: A Lancet Commission. Lancet 2013, 383, 12–13. [Google Scholar] [CrossRef]
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Balch, C.; Brennan, M.F.; Dare, A.; D’Cruz, A. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–11224. [Google Scholar] [CrossRef]
- Grimes, C.E.; Henry, J.A.; Maraka, J.; Mkandawire, N.C.; Cotton, M. Cost-effectiveness of Surgery in Low- and Middle-income Countries: A Systematic Review. World J. Surg. 2014, 38, 252–1263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; Food & Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018. [Google Scholar]
- Riad, A.M.; Knight, S.R.; Ewen, M.; Harrison on behalf of GlobalSurg Collaborative. O19° The effect of malnutrition on early outcomes after elective cancer surgery: An international prospective cohort study in 82 countries. Br. J. Surg. 2021, 108, znab282.204. [Google Scholar] [CrossRef]
- Vaid, S.; Bell, T.; Grim, R.; Ahuja, V. Predicting risk of death in general surgery patients on the basis of preoperative variables using American College of Surgeons National Surgical Quality Improvement Program data. Perm. J. 2012, 16, 10–17. [Google Scholar] [CrossRef]
- Burden, S.T.; Gibson, D.J.; Lal, S.; Hill, J.; Pilling, M.; Soop, M.; Ramesh, A.; Todd, C. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: Single-blind randomized controlled trial. J. Cachexia Sarcopenia Muscle 2017, 8, 437–446. [Google Scholar] [CrossRef]
- Gade, J.; Levring, T.; Hillingsø, J.; Hansen, C.P.; Andersen, J.R. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer—A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 225–233. [Google Scholar] [CrossRef]
- Smedley, F.; Bowling, T.; James, M.; Stokes, E.; Goodger, C.; O’Connor, O.; Oldale, C.; Jones, P.; Silk, D. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br. J. Surg. 2004, 91, 983–990. [Google Scholar] [CrossRef]
- Burden, S.; Todd, C.; Hill, J.; Lal, S. Pre-operative Nutrition Support in Patients Undergoing Gastrointestinal Surgery. Cochrane Database Syst. Rev. 2012, 11. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Muscle: Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopeni 2020, 11, 366–380. [Google Scholar] [CrossRef]
- National Institute for Health Research Global Health Research Unit on Global Surgery. Prioritizing research for patients requiring surgery in low-and middle-income countries. Br. J. Surg. 2019, 106, e113–e120. [Google Scholar] [CrossRef] [PubMed]
- DAC List of ODA Recipients. Available online: https://www.oecd.org/dac/financing-sustainable-development/development-finance-standards/DAC-List-ODA-Recipients-for-reporting-2021-flows.pdf (accessed on 17 January 2022).
- Naderifar, M.; Goli, H.; Ghaljaie, F. Snowball sampling: A purposeful method of sampling in qualitative research. Strides Dev. Med. Educ. 2017, 14. [Google Scholar] [CrossRef]
- Flick, U. Doing Triangulation and Mixed Methods; Sage: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Moran, D. What is the phenomenological approach? Revisiting intentional explication. Phenomenol. Mind 2018, 72–90. [Google Scholar] [CrossRef]
- CRANE Feasibility Study: Nutritional Intervention for Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries (CRANE). Available online: https://clinicaltrials.gov/ct2/show/NCT04448041 (accessed on 17 January 2022).
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Barcus, G.C.; Papathakis, P.C.; Schaffner, A.; Chimera, B. Nutrition Screening, Reported Dietary Intake, Hospital Foods, and Malnutrition in Critical Care Patients in Malawi. Nutrients 2021, 13, 1170. [Google Scholar] [CrossRef]
- Keller, D.S.; Flores-Gonzalez, J.R.; Ibarra, S.; Madhoun, N.; Tahilramani, R.; Mahmood, A.; Haas, E.M. Evaluating quality across minimally invasive platforms in colorectal surgery. Surg. Endosc. 2016, 30, 2207–2216. [Google Scholar] [CrossRef]
- Murphy, J.L.; Munir, F.; Davey, F.; Miller, L.; Cutress, R.; White, R.; Lloyd, M.; Roe, J.; Granger, C.; Burden, S. The provision of nutritional advice and care for cancer patients: A UK national survey of healthcare professionals. Supp. Care Cancer 2021, 29, 2435–2442. [Google Scholar] [CrossRef]
- Young, L.S.; Huong, P.T.T.; Lam, N.T.; Thu, N.N.; Van, H.T.; Hanh, N.L.; Tuyen, L.D.; Lien, D.T.K.; Hoc, T.H.; Tuyet, C.T. Nutritional status and feeding practices in gastrointestinal surgery patients at Bach Mai Hospital, Hanoi, Vietnam. Asia Pac. J. Clin. Nutr. 2016, 25, 513. [Google Scholar]
- Siribumrungwong, B.; Srithamma, B.; Kuntonpreeda, K.; Tomtitchong, P.; Paochareun, V. Prevalence of malnutrition and nutritional assessment in abdominal-surgical patients; a prospective cross-sectional study. J. Med. Assoc. Thai. 2011, 94, S19–S23. [Google Scholar]
- Leandro-Merhi, V.A.; de Aquino, J.L.B.; de Camargo, J.G.T.; Frenhani, P.B.; Bernardi, J.L.D.; McLellan, K.C.P. Clinical and nutritional status of surgical patients with and without malignant diseases: Cross-sectional study. Arq. de Gastroenterol. 2011, 48, 58–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Susetyowati, H.H.; Hakimi, M.; Asdie, A. Development, validation and reliability of the simple nutrition screening tool (SNST) for adult hospital patient in Indonesia. Pak. J. Nutr. 2014, 13, 157–163. [Google Scholar]
- Cheung, G.; Pizzola, L.; Keller, H. Geriatrics: Dietary, food service, and mealtime interventions to promote food intake in acute care adult patients. J. Nutr. Gerontol. Geriatr. 2013, 32, 175–212. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, M.-F.; Alligier, M.; Baclet, N.; Capelle, J.; Dousseaux, M.-P.; Eyraud, E.; Fayemendy, P.; Flori, N.; Guex, E.; Hennequin, V. Guidelines on Standard and Therapeutic Diets for Adults in Hospitals by the French Association of Nutritionist Dieticians (AFDN) and the French Speaking Society of Clinical Nutrition and Metabolism (SFNCM). Nutrients 2021, 13, 2434. [Google Scholar] [CrossRef]
- Jeffrey, D. The Hospital Food Standards Panel’s Report on Standards for Food and Drink in NHS Hospitals. Department of Health, 2014. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/523049/Hospital_Food_Panel_May_2016.pdf (accessed on 17 January 2022).
- Moran, A.; Krepp, E.M.; Johnson Curtis, C.; Lederer, A. An intervention to increase availability of healthy foods and beverages in New York City hospitals: The Healthy Hospital Food Initiative, 2010–2014. Prev. Chronic Dis. 2016, 13, E77. [Google Scholar] [CrossRef][Green Version]
- Nakahara, S.; Nguyen, D.H.; Bui, A.T.; Sugiyama, M.; Ichikawa, M.; Sakamoto, T.; Nakamura, T. Perioperative nutrition management as an important component of surgical capacity in low- and middle-income countries. Trop. Med. Int. Health 2017, 22, 784–796. [Google Scholar] [CrossRef]
- Kang, J.G.; Kim, M.H.; Kim, E.H.; Lee, S.H. Intraoperative intravenous lidocaine reduces hospital length of stay following open gastrectomy for stomach cancer in men. J. Clin. Anesth. 2012, 24, 465–470. [Google Scholar] [CrossRef]
- Nair, A.B.; Madan, J.; Dhanaki, N. Formulation and Nutritional Appraisal of Low Cost Enteral Formula. Int. J. Sci. Res. Publ. 2015, 5, 2250–3153. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowerbutts, A.M.; Knight, S.R.; Lapitan, M.C.M.; Qureshi, A.U.; Maimbo, M.; Yenli, E.M.T.-a.; Tabiri, S.; Ghosh, D.; Kingsley, P.A.; Sundar, S.; et al. A Qualitative Exploration of Nutrition Screening, Assessment and Oral Support Used in Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries. Nutrients 2022, 14, 863. https://doi.org/10.3390/nu14040863
Sowerbutts AM, Knight SR, Lapitan MCM, Qureshi AU, Maimbo M, Yenli EMT-a, Tabiri S, Ghosh D, Kingsley PA, Sundar S, et al. A Qualitative Exploration of Nutrition Screening, Assessment and Oral Support Used in Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries. Nutrients. 2022; 14(4):863. https://doi.org/10.3390/nu14040863
Chicago/Turabian StyleSowerbutts, Anne Marie, Stephen R. Knight, Marie Carmela M. Lapitan, Ahmad U. Qureshi, Mayaba Maimbo, Edwin Mwintiereh Ta-ang Yenli, Stephen Tabiri, Dhruva Ghosh, Pamela Alice Kingsley, Sudha Sundar, and et al. 2022. "A Qualitative Exploration of Nutrition Screening, Assessment and Oral Support Used in Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries" Nutrients 14, no. 4: 863. https://doi.org/10.3390/nu14040863
APA StyleSowerbutts, A. M., Knight, S. R., Lapitan, M. C. M., Qureshi, A. U., Maimbo, M., Yenli, E. M. T.-a., Tabiri, S., Ghosh, D., Kingsley, P. A., Sundar, S., Shaw, C. A., Valparaiso, A., Alviz, C. A., Bhangu, A., Theodoratou, E., Weiser, T. G., Harrison, E. M., & Burden, S. T. (2022). A Qualitative Exploration of Nutrition Screening, Assessment and Oral Support Used in Patients Undergoing Cancer Surgery in Low- and Middle-Income Countries. Nutrients, 14(4), 863. https://doi.org/10.3390/nu14040863






