A Dose–Response Relationship of Alcohol Consumption with Risk of Visual Impairment in Korean Adults: The Kangbuk Samsung Health Study
Abstract
1. Introduction
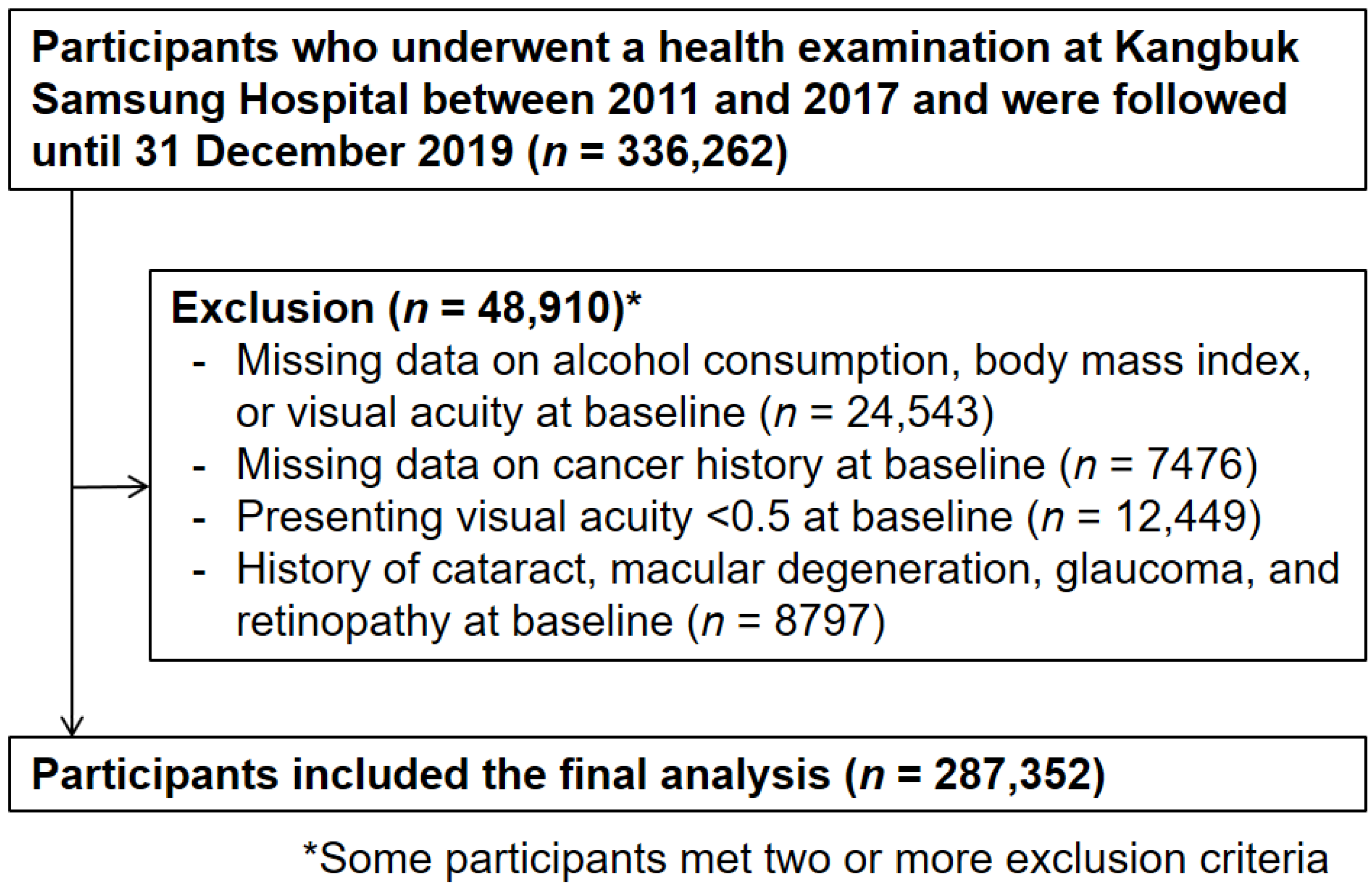
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, 1221–1234. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, 888–897. [Google Scholar] [CrossRef]
- Kulmala, J.; Era, P.; Tormakangas, T.; Parssinen, O.; Rantanen, T.; Heikkinen, E. Visual acuity and mortality in older people and factors on the pathway. Ophthalmic Epidemiol. 2008, 15, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dhital, A.; Pey, T.; Stanford, M.R. Visual loss and falls: A review. Eye 2010, 24, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Brown, M.M.; Stein, J.D.; Smiddy, W.E.; Ophthalmic Utility Research Study, G. Vision-related quality of life associated with unilateral and bilateral ocular conditions. Ophthalmology 2018, 125, 965–971. [Google Scholar] [CrossRef]
- Park, H.Y.; Ryu, H.; Kang, H.Y.; Lee, H.; Kwon, J.W. Clinical and Economic Burden of Visual Impairment in an Aging Society of South Korea. Asia-Pac. J. Public Health 2015, 27, 631–642. [Google Scholar] [CrossRef]
- Weih, L.M.; VanNewkirk, M.R.; McCarty, C.A.; Taylor, H.R. Age-specific causes of bilateral visual impairment. Arch. Ophthalmol. 2000, 118, 264–269. [Google Scholar] [CrossRef][Green Version]
- Levenson, J.H.; Kozarsky, A. Visual Acuity. In Clinical Methods: The History, Physical and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterwirths: Boston, MA, USA, 1990; pp. 563–564. [Google Scholar]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, 51–61. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Kolota, A.; Glabska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Oxidative Stress Parameters in the Liver of Growing Male Rats Receiving Various Alcoholic Beverages. Nutrients 2020, 12, 158. [Google Scholar] [CrossRef]
- Dickson, J.M.; Naylor, G.; Colver, G.; Powers, H.J.; Masters, P. Multiple vitamin deficiencies in a patient with a history of chronic alcohol excess and self-neglect in the UK. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hoyumpa, A.M. Mechanisms of vitamin deficiencies in alcoholism. Alcohol. Clin. Exp. Res. 1986, 10, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009, 44, 136–140. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.J.; Wong, T.Y. Alcohol and eye diseases. Surv. Ophthalmol. 2008, 53, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Z.; Li, Y.; Zhang, X.; Klein, R.; Mokdad, A.H.; Saaddine, J.B.; Balluz, L. Alcohol consumption, drinking pattern, and self-reported visual impairment. Ophthalmic Epidemiol. 2012, 19, 8–15. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Wu, S.; Sun, Y.; Song, Z.; Jin, D.; Liu, P. Alcohol consumption and visual impairment in a rural Northern Chinese population. Ophthalmic Epidemiol. 2014, 21, 384–390. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Moreau, G.; Ozguler, A.; Srour, B.; Cougnard-Gregoire, A.; Goldberg, M.; Zins, M.; Delcourt, C. Unhealthy behaviours and risk of visual impairment: The CONSTANCES population-based cohort. Sci. Rep. 2018, 8, 6569. [Google Scholar] [CrossRef]
- Klein, R.; Lee, K.E.; Gangnon, R.E.; Klein, B.E. Relation of smoking, drinking, and physical activity to changes in vision over a 20-year period: The Beaver Dam Eye Study. Ophthalmology 2014, 121, 1220–1228. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S.; Choi, Y.; Zhang, Y.; Cho, J.; Kwon, M.J.; Hyun, Y.Y.; Lee, K.B.; Kim, H.; Jung, H.S.; et al. Metabolically healthy obesity and development of chronic kidney disease: A cohort study. Ann. Intern. Med. 2016, 164, 305–312. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Chun, M.Y. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med. 2012, 33, 144–151. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Jung, H.S.; Yun, K.E.; Kwon, M.J.; Choi, Y.; Kim, C.W.; Cho, J.; Suh, B.S.; Cho, Y.K.; et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J. Hepatol. 2015, 63, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ryu, S.; Kim, Y.; Cho, Y.K.; Sung, E.; Kim, H.N.; Ahn, J.; Jung, H.S.; Yun, K.E.; Kim, S.; et al. Low levels of alcohol consumption, obesity, and development of fatty liver with and without evidence of advanced fibrosis. Hepatology 2020, 71, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; Murray, C.J.L. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Brooks, P.J.; Enoch, M.A.; Goldman, D.; Li, T.K.; Yokoyama, A. The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009, 6, e50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.J.; Kim, T.Y.; Song, J.Y.; Cho, Y.H.; Park, Y.C.; Chung, H.W. Association of ALDH2 polymorphism with sensitivity to acetaldehyde-induced micronuclei and facial flushing after alcohol intake. Toxicology 2005, 210, 169–174. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Wen, C.P.; David Cheng, T.Y.; Tsai, S.P.; Chan, H.T.; Hsu, H.L.; Hsu, C.C.; Eriksen, M.P. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009, 12, 497–506. [Google Scholar] [CrossRef]
- Han, S.Y.; Chang, Y.; Shin, H.; Choi, C.Y.; Ryu, S. Visual acuity and risk of overall, injury-related, and cardiovascular mortality: The Kangbuk Samsung Health Study. Eur. J. Prev. Cardiol. 2021, zwab025. [Google Scholar] [CrossRef]
- Freeman, E.E.; Egleston, B.L.; West, S.K.; Bandeen-Roche, K.; Rubin, G. Visual acuity change and mortality in older adults. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4040–4045. [Google Scholar] [CrossRef]
- Aljied, R.; Aubin, M.J.; Buhrmann, R.; Sabeti, S.; Freeman, E.E. Prevalence and determinants of visual impairment in Canada: Cross-sectional data from the Canadian Longitudinal Study on Aging. Can. J. Ophthalmol. Can. J. Opthalmol. 2018, 53, 291–297. [Google Scholar] [CrossRef]
- Royston, P.; Parmar, M.K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 2002, 21, 2175–2197. [Google Scholar] [CrossRef]
- Wilsnack, R.W.; Wilsnack, S.C.; Kristjanson, A.F.; Vogeltanz-Holm, N.D.; Gmel, G. Gender and alcohol consumption: Patterns from the multinational GENACIS project. Addiction 2009, 104, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; di Padova, C.; Pozzato, G.; Terpin, M.; Baraona, E.; Lieber, C.S. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N. Engl. J. Med. 1990, 322, 95–99. [Google Scholar] [CrossRef]
- Taylor, J.L.; Dolhert, N.; Friedman, L.; Mumenthaler, M.; Yesavage, J.A. Alcohol elimination and simulator performance of male and female aviators: A preliminary report. Aviat. Space Environ. Med. 1996, 67, 407–413. [Google Scholar] [PubMed]
- Casares-López, M.; Castro-Torres, J.J.; Ortiz-Peregrina, S.; Martino, F.; Ortiz, C. Changes in Visual Performance under the Effects of Moderate-High Alcohol Consumption: The Influence of Biological Sex. Int. J. Environ. Res. Public Health 2021, 18, 6790. [Google Scholar] [CrossRef] [PubMed]
- White, A.M. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. Curr. Rev. 2020, 40, 1. [Google Scholar] [CrossRef]
- Devaux, M.; Sassi, F. Social disparities in hazardous alcohol use: Self-report bias may lead to incorrect estimates. Eur. J. Public Health 2016, 26, 129–134. [Google Scholar] [CrossRef]
- Minagawa, Y. Gender differences in alcohol choice among Russians: Evidence from a quantitative study. Eur. Addict. Res. 2013, 19, 82–88. [Google Scholar] [CrossRef]
- Choe, S.A.; Yoo, S.; JeKarl, J.; Kim, K.K. Recent trend and associated factors of harmful alcohol use based on age and gender in Korea. J. Korean Med. Sci. 2018, 33, e23. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R. Lifestyle exposures and eye diseases in adults. Am. J. Ophthalmol. 2007, 144, 961–969. [Google Scholar] [CrossRef]
- Cumurcu, T.; Gunduz, A.; Cumurcu, B.E.; Gul, I.G.; Akpolat, N.; Karlidag, R. The changes in tear film parameters and impression cytology in heavily drinking men. Cornea 2013, 32, 237–241. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Nam, W.H.; Yi, K.; Choi, D.G.; Hyon, J.Y.; Wee, W.R.; Shin, Y.J. Oral alcohol administration disturbs tear film and ocular surface. Ophthalmology 2012, 119, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, J.M.; Hickman, S.J. Treatment and Outcomes in Nutritional Optic Neuropathy. Curr. Treat. Options Neurol. 2019, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, C.; Van Stavern, G.; McClelland, C. Leber hereditary optic neuropathy: Current perspectives. Clin. Ophthalmol. 2015, 9, 1165–1176. [Google Scholar] [PubMed]
- Charnwood, L. Influence of alcohol on fusion. Br. J. Ophthalmol. 1950, 34, 733–736. [Google Scholar] [CrossRef]
- Savolainen, K.; Riihimaki, V.; Vaheri, E.; Linnoila, M. Effects of xylene and alcohol on vestibular and visual functions in man. Scand. J. Work Environ. Health 1980, 6, 94–103. [Google Scholar] [CrossRef]
- Crowdy, K.A.; Marple-Horvat, D.E. Alcohol affects eye movements essential for visually guided stepping. Alcohol. Clin. Exp. Res. 2004, 28, 402–407. [Google Scholar] [CrossRef]
- Zhuang, X.; Kang, P.; King, A.; Cao, D. Alcohol Intoxication Impairs Mesopic Rod and Cone Temporal Processing in Social Drinkers. Alcohol. Clin. Exp. Res. 2015, 39, 1842–1849. [Google Scholar] [CrossRef]
- Brasil, A.; Castro, A.J.; Martins, I.C.; Lacerda, E.M.; Souza, G.S.; Herculano, A.M.; Rosa, A.A.; Rodrigues, A.R.; Silveira, L.C. Colour Vision Impairment in Young Alcohol Consumers. PLoS ONE 2015, 10, e0140169. [Google Scholar] [CrossRef]
- Castro, J.J.; Ortiz, C.; Pozo, A.M.; Anera, R.G.; Soler, M. A visual test based on a freeware software for quantifying and displaying night-vision disturbances: Study in subjects after alcohol consumption. Theor. Biol. Med. Model. 2014, 11, S1. [Google Scholar] [CrossRef][Green Version]
- Schou, A.L.; Molbak, M.L.; Schnor, P.; Gronbaek, M.; Tolstrup, J.S. Alcohol consumption, smoking and development of visible age-related signs: A prospective cohort study. J. Epidemiol. Community Health 2017, 71, 1177–1184. [Google Scholar]
- Foong, A.W.; Fong, C.W.; Wong, T.Y.; Saw, S.M.; Heng, D.; Foster, P.J. Visual acuity and mortality in a chinese population. The Tanjong Pagar Study. Ophthalmology 2008, 115, 802–807. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R. Toxic optic neuropathy. Indian J. Ophthalmol. 2011, 59, 137–141. [Google Scholar] [CrossRef]
- Peponis, V.; Kyttaris, V.C.; Chalkiadakis, S.E.; Bonovas, S.; Sitaras, N.M. Ocular side effects of anti-rheumatic medications: What a rheumatologist should know. Lupus 2010, 19, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L.; Friedman, G.D.; Armstrong, M.A.; Kipp, H. Wine, liquor, beer, and mortality. Am. J. Epidemiol. 2003, 158, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J. Trends in Alcohol Consumption for Korean Adults from 1998 to 2018: Korea National Health and Nutritional Examination Survey. Nutrients 2021, 13, 609. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | Lifetime Drinking Status | |||||
|---|---|---|---|---|---|---|---|
| Lifetime Abstainer | Current Abstainer | 0 to <10 g/Day | 10 to <20 g/Day | 20 to <40 g/Day | ≥40 g/Day | ||
| Number | 287,352 | 9311 | 24,810 | 133,807 | 52,288 | 37,445 | 29,691 |
| Age (years) a | 37.8 (7.8) | 43.9 (10.4) | 37.8 (8.1) | 36.9 (7.2) | 37.5 (7.6) | 38.7 (8.0) | 39.3 (8.0) |
| Male (%) | 57.6 | 17.4 | 29.0 | 43.4 | 75.9 | 85.1 | 91.3 |
| Current smoker (%) | 22.7 | 3.9 | 7.0 | 13.1 | 29.0 | 40.8 | 48.2 |
| HEPA (%) | 15.8 | 15.3 | 13.8 | 14.4 | 16.3 | 18.3 | 19.9 |
| High education level (%) c | 84.7 | 73.6 | 81.2 | 86.8 | 86.2 | 83.9 | 80.0 |
| Hypertension (%) | 9.9 | 10.8 | 6.2 | 6.4 | 11.0 | 15.5 | 19.7 |
| Diabetes (%) | 3.3 | 4.2 | 2.6 | 2.2 | 3.4 | 4.8 | 6.2 |
| History of CVD (%) | 0.8 | 1.4 | 0.8 | 0.6 | 0.9 | 1.0 | 1.3 |
| Medication for dyslipidemia (%) | 1.9 | 4.6 | 1.7 | 1.4 | 1.9 | 2.4 | 2.9 |
| Obesity (%) d | 28.4 | 19.9 | 19.7 | 21.5 | 33.7 | 39.7 | 45.9 |
| Body mass index (kg/m2) a | 23.3 (3.4) | 22.4 (3.3) | 22.4 (3.4) | 22.7 (3.3) | 23.9 (3.2) | 24.4 (3.2) | 24.9 (3.2) |
| Systolic BP (mmHg) a | 109.4 (13.0) | 107.1 (13.3) | 104.2 (12.5) | 106.6 (12.3) | 111.8 (12.3) | 114.4 (12.3) | 116.4 (12.3) |
| Diastolic BP (mmHg) a | 70.1 (9.9) | 67.9 (9.3) | 66.8 (9.3) | 68.0 (9.2) | 71.7 (9.6) | 73.8 (9.9) | 75.5 (10.0) |
| Glucose (mg/dL) a | 94.8 (13.9) | 94.0 (13.1) | 92.2 (12.9) | 93.0 (12.0) | 95.6 (14.1) | 97.8 (15.9) | 99.8 (17.5) |
| Total cholesterol (mg/dL) a | 193.3 (34.0) | 193.6 (34.9) | 189.4 (33.5) | 189.9 (33.2) | 195.6 (33.9) | 198.6 (34.3) | 201.1 (35.1) |
| LDL-C (mg/dL) a | 120.2 (32.0) | 120.4 (32.5) | 114.4 (31.0) | 117.3 (31.2) | 123.5 (32.2) | 125.1 (32.4) | 125.8 (32.8) |
| HDL-C (mg/dL) a | 58.8 (15.4) | 61.0 (15.2) | 60.8 (15.2) | 60.3 (15.4) | 56.8 (15.1) | 56.5 (15.1) | 56.6 (14.9) |
| Triglycerides (mg/dL) b | 90 (64–135) | 80 (59–114) | 77 (57–110) | 80 (59–116) | 99 (69–147) | 112 (77–164) | 123 (85–182) |
| AST (U/L) b | 19 (16–24) | 19 (16–23) | 18 (15–22) | 18 (16–22) | 20 (17–25) | 21 (18–27) | 23 (19–29) |
| ALT (U/L) b | 18 (13–28) | 15 (12–22) | 15 (11–22) | 16 (12–24) | 20 (14–31) | 22 (16–33) | 24 (17–36) |
| GGT (U/L) b | 20 (13–35) | 14 (11–21) | 15 (11–22) | 16 (12–25) | 25 (16–40) | 32 (21–54) | 42 (26–71) |
| hsCRP (mg/L) b | 0.4 (0.2–0.9) | 0.4 (0.2–0.8) | 0.4 (0.2–0.9) | 0.4 (0.2–0.8) | 0.5 (0.2–1.0) | 0.5 (0.3–1.0) | 0.5 (0.3–1.0) |
| HOMA-IR b | 1.21 (0.80–1.80) | 1.16 (0.76–1.73) | 1.14 (0.76–1.67) | 1.16 (0.77–1.73) | 1.25 (0.82–1.86) | 1.29 (0.85–1.93) | 1.34 (0.88–2.02) |
| Total energy intake (kcal/day) b,e | 1523.5 (1157.9–1924.9) | 1446.0 (1096.4–1838.1) | 1464.0 (1091.8–1867.0) | 1480.3 (1119.1–1870.8) | 1548.8 (1198.3–1947.4) | 1594.7 (1235.9–2007.8) | 1659.6 (1285.1–2095.5) |
| Alcohol Consumption Category | Person-Years (PY) | Incident Cases | Incidence Density (103 PY) | Age & Sex Adjusted HRs (95% CI) | Multivariable-Adjusted HR (95% CI) a | HR (95% CI) b in Model Using Time-Dependent Variables |
|---|---|---|---|---|---|---|
| Total (n = 287,352) | ||||||
| Lifetime abstainer | 40,090.3 | 412 | 10.3 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 0.1 to <10 g/day | 639,831.4 | 3909 | 6.1 | 1.08 (0.97–1.19) | 1.07 (0.96–1.19) | 1.09 (0.98–1.21) |
| 10 to <20 g/day | 256,412.5 | 1359 | 5.3 | 1.18 (1.05–1.32) | 1.15 (1.03–1.30) | 1.19 (1.06–1.34) |
| 20 to <40 g/day | 182,329.7 | 957 | 5.2 | 1.19 (1.05–1.35) | 1.15 (1.01–1.30) | 1.21 (1.07–1.38) |
| ≥40 g/day | 140,300.0 | 781 | 5.6 | 1.29 (1.13–1.47) | 1.23 (1.08–1.40) | 1.31 (1.15–1.49) |
| p for trend c | <0.001 | <0.001 | <0.001 | |||
| Current abstainer | 126,528.8 | 902 | 7.1 | 1.09 (0.97–1.23) | 1.09 (0.97–1.23) | 1.17 (1.04–1.32) |
| Women (n = 121,819) | ||||||
| Lifetime abstainer | 32,802.1 | 386 | 11.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 0.1 to <10 g/day | 352,293.3 | 2756 | 7.8 | 1.05 (0.94–1.17) | 1.05 (0.94–1.17) | 1.07 (0.96–1.19) |
| 10 to <20 g/day | 57,145.8 | 486 | 8.5 | 1.20 (1.04–1.37) | 1.17 (1.02–1.34) | 1.22 (1.06–1.40) |
| 20 to <40 g/day | 24,653.1 | 214 | 8.7 | 1.24 (1.04–1.47) | 1.19 (1.004–1.41) | 1.29 (1.08–1.53) |
| ≥40 g/day | 11,400.7 | 101 | 8.9 | 1.32 (1.06–1.64) | 1.24 (0.99–1.55) | 1.30 (1.03–1.64) |
| p for trend c | <0.001 | 0.003 | <0.001 | |||
| Current abstainer | 88,359.0 | 702 | 7.9 | 1.02 (0.90–1.16) | 1.03 (0.90–1.16) | 1.12 (0.98–1.27) |
| Men (n = 165,533) | ||||||
| Lifetime abstainer | 7288.1 | 26 | 3.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 0.1 to <10 g/day | 287,538.1 | 1153 | 4.0 | 1.42 (0.96–2.10) | 1.40 (0.95–2.07) | 1.32 (0.91–1.92) |
| 10 to <20 g/day | 199,266.7 | 873 | 4.4 | 1.51 (1.02–2.24) | 1.48 (1.00–2.18) | 1.40 (0.96–2.05) |
| 20 to <40 g/day | 157,676.6 | 743 | 4.7 | 1.53 (1.04–2.27) | 1.47 (0.99–2.18) | 1.43 (0.98–2.08) |
| ≥40 g/day | 128,899.3 | 680 | 5.3 | 1.68 (1.13–2.48) | 1.59 (1.07–2.36) | 1.56 (1.07–2.29) |
| p for trend c | <0.001 | 0.005 | <0.001 | |||
| Current abstainer | 38,169.7 | 200 | 5.2 | 1.70 (1.13–2.56) | 1.69 (1.12–2.55) | 1.59 (1.07–2.37) |
| Drinking Pattern | Multivariable-Adjusted HR (95% CI) a | ||
|---|---|---|---|
| Total | Women | Men | |
| Frequency of drinking (drinks/week) | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1–2 | 1.09 (0.98–1.21) | 1.07 (0.95–1.19) | 1.49 (1.002–2.21) |
| 3–4 | 1.18 (1.04–1.33) | 1.13 (0.96–1.32) | 1.62 (1.09–2.41) |
| 5–6 | 1.18 (0.98–1.41) | 1.02 (0.74–1.42) | 1.67 (1.09–2.56) |
| 7 | 1.34 (0.97–1.85) | 1.31 (0.75–2.27) | 1.83 (1.06–3.13) |
| p for trend | 0.002 | 0.173 | 0.004 |
| Number of drinks consumed per drinking day | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1–2 | 1.06 (0.95–1.18) | 1.02 (0.91–1.14) | 1.49 (0.99–2.23) |
| 3–5 | 1.11 (1.00–1.25) | 1.09 (0.97–1.23) | 1.43 (0.96–2.13) |
| ≥6 | 1.27 (1.13–1.43) | 1.33 (1.16–1.53) | 1.61 (1.08–2.39) |
| p for trend | <0.001 | <0.001 | 0.003 |
| Frequency of binge drinking b | |||
| Never | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| <Once a month | 1.06 (0.99–1.14) | 1.08 (0.99–1.18) | 0.99 (0.87–1.13) |
| Once a month | 1.09 (1.004–1.19) | 1.15 (1.02–1.30) | 1.02 (0.89–1.15) |
| ≥Once a week | 1.22 (1.13–1.32) | 1.25 (1.10–1.42) | 1.15 (1.03–1.29) |
| p for trend | <0.001 | <0.001 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Chang, Y.; Kim, Y.; Choi, C.Y.; Ryu, S. A Dose–Response Relationship of Alcohol Consumption with Risk of Visual Impairment in Korean Adults: The Kangbuk Samsung Health Study. Nutrients 2022, 14, 791. https://doi.org/10.3390/nu14040791
Han SY, Chang Y, Kim Y, Choi CY, Ryu S. A Dose–Response Relationship of Alcohol Consumption with Risk of Visual Impairment in Korean Adults: The Kangbuk Samsung Health Study. Nutrients. 2022; 14(4):791. https://doi.org/10.3390/nu14040791
Chicago/Turabian StyleHan, So Young, Yoosoo Chang, Yejin Kim, Chul Young Choi, and Seungho Ryu. 2022. "A Dose–Response Relationship of Alcohol Consumption with Risk of Visual Impairment in Korean Adults: The Kangbuk Samsung Health Study" Nutrients 14, no. 4: 791. https://doi.org/10.3390/nu14040791
APA StyleHan, S. Y., Chang, Y., Kim, Y., Choi, C. Y., & Ryu, S. (2022). A Dose–Response Relationship of Alcohol Consumption with Risk of Visual Impairment in Korean Adults: The Kangbuk Samsung Health Study. Nutrients, 14(4), 791. https://doi.org/10.3390/nu14040791







