Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Volatile Compounds and Odor Description
2.3. Animal Care and Experimental Design
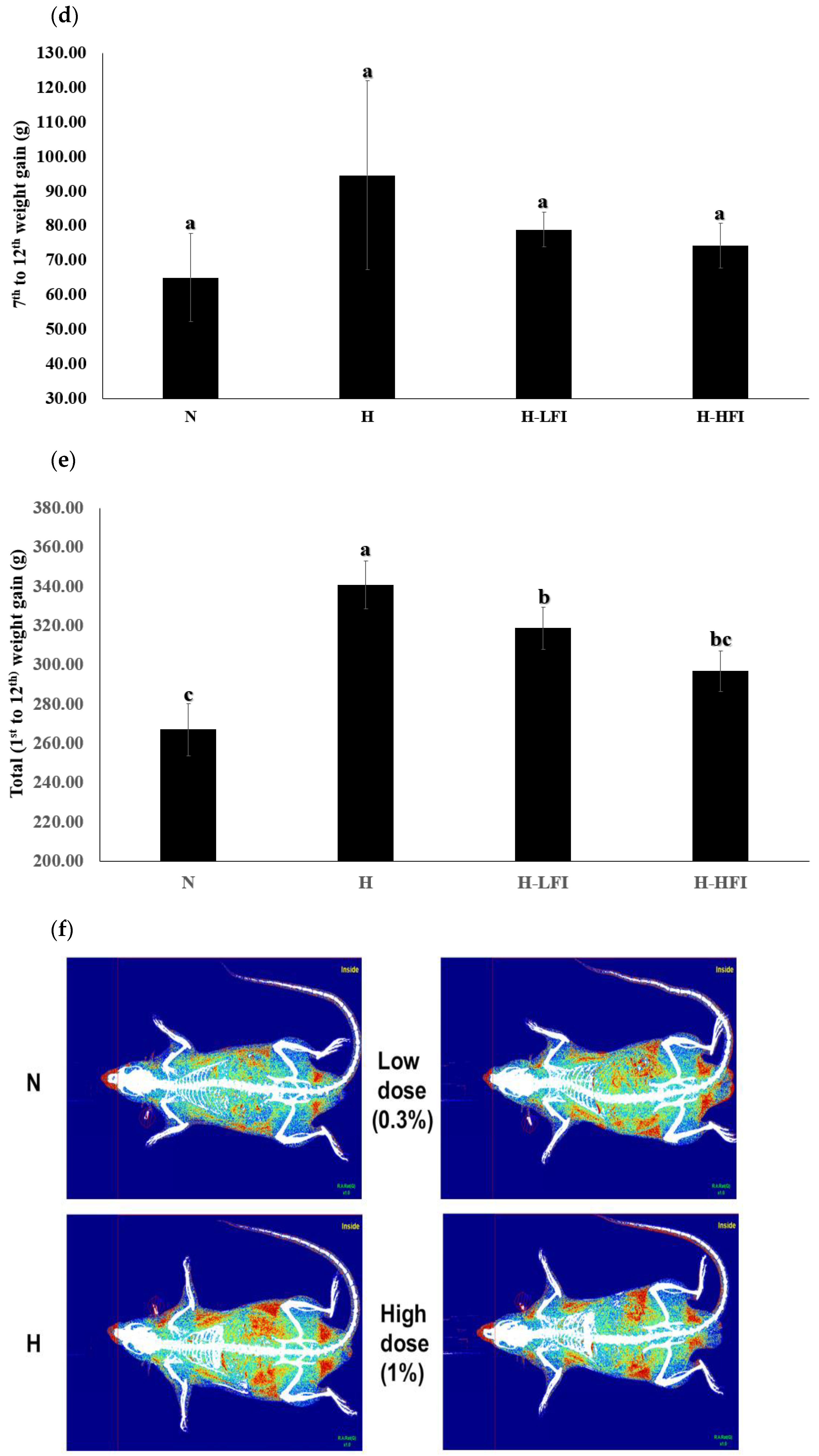
2.4. Body Composition Assessment Using Dual-Energy X-ray Absorptiometry (DXA)
2.5. Plasma Biomarker Analysis
2.6. Blood Glucose Assessment
2.7. Blood Pressure Assessment
2.8. Statistical Analysis
3. Results
3.1. Volatile Compounds
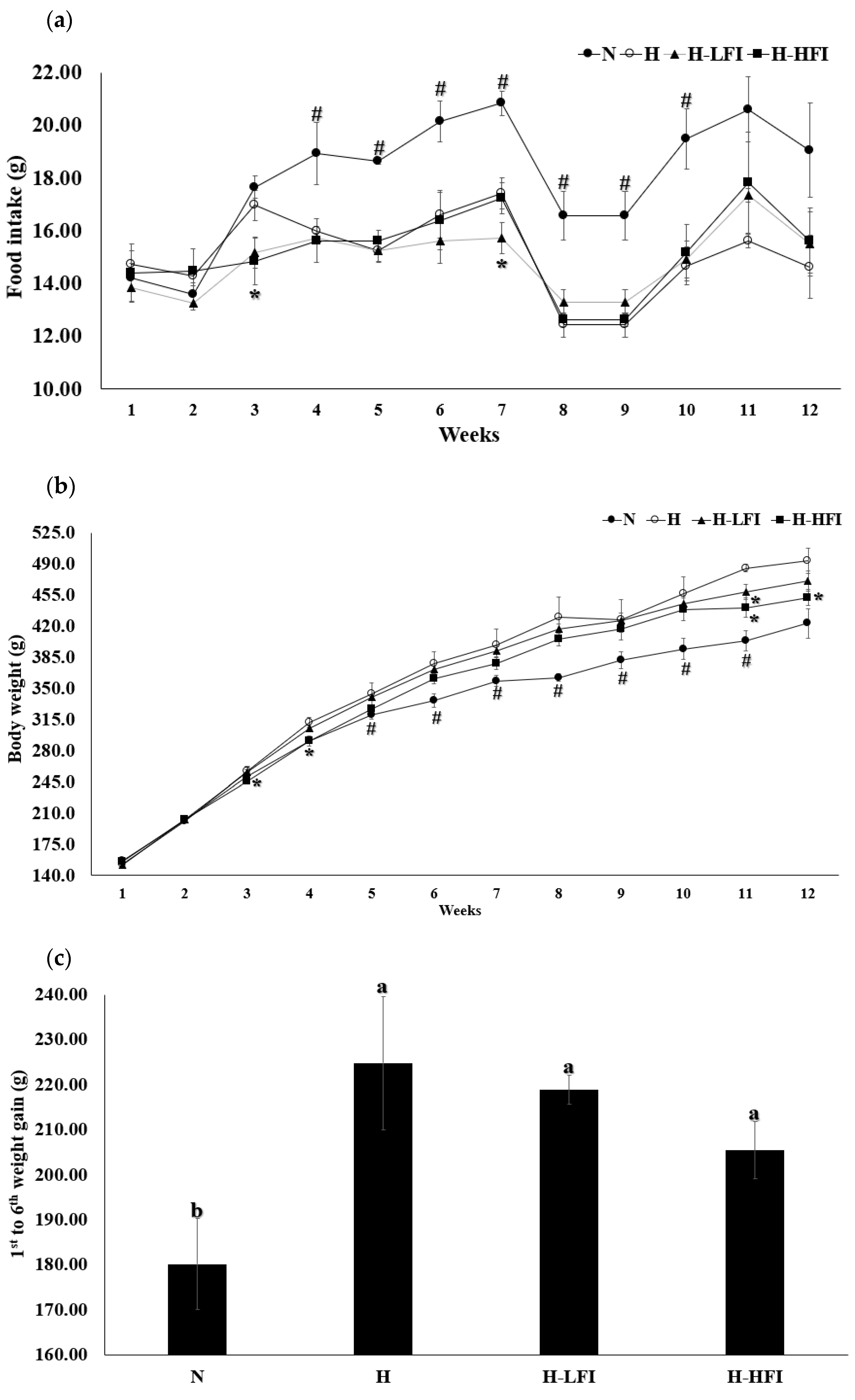
3.2. Food Intake, Body Weight, Body Composition, and Length
3.3. Organ Weight
3.4. Plasma Analysis
3.5. Blood Glucose
3.6. Blood Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, S.J.; Cho, J.J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.C. Inhalation of Patchouli (Pogostemon Cablin Benth.) Essential Oil Improved Metabolic Parameters in Obesity-Induced Sprague Dawley Rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef]
- Baek, H.; Kim, S.; Lee, I.; Kang, S.; Yoo, J.; Yoon, W.; Kim, Y.; Kim, H.; Kim, J. Anti-Obesity and Anti-Lipidemic Effects of Linalool in High-Fat Diet-Induced Obese Mice. J. Biomed. Res. 2012, 13, 229–235. [Google Scholar] [CrossRef]
- Després, J.P. Is Visceral Obesity the Cause of the Metabolic Syndrome? Ann. Med. 2006, 38, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Olson, D.P. Central Nervous System Control of Metabolism. Nature 2012, 491, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Go, G.W. Pinus densiflora Bark Extract (PineXol) Decreases Adiposity in Mice by Down-Regulation of Hepatic De Novo Lipogenesis and Adipogenesis in White Adipose Tissue. J. Microbiol. Biotechnol. 2017, 27, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Depoortere, I.; Hatt, H. Therapeutic Potential of Ectopic Olfactory and Taste Receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138. [Google Scholar] [CrossRef]
- Batubara, I.; Suparto, I.H.; Sa’diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella Oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef]
- He, W.; Huang, B.K. A Review of Chemistry and Bioactivities of a Medicinal Spice: Foeniculum vulgare. J. Med. Plants Res. 2011, 5, 3595–3600. [Google Scholar] [CrossRef]
- Bae, J.Y.; Kim, J.E.; Choue, R.W.; Lim, H.J. Fennel (Foeniculum vulgare) and Fenugreek (Trigonella Foenum-Graecum) Tea Drinking Suppresses Subjective Short-Term Appetite in Overweight Women. Clin. Nutr. Res. 2015, 4, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Chen, J.; Niu, Y.; Chen, F. Characterization of the Key Odorants of Fennel Essential Oils of Different Regions Using GC–MS and GC–O Combined With Partial Least Squares Regression. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1063, 226–234. [Google Scholar] [CrossRef]
- Elghazaly, N.A.; Radwan, E.H.; Zaatout, H.H.; Elghazaly, M.M.; Allam, N.E. Beneficial Effects of Fennel (Foeniculum vulgare) in Treating Obesity in Rats. Obes. Manag. 2019, 1, 16–33. [Google Scholar] [CrossRef]
- Punetha, D.; Tewari, G.; Pande, C.; Bhatt, S. Effect of Climatic Conditions on the Volatile Compounds of the Aerial Parts of Foeniculum vulgare Mill. J. Essent. Oil Bear. Plants 2019, 22, 1093–1103. [Google Scholar] [CrossRef]
- Diaz-Maroto, M.C.; Pérez-Coello, M.S.; Esteban, J.; Sanz, J. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from Central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Lee, D.S.; Kang, J.Y.; Kim, J.M.; Park, S.K.; Kang, J.E.; Kwon, B.S.; Park, S.H.; Park, S.B.; Ha, G.J.; et al. Memory Improvement Effect of Artemisia Argyi H. Fermented With Monascus Purpureus on Streptozotocin-Induced Diabetic Mice. Korean J. Food Sci. Technol. 2017, 49, 550–558. [Google Scholar] [CrossRef]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Giant Fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050–1062. [Google Scholar] [PubMed]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, F.M.; Saleh, M.A.; Abdel-Azim, N.S.; Shams, K.A.; Ismail, S.I.; Shahat, A.A.; Saleh, I.A. Evaluation of the essential oil of Foeniculum vulgare Mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Mirdehghan Ashkezari, S.M.; Bahmanyar, H.; Azizpour, H.; Mohammadi, M.; Najafipour, I. Investigation of Operating Parameters on Ultrasound-Assisted Extraction of Anethole in Fennel Essential Oil. J. Chem. Pet. Eng. 2021, 55, 339–351. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, T.; Tanaka, S.; Bakhshishayan, S.; Kogo, M.; Yamamoto, T. Olfactory Stimulation Modulates the Blood Glucose Level in Rats. Int. J. Med. Sci. 2018, 15, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa Intake Reduces Plasma and Liver Cholesterol, Lessens Obesity-Associated Inflammation, and Helps to Prevent Hepatic Steatosis in Obese Db/Db Mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Hur, M.H.; Kim, C.; Kim, C.H.; Ahn, H.C.; Ahn, H.Y. The Effects of Inhalation of Essential Oils on the Body Weight, Food Efficiency Rate and Serum Leptin of Growing SD Rats. J. Korean Acad. Nurs. 2006, 36, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-Anethole Ameliorates Obesity via Induction of Browning in White Adipocytes and Activation of Brown Adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef]
- You, M.; Fan, R.; Kim, J.; Shin, S.H.; Chung, S. Alpha-Linolenic Acid-Enriched Butter Promotes Fatty Acid Remodeling and Thermogenic Activation in the Brown Adipose Tissue. Nutrients 2020, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- Latif, R.; Lodhi, G.M.; Aslam, M. Effects of Amlodipine on Serum Testosterone, Testicular Weight and Gonado-Somatic Index in Adult Rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 8–10. [Google Scholar]
- Nejatbakhsh, R.; Riyahi, S.; Farrokhi, A.; Rostamkhani, S.; Mahmazi, S.; Yazdinezhad, A.; Kazemi, M.; Shokri, S. Ameliorating Effects of Fennel and Cumin Extracts on Sperm Quality and Spermatogenic Cells Apoptosis by Inducing Weight Loss and Reducing Leptin Concentration in Diet-Induced Obese Rats. Andrologia 2017, 49, e12748. [Google Scholar] [CrossRef]
- Tsolakis, C.; Xekouki, P.; Kaloupsis, S.; Karas, D.; Messinis, D.; Vagenas, G.; Dessypris, A. The Influence of Exercise on Growth Hormone and Testosterone in Prepubertal and Early-Pubertal Boys. Hormones 2003, 2, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdemir, F.; Atilgan, D.; Markoc, F.; Boztepe, O.; Suha-Parlaktas, B.; Sahin, S. The Effect of Diet Induced Obesity on Testicular Tissue and Serum Oxidative Stress Parameters. Actas Urol. Esp. 2012, 36, 153–159. [Google Scholar] [CrossRef]
- Parsaeyan, N. The Effect of Foeniculum vulgare (Fennel) Extract on Lipid Profile, Lipid Peroxidation and Liver Enzymes of Diabetic Rat. Iran. J. Diabetes Obes. 2016, 8, 24–29. [Google Scholar]
- Kooti, W.; Moradi, M.; Akbari, S.A.; Ahvazi, N.S.; Samani, M.A.; Larky, D.A. Therapeutic and Pharmacological Potential of Foeniculum vulgare Mill.: A Review. J. HerbMed Pharmacol. 2015, 4, 1–9. [Google Scholar]
- Tamburlin, I.S.; Roux, E.; Feuillée, M.; Labbé, J.; Aussagues, Y.; El Fadle, F.E.; Fraboul, F.; Bouvier, G. Toxicological safety assessment of essential oils used as food supplements to establish safe oral recommended doses. Food Chem. Toxicol. 2021, 157, 112603. [Google Scholar] [CrossRef] [PubMed]
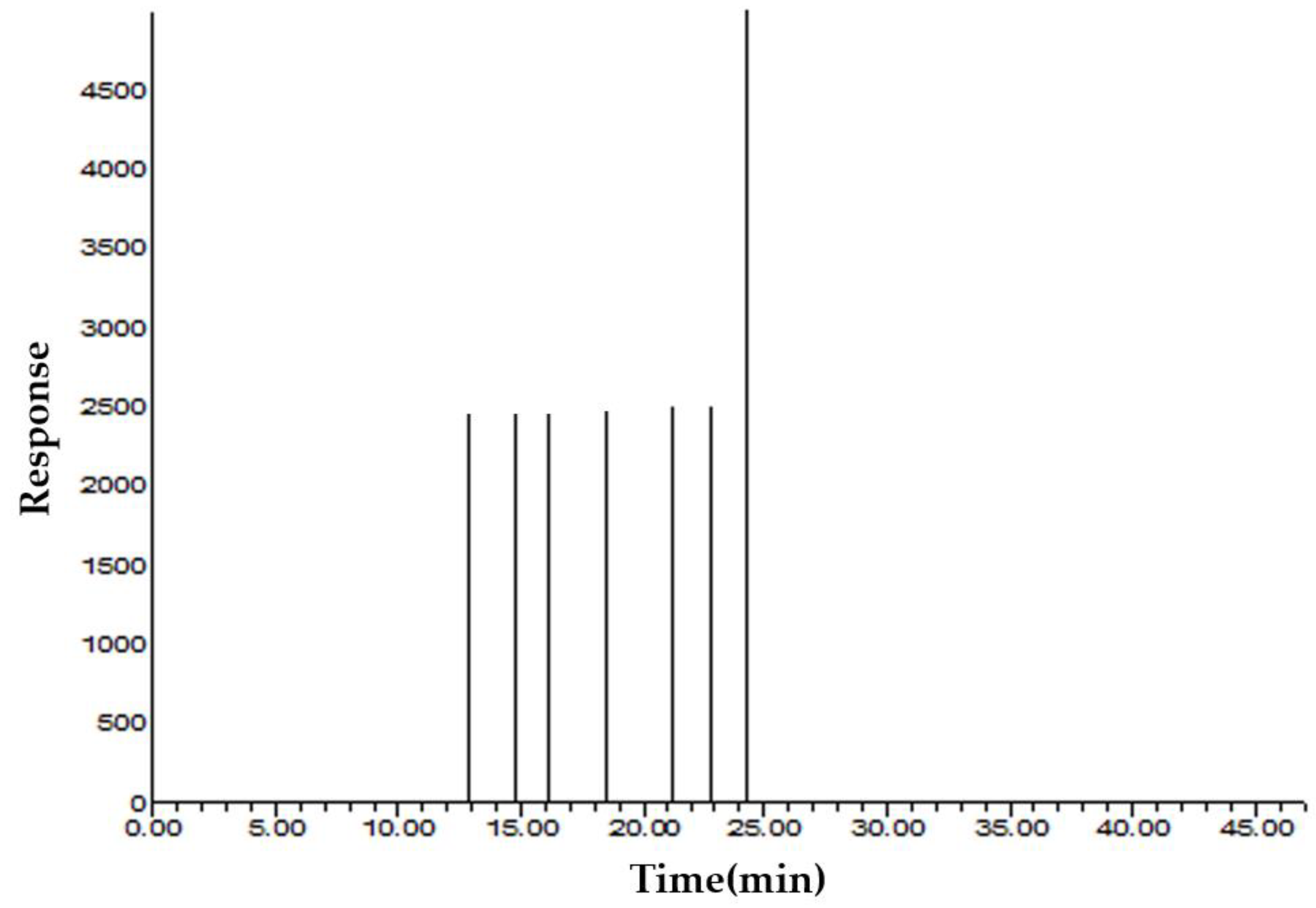
| Major Compound | RT a | RI b | Mean ± SD | Mean ± SD | Odor Intensity | Odor Description | I.D. c |
|---|---|---|---|---|---|---|---|
| (min) | (μg/100 g) | (%) | |||||
| Aldehyde (1) | |||||||
| p-Anisaldehyde | 22.57 | 1276 | 304.17 ± 38.79 | 1.18 ± 0.15 | 1 | Fennel | MS d/RI |
| Hydrocarbons (5) | |||||||
| α-Pinene | 13.03 | 961 | 1635.87 ± 139.95 | 6.33 ± 0.54 | 1 | Herb | MS/RI |
| Limonene | 16.11 | 1056 | 733.77 ± 18.60 | 2.84 ± 0.07 | 1 | Fennel | MS/RI |
| Estragole | 21.16 | 1224 | 1556.21 ± 40.28 | 6.02 ± 0.15 | 1 | Fennel | MS/RI |
| cis-Anethole | 22.55 | 1275 | 382.86 ± 35.95 | 1.48 ± 0.16 | 2 | Fennel | MS |
| trans-Anethole | 23.69 | 1317 | 13,100.47 ± 3971.88 | 50.69 ± 15.37 | 2 | Fennel | MS |
| Ketone (1) | |||||||
| Fenchone | 18.23 | 1123 | 4956.46 ± 117.31 | 19.18 ± 4.32 | 1 | Herb, fragrance | MS |
| Parameters | N | H | H-LFI | H-HFI |
|---|---|---|---|---|
| Growth parameters | ||||
| Food intake (g/day) | 18.03 ± 0.70 a1 | 15.09 ± 0.64 b | 14.92 ± 0.37 b | 15.21 ± 0.78 b |
| Initial length (cm) | 17.3 ± 0.3 a | 16.7 ± 0.1 b | 16.7 ± 0.1 b | 16.5 ± 0.2 b |
| Final length (cm) | 23.4 ± 0.3 b | 23.4 ± 0.1 b | 24.1 ± 0.1 a | 23.9 ± 0.1 a |
| Length gain (cm) | 6.1 ± 0.2 c | 6.6 ± 0.1 b | 7.4 ± 0.1 a | 7.4 ± 0.2 a |
| Energy intake (kcal/day) | 50.48 ± 1.93 b | 69.40 ± 2.94 a | 68.64 ± 1.68 a | 69.95 ± 3.58 a |
| BMI | 7.71 ± 0.45 b | 9.17 ± 0.30 a | 8.13 ± 0.13 b | 7.92 ± 0.15 b |
| FER (%) | 17.65 ± 1.29 b | 26.93 ± 1.83 a | 25.45 ± 1.48 a | 23.27 ± 1.49 a |
| Organ weights | ||||
| Brain (g/kg) | 3.91 ± 0.17 a | 3.57 ± 0.25 a | 3.94 ± 0.06 a | 3.98 ± 0.39 a |
| Liver (g/kg) | 24.84 ± 0.29 a | 24.81 ± 5.46 a | 24.44 ± 0.64 a | 25.41 ± 2.28 a |
| Kidney (g/kg) | 5.71 ± 0.18 a | 4.46 ± 0.11 b | 5.46 ± 0.14 ab | 5.36 ± 0.72 ab |
| Heart (g/kg) | 3.02 ± 0.05 a | 2.34 ± 0.12 b | 3.01 ± 0.10 a | 2.89 ± 0.47 ab |
| WAT (g/kg) | 26.76 ± 1.71 b | 41.37 ± 1.64 a | 32.22 ± 4.51 b | 29.30 ± 1.06 b |
| BAT (g/kg) | 0.53 ± 0.04 b | 0.62 ± 0.07 b | 1.04 ± 0.06 a | 0.55 ± 0.08 b |
| Lung (g/kg) | 3.45 ± 0.11 ab | 3.10 ± 0.33 b | 3.87 ± 0.06 a | 3.89 ± 0.32 a |
| Adrenal galnds(g/kg) | 0.12 ± 0.01 b | 0.12 ± 0.01 b | 0.13 ± 0.01 b | 0.15 ± 0.01 b |
| Spleen (g/kg) | 1.67 ± 0.01 a | 1.34 ± 0.03 b | 1.47 ± 0.19 ab | 1.75 ± 0.14 a |
| Testicular (g/kg) | 9.81 ± 0.57 a | 7.52 ± 0.45 b | 9.30 ± 0.17 a | 9.53 ± 0.48 a |
| Epididymis (g/kg) | 2.87 ± 0.12 a | 2.59 ± 0.20 a | 2.89 ± 0.07 a | 2.70 ± 0.26 a |
| Plasma biomarkers | ||||
| TC (mg/dL) | 128.91 ± 3.63 b | 131.45 ± 2.07 b | 124.07 ± 4.05 b | 173.44 ± 1.77 a |
| HDL (mg/dL) | 35.50 ± 0.79 d | 42.51 ± 0.44 c | 49.90 ± 1.13 b | 52.82 ± 0.25 a |
| LDL (mg/dL) | 46.72 ± 1.78 c | 75.62 ± 1.59 b | 55.95 ± 5.41 c | 109.85 ± 5.63 a |
| AI (mg/dL) | 2.61 ± 0.05 a | 2.10 ± 0.10 c | 1.44 ± 0.04 d | 2.27 ± 0.04 b |
| CRF (mg/dL) | 3.61 ± 0.05 a | 3.17 ± 0.10 b | 2.44 ± 0.04 c | 3.27 ± 0.04 b |
| LHR (mg/dL) | 131.70 ± 7.94 c | 177.92 ± 5.61 b | 112.05 ± 8.29 c | 207.93 ± 9.69 a |
| TG (mg/dL) | 94.20 ± 3.03 b | 102.52 ± 3.14 a | 88.75 ± 2.02 b | 111.91 ± 6.61 a |
| Cortisol (ng/dL) | 4.29 ± 0.54 a | 4.38 ± 0.37 a | 4.50 ± 0.05 a | 4.07 ± 0.07 a |
| Insulin (ng/mL) | 0.42 ± 0.17 d | 4.13 ± 0.14 a | 1.79 ± 0.13 c | 2.83 ± 0.04 b |
| HOMA-IR | 2.19 ± 1.10 d | 22.57 ± 2.78 a | 8.54 ± 1.25 c | 14.05 ± 0.39 b |
| Leptin (pg/mL) | 2842.79 ± 173.45 a | 3926.00 ± 225.35 a | 3863.55 ± 790.87 a | 2711.10 ± 936.96 a |
| Testosterone (pg/mL) | 0.43 ± 0.11 b | 0.29 ± 0.07 b | 1.38 ± 0.19 a | 0.31 ± 0.01 b |
| ALT (Karmen/mL) | 5.16 ± 2.71 a | 8.29 ± 3.73 a | 10.25 ± 8.53 a | 3.05 ± 0.79 a |
| AST (Karmen/mL) | 40.00 ± 24.28 a | 70.63 ± 1.36 aA2 | 54.08 ± 7.54 aB | 56.46 ± 1.36 aB |
| Blood Glucose | Weak | ||
|---|---|---|---|
| (mg/dL) | Initial Period | Obesity Induced Period | Final Period |
| N | 102.3 ± 7.2 a1 | 108.7 ± 5.5 b | 114.0 ± 12.5 a |
| H | 108.0 ± 3.6 a | 126.7 ± 6.5 a | 122.7 ± 11.0 a |
| H-LFI | 101.7 ± 8.1 a | 107.2 ± 6.6 b | 117.6 ± 8.0 a |
| H-HFI | 112.3 ± 3.8 a | 109.7 ± 3.1 b | 111.7 ± 1.5 a |
| Systolic(mmHg) | Weak | |
|---|---|---|
| Initial Period | Final Period | |
| N | 154.7 ± 3.2 a1 | 199.3 ± 3.8 bc |
| H | 153.0 ± 5.3 a | 215.0 ± 7.0 a |
| H-LFI | 157.3 ± 12.4 a | 190.0 ± 2.0 c |
| H-HFI | 148.7 ± 4.9 a | 201.0 ± 1.0 b |
| Diastolic(mmHg) | ||
| N | 69.7 ± 1.5 a | 133.3 ± 12.1 a |
| H | 52.0 ± 5.3 a | 114.0 ± 4.0 ab |
| H-LFI | 60.3 ± 10.1 a | 131.3 ± 5.5 ab |
| H-HFI | 55.0 ± 19.5 a | 113.3 ± 5.5 b |
| Pulse(beats/min) | ||
| N | 422.0 ± 13.1 a | 377.0 ± 14.9 b |
| H | 415.7 ± 18.6 a | 415.3 ± 8.3 aA2 |
| H-LFI | 408.7 ± 8.1 a | 381.3 ± 7.44 bB |
| H-HFI | 424.0 ± 22.7 a | 382.3 ± 6.4 abB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.J.; Yoon, S.; Jo, S.M.; Jeong, H.; Youn, M.Y.; Kim, Y.J.; Kim, J.K.; Shin, E.-C. Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients 2022, 14, 741. https://doi.org/10.3390/nu14040741
Hong SJ, Yoon S, Jo SM, Jeong H, Youn MY, Kim YJ, Kim JK, Shin E-C. Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients. 2022; 14(4):741. https://doi.org/10.3390/nu14040741
Chicago/Turabian StyleHong, Seong Jun, Sojeong Yoon, Seong Min Jo, Hyangyeon Jeong, Moon Yeon Youn, Young Jun Kim, Jae Kyeom Kim, and Eui-Cheol Shin. 2022. "Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats" Nutrients 14, no. 4: 741. https://doi.org/10.3390/nu14040741
APA StyleHong, S. J., Yoon, S., Jo, S. M., Jeong, H., Youn, M. Y., Kim, Y. J., Kim, J. K., & Shin, E.-C. (2022). Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients, 14(4), 741. https://doi.org/10.3390/nu14040741







