Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus sunki Hort. ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Polymethoxyflavone-Rich Fraction (PRF)
2.2. Animals and Experimental Design
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Collection of Serum and Tissue Samples
2.5. Biochemical Parameter Assays
2.6. Histological Analysis
2.7. Western Blot Analysis
2.8. RNA-Seq Library Preparation and Sequencing
2.9. Data Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of PRF on the Body Weight (BW) in HFD-Induced Obese Mice
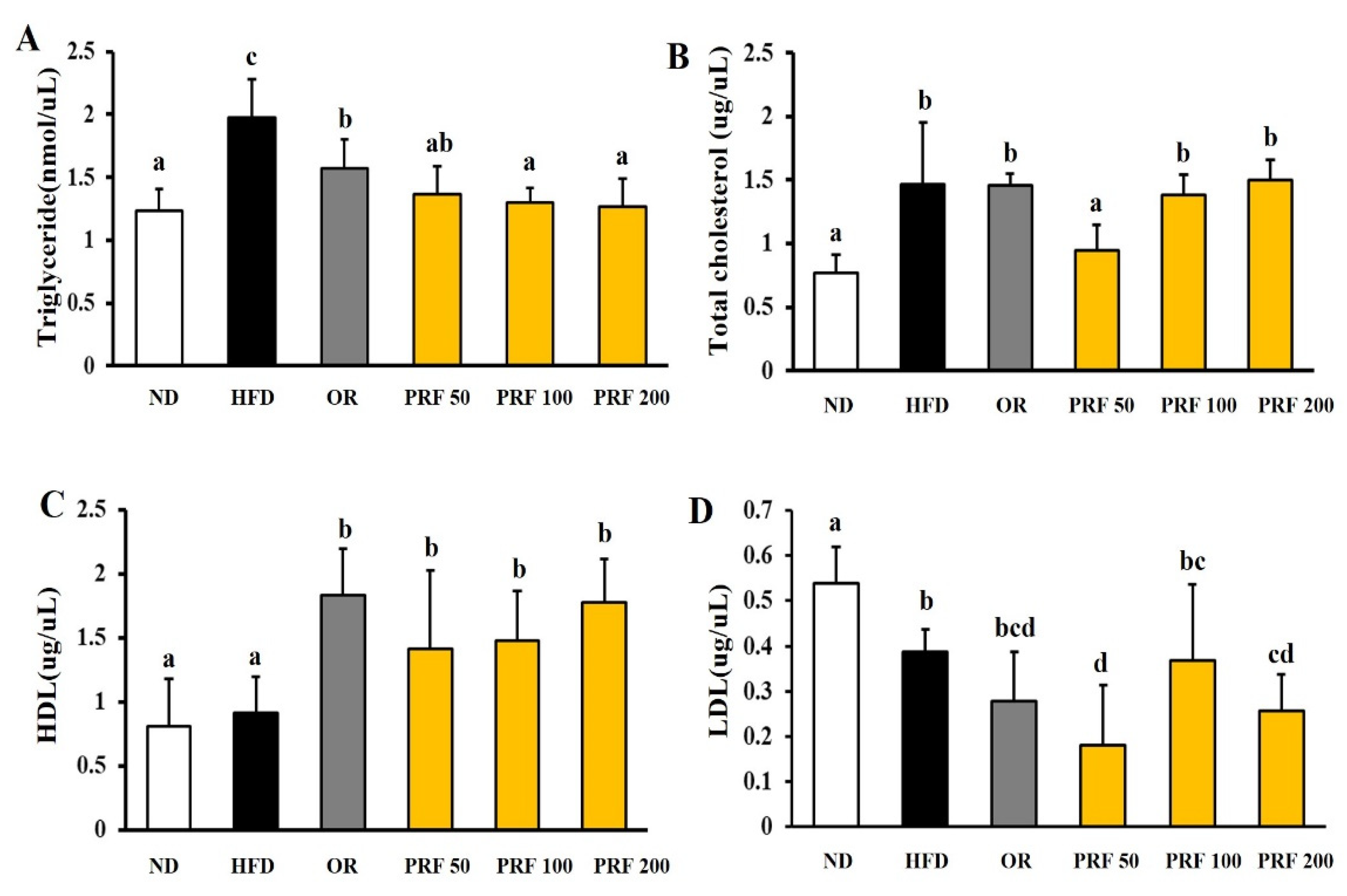
3.2. Effects of PRF on Serum Lipid Profiles in HFD-Induced Obese Mice
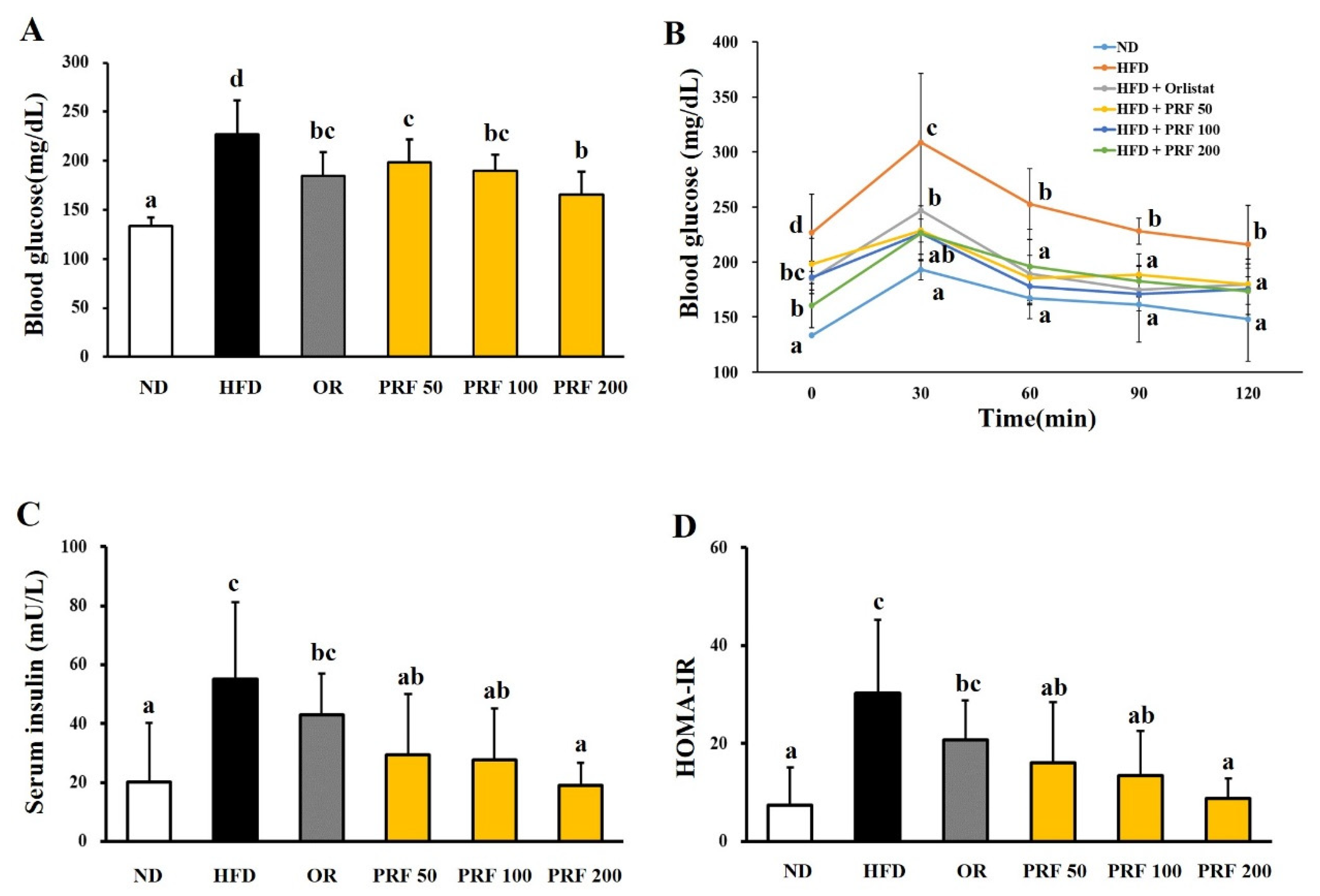
3.3. Effects of PRF on the Insulin Resistance in HFD-Induced Obese Mice
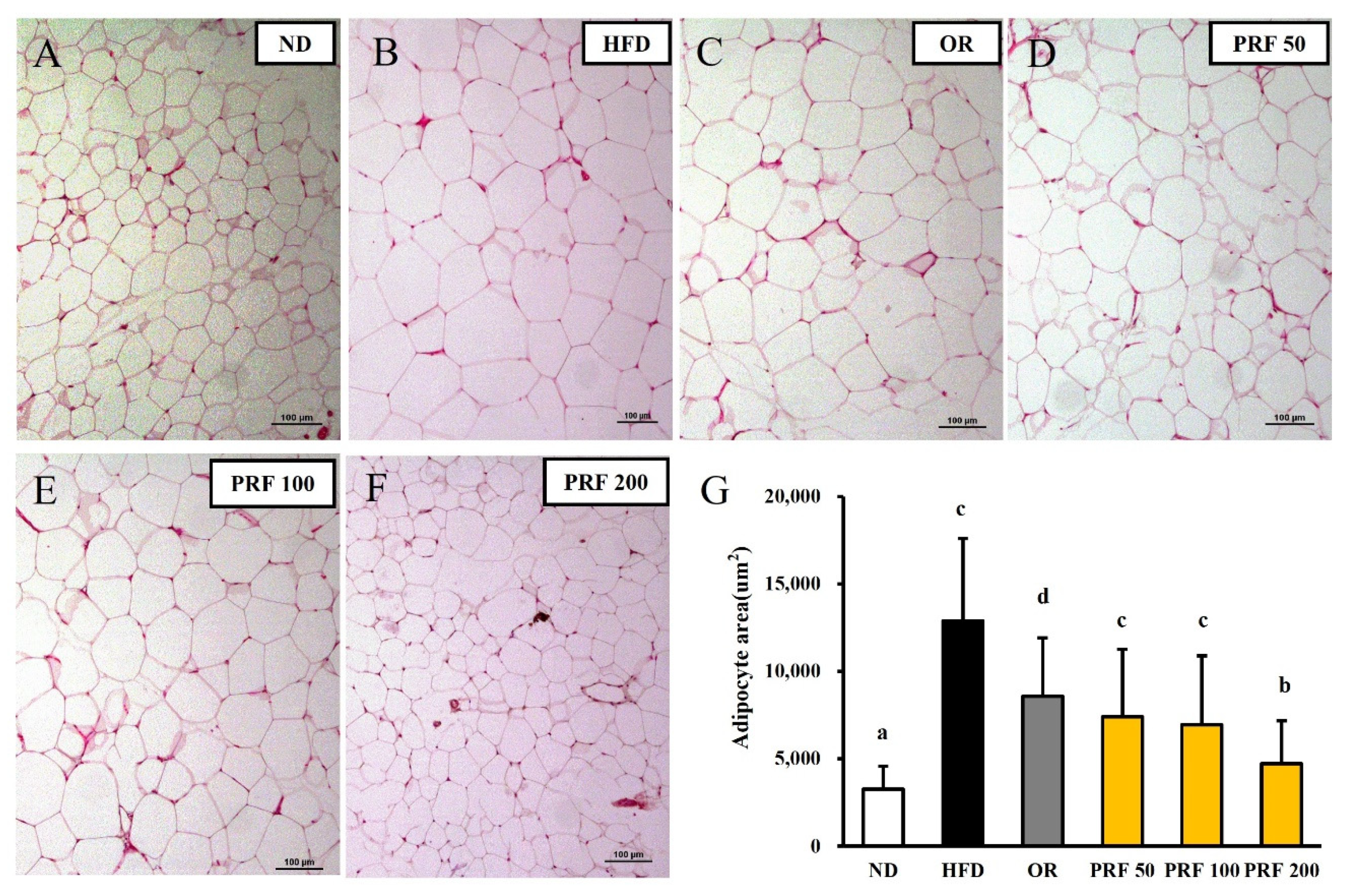
3.4. Effects of PRF on the Hypertrophy of Adipocytes in HFD-Induced Obese Mice
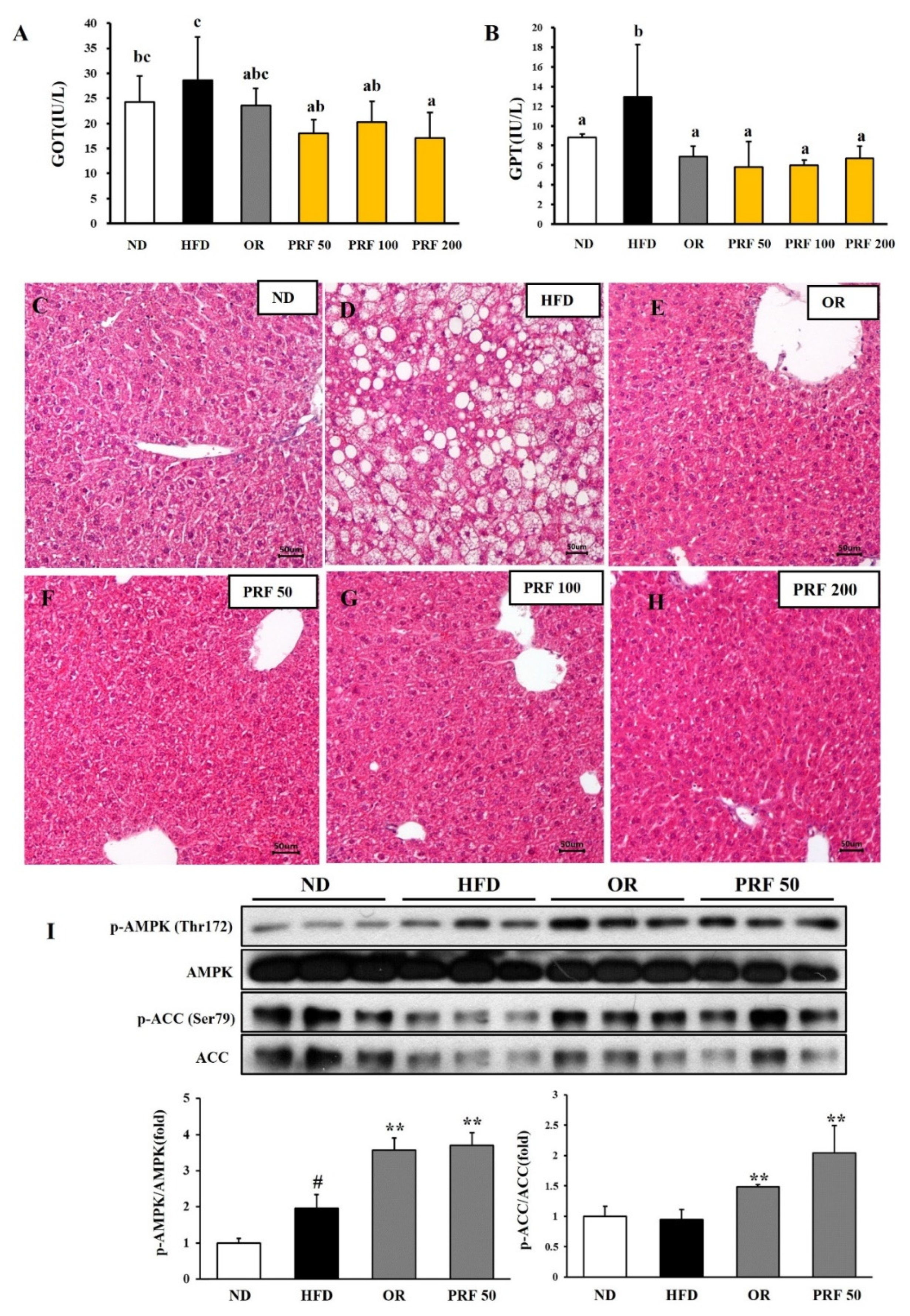
3.5. Effects of PRF on Fatty-Acid Oxidation and Lipolysis in HFD-Induced Obese Mice
3.6. Effects of PRF on Liver Function in HFD-Induced Obese Mice
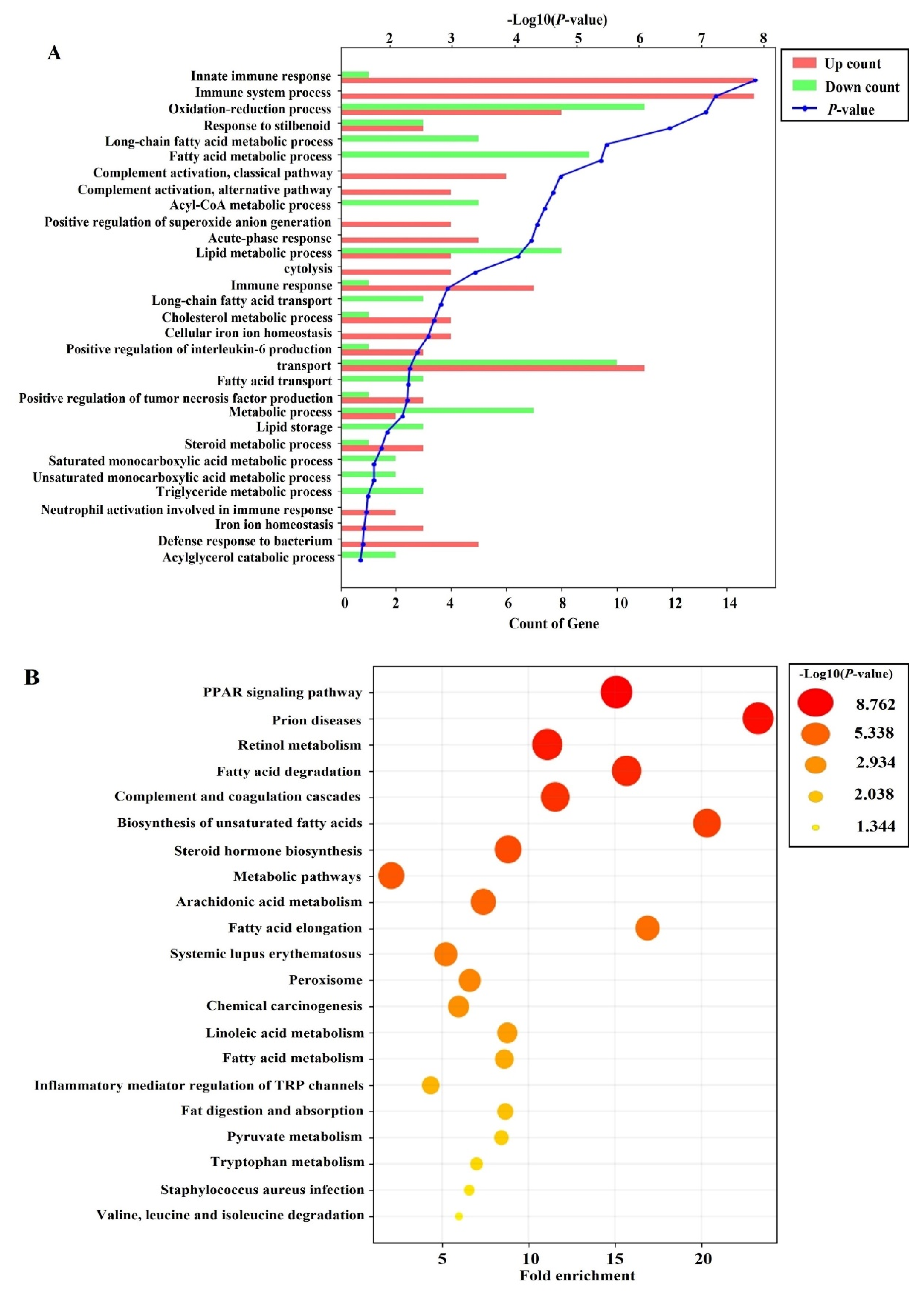
3.7. Effects of PRF on the Liver Transcriptome in HFD-Induced Obese Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klaauw, A.A.; Farooqi, I.S. The Hunger genes: Pathway to obesity. Cell 2015, 26, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Blueher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA-J. Am. Med. Assoc. 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadde, K.M.; Martin, C.K.; Verthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, A.; Brunani, A.; Capodaglio, P.; Berselli, M.E.; Villa, V.; Corti, S.; Leonardi, M.; Raggi, A. ICF-OB: A multidisciplinary questionnaire based on the international classification of functioning, disability and health to address disability in obesity. Eur. J. Physical. Rehab. Med. 2018, 54, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. New therapeutic approaches for the treatment of obesity. Sci. Transl. Med. 2016, 8, 323rv2. [Google Scholar] [CrossRef]
- Chien, M.Y.; Ku, Y.H.; Chang, J.M.; Yang, C.M.; Chen, C.H. Effects of herbal mixture extracts on obesity in rats fed a high-fat diet. J. Food. Drug. Anal. 2016, 24, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Rio, D.D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox. Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, W.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Func. Food. 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of obesity and type 2 diabetes with aged citrus peel (Chenpi) extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.L.; Li, S.Z.; Xio, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Lim, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Zhu, X.; Xhen, T.; Peng, B.; Lu, M.; Zheng, H.; Huang, Q.; Ho, C.T.; Chenu, Y.; Cao, Y. Prevention of obesity and hyperlipidemia by heptamethoxyflavone in high-fat diet-induced rats. J. Agric. Food Chem. 2019, 67, 2476–2489. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saitoa, K.; Choi, S.S.; Wanga, X.X.; Choi, B.K.; Yonezawaa, T.; Teruyab, T.; Nagaia, K.; Woo, J.T. Effects of a Citrus depressa Hayata (shiikuwasa) extract on obesity in high-fat diet-induced obese mice. Phytomedicine 2011, 18, 648–654. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 20, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Kim, M.H.; Ko, H.C.; Kim, S.J. Immature citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol. Pharm. Bull. 2012, 35, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.C.; Jang, M.G.; Kang, C.H.; Lee, N.H.; Kang, S.I.; Lee, S.R.; Park, D.B.; Kim, S.J. Preparation of a polymethoxyflavone-rich fraction (PRF) of Citrus sunki Hort. ex Tanaka and its antiproliferactive effects. Food Chem. 2010, 123, 484–488. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 31, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jio, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Tuner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.G.; Oh, J.M.; Ko, H.C.; Kim, J.-W.; Baek, S.; Jin, Y.J.; Hur, S.-P.; Kim, S.-J. Clerodendrum trichotomum extract improves metabolic derangements in high fructose diet-fed rats. Anim. Cells Syst. 2021, 25, 396–404. [Google Scholar] [CrossRef]
- Andrews, S. FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 7 December 2010).
- Hannon Lab. FASTX Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 7 December 2021).
- Bushnell, B. BBMap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 7 December 2021).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ford, E.S.; Li, C.; Zhao, G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J. Diabetes 2010, 2, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.C.; Chang, W.T.; Li, S.; Wu, J.C.; Badmeav, V.; Ho, C.T.; Pan, M.H. Citrus peel extracts attenuated obesity and modulated gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2018, 9, 3363–3373. [Google Scholar] [CrossRef]
- Malkanthi, E.; Judy, W.V.; Wilson, D.; Rumberger, J.A.; Guthrie, N. Randomized, double-blind, placebo-controlled, clinical study on the effect of Diabetinol® on glycemic control of subjects with impaired fasting glucose. Diabetes Metab. Syndr. Obes. 2015, 8, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Cha, B.Y.; Choi, S.S.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef]
- Pan, M.H.; Yang, G.; Li, S.; Li, M.Y.; Tsai, M.L.; Wu, J.C.; Badmaev, V.; Ho, C.T.; Lai, C.S. Combination of citrus polymethoxyflavones, green tea polyphenols and Lychee extracts suppresses obesity and hepatic steatosis in high-fat diet induced obese mice. Mol. Nutr. Food Res. 2017, 61, 1601104. [Google Scholar] [CrossRef]
- Sundarama, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Yang, J.; Eliasson, B.; Smith, U.; Cushman, S.W.; Sherman, A. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetics. Obesity 2012, 20, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.K.; Ciaraldi, T.P.; Henry, R.R.; Wittgrove, A.C.; Phillips, S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2013, 2, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Shen, W.J.; Muliro, K.; Patel, S.; Souza, S.C.; Roth, R.A.; Kraemer, F.B. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J. Biol. Chem. 2001, 276, 45456–45461. [Google Scholar] [CrossRef] [Green Version]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. Fat signal-lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. J. Am. J. Physiol. Endocrnol. Metabol. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, G.R.; Michell, B.J.; van Denderen, B.J.; Watt, M.J.; Carey, A.L.; Fam, B.C.; Andrikopoulos, S.; Proietto, J.; Gorgun, C.Z.; Carling, D.; et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006, 4, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Salmine, A.; Hyttinen, J.M.; Kaarniranat, K. AMP-activated protein kinase inhibit NF-kB signalling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Carmen, G.Y.; Victor, S.M. Signalling mechanisms regulating lipolysis. Cell Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef]
- Benetti, E.; SA Patel, N.; Collino, M. The role of PPARβ/δ in the management of metabolic syndrome and its associated cardiovascular complications. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 273–284. [Google Scholar] [CrossRef]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016, 157, 70–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, J.; Kim, S.; Yoon, H.G.; You, Y.; Kim, O.K.; Choi, K.C.; Lee, Y.H.; Lee, J.; Park, J.; Jun, W. Water Extract of Curcuma longa L. Ameliorates Non-Alcoholic Fatty Liver Disease. Nutrients 2019, 11, 2536. [Google Scholar] [CrossRef] [Green Version]
- Luiken, J.J.F.P.; Bonen, A.; Glatz, J.F.C. Cellular fatty acid uptake is acutely regulated by membrane-associated fatty acid-binding proteins. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Khristi, V.; Ratri, A.; Ghosh, S.; Pathak, D.; Borosha, S.; Dai, E.; Roy, R.; Charkravarthi, V.P.; Wolfe, M.W.; Rumi, M.K. Disruption of ESR1 alters the expression of genes regulating hepatic lipid and carbohydrate metabolism in male rats. Mol. Cell. Endocrinol. 2019, 490, 47–56. [Google Scholar] [CrossRef] [PubMed]


| ND | HFD | OR | PRF 50 | PRF 100 | PRF 200 | |
|---|---|---|---|---|---|---|
| Initial weight (g) | 27.64 ± 1.26 a | 34.60 ± 6.20 b | 32.53 ± 3.78 b | 33.01 ± 4.61 b | 32.70 ± 3.11 b | 32.84 ± 2.83 b |
| Final weight (g) | 28.73 ± 0.30 a | 43.26 ± 5.68 c | 37.36 ± 3.98 b | 37.54 ± 5.12 b | 37.07 ± 3.56 b | 36.71 ± 4.66 b |
| Weight gain (g) | 1.26 ± 0.30 a | 8.66 ± 1.20 c | 4.83 ± 1.93 b | 4.53 ± 2.10 b | 4.37 ± 2.17 b | 3.87 ± 2.22 b |
| Food intake (g/mouse/day) | 4.08 ± 0.51 a | 2,76 ± 0.18 b | 3.05 ± 0.34 b | 2.89 ± 0.50 b | 2.61 ± 0.23 b | 2.74 ± 0.56 b |
| Liver (g) | 1.37 ± 0.08 bc | 1.49 ± 0.41 c | 1.09 ± 0.18 a | 1.36 ± 0.15 abc | 1.19 ± 0.17 ab | 1.25 ± 0.08 ab |
| Epididymal fat (g) | 0.67 ± 0.17 a | 2.55 ± 0.60 c | 2.11 ± 0.20 bc | 1.70 ± 0.41 b | 1.93 ± 0.41 b | 1.74 ± 0.77 b |
| Kidney (g) | 0.30 ± 0.03 a | 0.36 ± 0.02 b | 0.31 ± 0.04 a | 0.35 ± 0.03 b | 0.34 ± 0.02 b | 0.34 ± 0.03 b |
| Spleen (g) | 0.08 ± 0.01 a | 0.13 ± 0.02 b | 0.07 ± 0.02 a | 0.09 ± 0.01 a | 0.08 ± 0.02 a | 0.09 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-J.; Jang, M.-G.; Kim, J.-W.; Baek, S.; Ko, H.-C.; Hur, S.-P.; Kim, S.-J. Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus sunki Hort. ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 865. https://doi.org/10.3390/nu14040865
Jin Y-J, Jang M-G, Kim J-W, Baek S, Ko H-C, Hur S-P, Kim S-J. Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus sunki Hort. ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet. Nutrients. 2022; 14(4):865. https://doi.org/10.3390/nu14040865
Chicago/Turabian StyleJin, Yeong-Jun, Mi-Gyeong Jang, Jae-Won Kim, Songyee Baek, Hee-Chul Ko, Sung-Pyo Hur, and Se-Jae Kim. 2022. "Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus sunki Hort. ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet" Nutrients 14, no. 4: 865. https://doi.org/10.3390/nu14040865
APA StyleJin, Y.-J., Jang, M.-G., Kim, J.-W., Baek, S., Ko, H.-C., Hur, S.-P., & Kim, S.-J. (2022). Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus sunki Hort. ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet. Nutrients, 14(4), 865. https://doi.org/10.3390/nu14040865








