Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Experimental Diets
2.4. Exercise
2.5. Glucose Transport
2.5.1. Muscle Preparation
2.5.2. Transport Assay
2.6. Biochemical Assay
2.6.1. Muscle Metabolites
2.6.2. Blood Parameters
2.6.3. Immunoblot Analysis
2.7. Statistics
3. Results
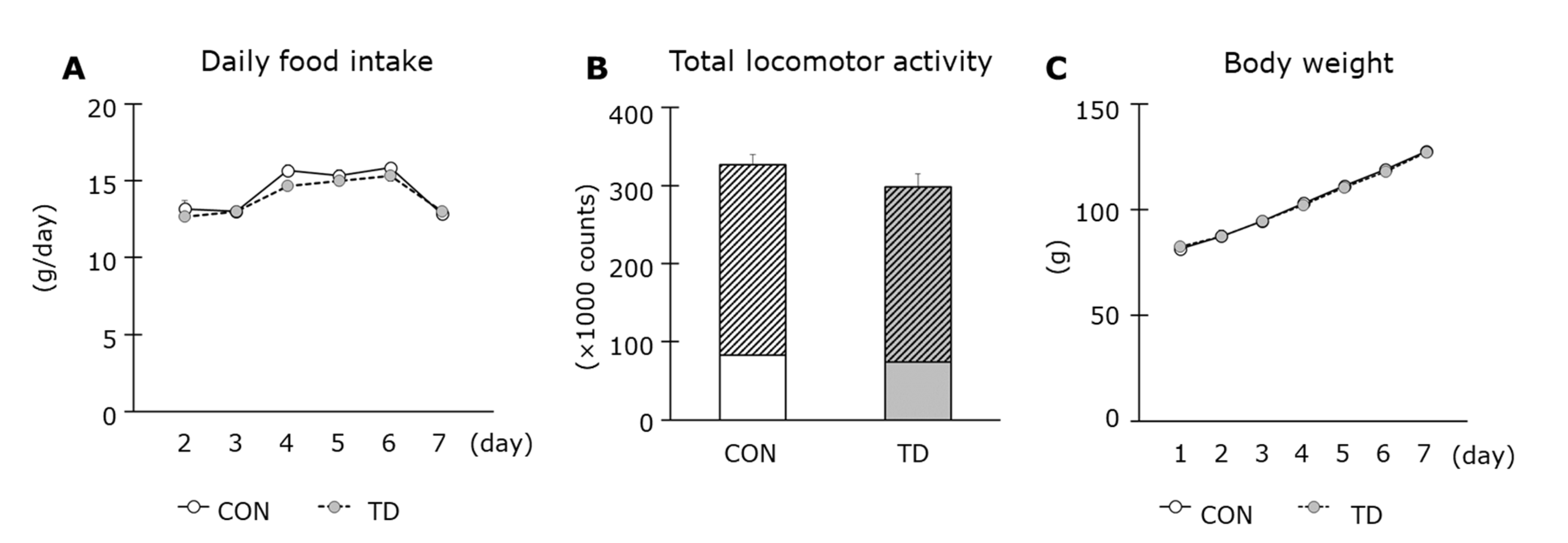
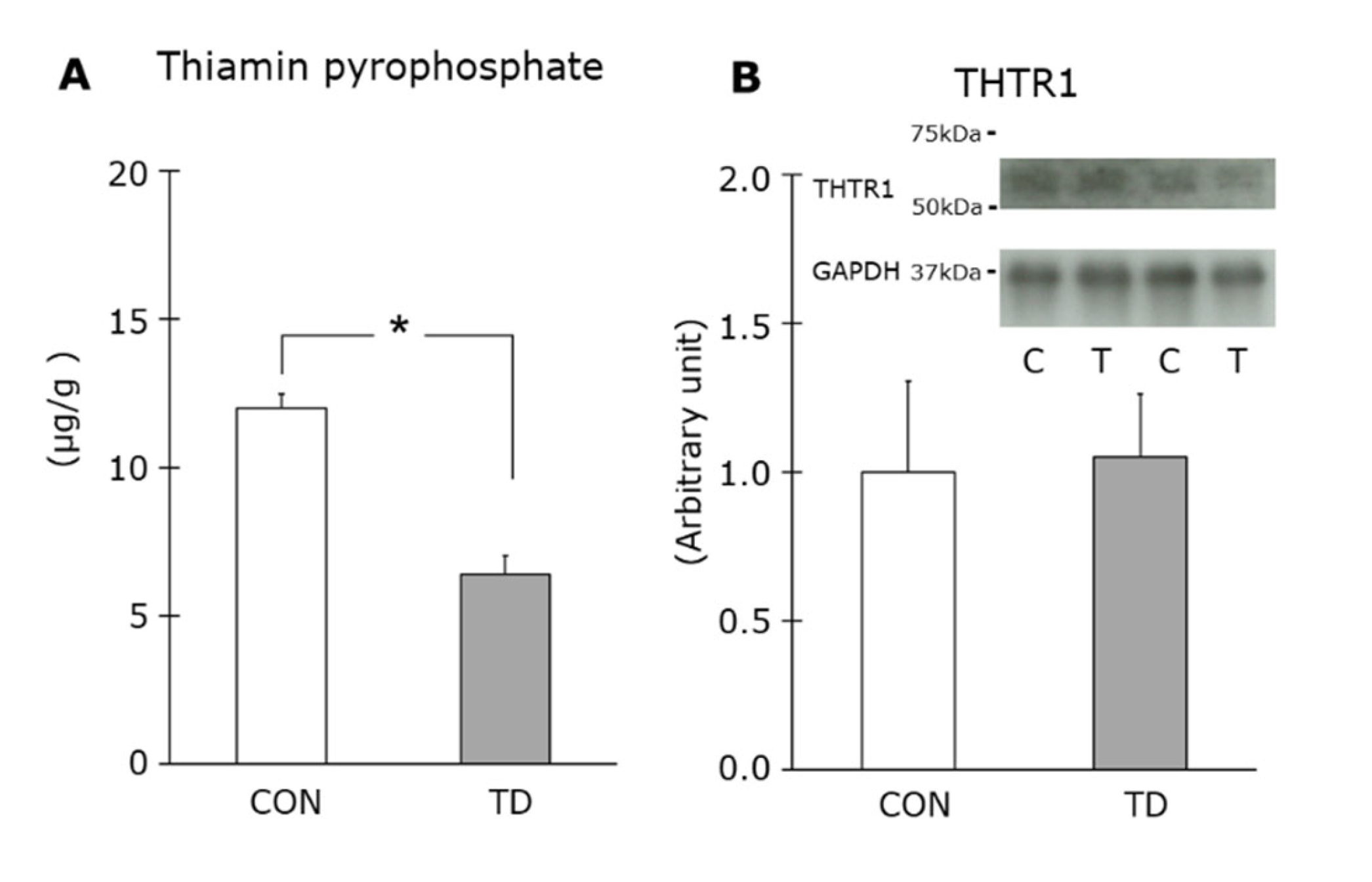
3.1. Thiamin Deficiency-Associated Symptoms and Muscle Thiamin Content
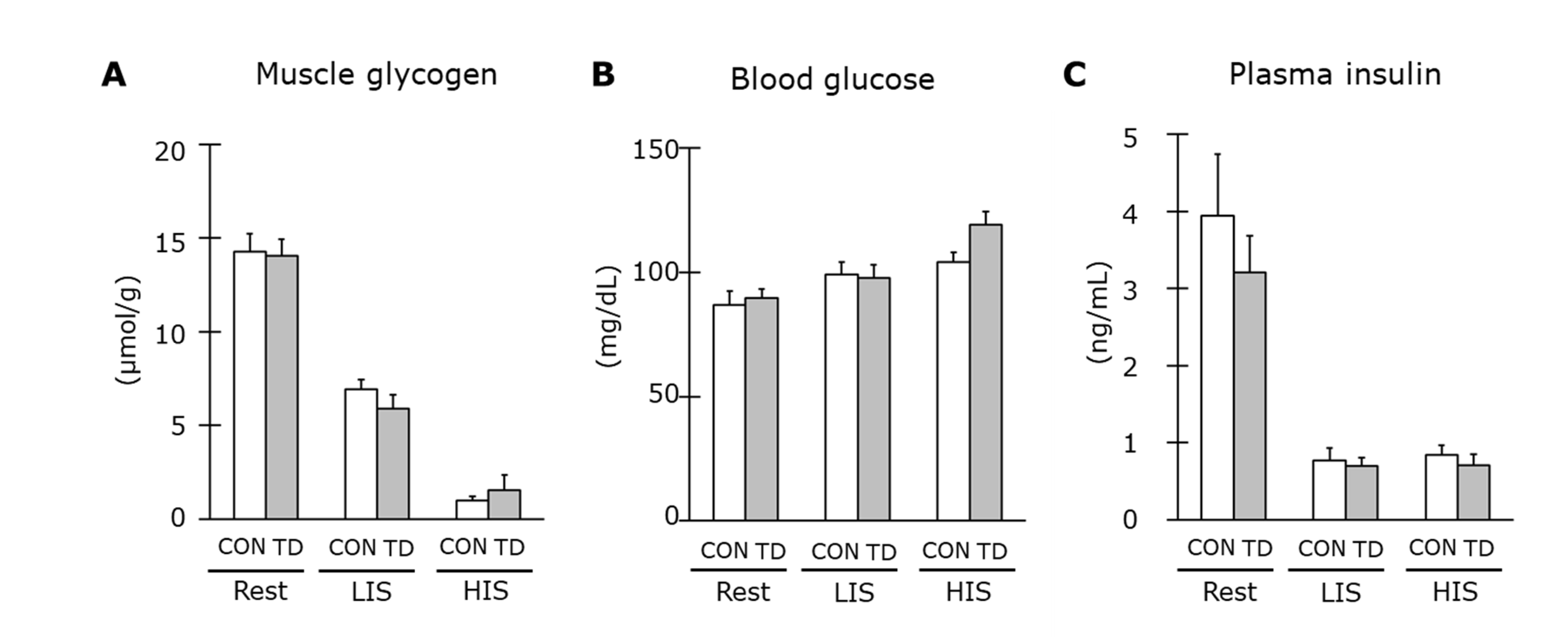
3.2. Muscle Glycogen and Related Regulations at Rest
3.3. Mitochondria Content and Related Molecule for Mitochondrial Biogenesis
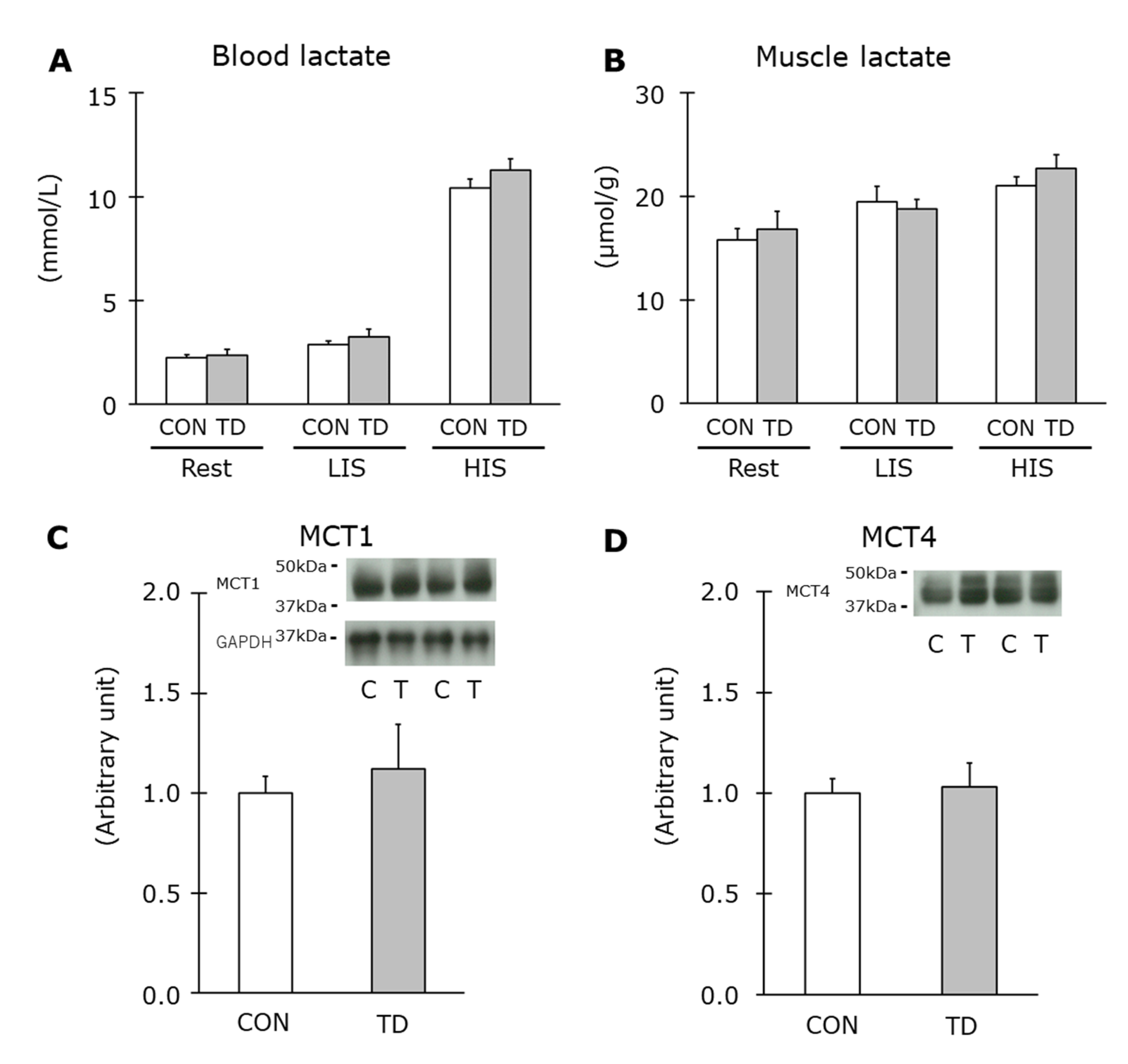
3.4. Muscle Glycogen and Related Regulations Immediately after Exercise
3.5. AMPK Phosphorylation Levels at Rest and Immediately after Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ihara, H.; Hashizume, N. Nutrition and deficiency: Vitamin B1. In The Vitamin Society of Japan eds. General Dictionary of Vitamin, 1st ed.; Asakura Publishing Co., Ltd.: Tokyo, Japan, 2011; pp. 166–171. (In Japanese) [Google Scholar]
- Shibata, K.; Fukuwatari, T. The body vitamin B1 levels of rats fed a diet containing the minimum requirement of vitamin B1 is reduced by exercise. J. Nutr. Sci. Vitaminol. 2013, 59, 87–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, A.; Shimoyama, Y.; Ishikawa, T.; Murayama, N. Dietary thiamin and riboflavin intake and blood thiamin and riboflavin concentrations in college swimmers undergoing intensive training. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Itokawa, Y. Effects of thiamine supplementation on exercise-induced fatigue. Metab. Brain Dis. 1996, 11, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.R.; Webster, M.J.; Erdmann, L.D. Allithiamine Ingestion Does Not Enhance Isokinetic Parameters of Muscle Performance. Int. J. Sport Nutr. 1997, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.R.; Branz, M.; Webster, M.J.; Scheett, T.P. The effect of a thiamin derivative on exercise performance. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 75, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Mizuma, H.; Tanaka, M.; Jin, G.; Tahara, T.; Mizuno, K.; Yamato, M.; Okuyama, K.; Eguchi, A.; Akimoto, K.; et al. Thiamine tetrahydrofurfuryl disulfide improves energy metabolism and physical performance during physical-fatigue loading in rats. Nutr. Res. 2009, 29, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Baek, S.H.; Choi, S.W. The effects of endurance training and thiamine supplementation on anti-fatigue during exercise. J. Exerc. Nutr. Biochem. 2013, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, E.J.; Van Dokkum, W.; Schrijver, J.; Wesstra, J.A.; Van de Weerd, H.; Hermus, R.J.J. Effect of marginal vitamin intake on physiological performance in man. Int. J. Sport Med. 1984, 5, 28–31. [Google Scholar] [CrossRef]
- Van der Beek, E.J.; van Dokkum, W.; Schrijver, J.; Wedel, M.; Gaillard, A.W.; Wesstra, A.; van de Weerd, H.; Hermus, R.J. Thiamin, riboflavin, and vitamins B-6 and C: Impact of combined restricted intake on functional performance in man. Am. J. Clin. Nutr. 1988, 48, 1451–1462. [Google Scholar] [CrossRef]
- van der Beek, E.J.; Van Dokkum, W.; Wedel, M.; Schrijver, J.; van den Berg, H. Thiamin, riboflavin and vitamin B6: Impact of restricted intake on physical performance in man. J. Am. Coll. Nutr. 1994, 13, 629–640. [Google Scholar] [CrossRef]
- Hernandez-Vazquez, A.D.J.; Garcia-Sanchez, J.A.; Moreno-Arriola, E.; Salvador-Adriano, A.; Ortega-Cuellar, D.; Velazquez-Arellano, A. Thiamine Deprivation Produces a Liver ATP Deficit and Metabolic and Genomic Effects in Mice: Findings Are Parallel to Those of Biotin Deficiency and Have Implications for Energy Disorders. J. Nutr. Nutr. 2016, 9, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Alimov, A.; Wang, H.; Frank, J.; Katz, W.; Xu, M.; Ke, Z.-J.; Luo, J. Thiamine deficiency induces anorexia by inhibiting hypothalamic AMPK. Neuroscience 2014, 267, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.R.; Whitfield, J.; Hoffman, N.J. Interactive Roles for AMPK and Glycogen from Cellular Energy Sensing to Exercise Metabolism. Int. J. Mol. Sci. 2018, 19, 3344. [Google Scholar] [CrossRef]
- Koshinaka, K.; Kawasaki, E.; Hokari, F.; Kawanaka, K. Effect of acute high-intensity intermittent swimming on post-exercise insulin responsiveness in epitrochlearis muscle of fed rats. Metabolism 2009, 58, 246–253. [Google Scholar] [CrossRef]
- Liang, C.C. Metabolic changes in rats during developing thiamin deficiency.(Short Communications). Biochem. J. 1975, 146, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Nakagawasai, O.; Tadano, T.; Hozumi, S.; Taniguchi, R.; Tan-No, K.; Esashi, A.; Niijima, F.; Kisara, K. Characteristics of depressive behavior induced by feeding thiamine-deficient diet in mice. Life Sci. 2001, 69, 1181–1191. [Google Scholar] [CrossRef]
- Gralak, M.A.; Dębski, B.; Drywień, M. Thiamine deficiency affects glucose transport and β-oxidation in rats. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1629–1635. [Google Scholar] [CrossRef]
- Koshinaka, K.; Honda, A.; Masuda, H.; Sato, A. Effect of Quercetin Treatment on Mitochondrial Biogenesis and Exercise-Induced AMP-Activated Protein Kinase Activation in Rat Skeletal Muscle. Nutrients 2020, 12, 729. [Google Scholar] [CrossRef]
- Kawanaka, K.; Tabata, I.; Tanaka, A.; Higuchi, M. Effects of high-intensity intermittent swimming on glucose transport in rat epitrochlearis muscle. J. Appl. Physiol. 1998, 84, 1852–1857. [Google Scholar] [CrossRef]
- Cartee, G.; Douen, A.G.; Ramlal, T.; Klip, A.; Holloszy, J.O. Stimulation of glucose transport in skeletal muscle by hypoxia. J. Appl. Physiol. 1991, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.A.; Gulve, E.A.; Holloszy, J.O. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J. Appl. Physiol. 1994, 76, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen-de Beer RJ, A.C.; Van Zelst, B.D.; Wardle, R.; Kooij, P.G.; de Rijke, Y.B. Simultaneous measurement of whole blood vitamin B1 and vitamin B6 using LC-ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2017, 1063, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Passonneau, J.; Lauderdale, V. A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 1974, 60, 405–412. [Google Scholar] [CrossRef]
- Passonneau, J.V.; Lowry, O.H. Enzymatic Analysis. A Practical Guide; The Humana Press Inc.: Totowa, NJ, USA, 1993. [Google Scholar]
- Fleming, J.C.; Tartaglini, E.; Steinkamp, M.P.; Schorderet, D.F.; Cohen, N.; Neufeld, E.J. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat. Genet. 1999, 22, 305–308. [Google Scholar] [CrossRef]
- Dutta, B.; Huang, W.; Molero, M.; Kekuda, R.; Leibach, F.H.; Devoe, L.D.; Ganapathy, V.; Prasad, P.D. Cloning of the Human Thiamine Transporter, a Member of the Folate Transporter Family. J. Biol. Chem. 1999, 274, 31925–31929. [Google Scholar] [CrossRef]
- Leick, L.; Fentz, J.; Biensø, R.S.; Knudsen, J.G.; Jeppesen, J.; Kiens, B.; Wojtaszewski, J.; Pilegaard, H. PGC-1α is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am. J. Physiol. Metab. 2010, 299, E456–E465. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; Pierre, J.S.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism—Emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- Manning Fox, J.E.; Meredith, D.; Halestrap, A.P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J. Physiol. 2000, 529, 285–293. [Google Scholar]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family?from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch.-Eur. J. Physiol. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E. Calorie Restriction Increases Muscle Mitochondrial Biogenesis in Healthy Humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Tadano, T.; Sakai, K.; Kisara, K.; Ogura, Y. Muricide induced by thiamine deficiency in the rats. Folia Pharmacol. Jpn. 1978, 74, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Barrett, R.J.; Schenker, S. Thiamin deficiency induced muricide behavior in rats. Physiol. Behav. 1979, 23, 211–214. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Exp. 2010, 67, 3407–3423. [Google Scholar] [CrossRef]
- Takimoto, M.; Takeyama, M.; Hamada, T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism 2013, 62, 1633–1640. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Takahashi, Y.; Machida, M.; Takeda, K.; Takemasa, T.; Hatta, H. Effect of AMPK activation on monocarboxylate transporter (MCT)1 and MCT4 in denervated muscle. J. Physiol. Sci. 2014, 64, 59–64. [Google Scholar] [CrossRef]
- Spriet, L.L.; Heigenhauser, G.J. Regulation of Pyruvate Dehydrogenase (PDH) Activity in Human Skeletal Muscle During Exercise. Exerc. Sport Sci. Rev. 2002, 30, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Howlett, R.A.; Heigenhauser, G.J.F.; Hultman, E.; Hollidge-Horvat, M.G.; Spriet, L.L. Effects of dichloroacetate infusion on human skeletal muscle metabolism at the onset of exercise. Am. J. Physiol. Metab. 1999, 277, E18–E25. [Google Scholar] [CrossRef] [PubMed]
- Manore, M. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am. J. Clin. Nutr. 2000, 72, 598S–606S. [Google Scholar] [CrossRef] [PubMed]
- Woolf, K.; Manore, M.M. B-Vitamins and Exercise: Does Exercise Alter Requirements? Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 453–484. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, A.; Sato, S.; Omori, G.; Koshinaka, K. Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle. Nutrients 2022, 14, 710. https://doi.org/10.3390/nu14030710
Sato A, Sato S, Omori G, Koshinaka K. Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle. Nutrients. 2022; 14(3):710. https://doi.org/10.3390/nu14030710
Chicago/Turabian StyleSato, Akiko, Shinji Sato, Go Omori, and Keiichi Koshinaka. 2022. "Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle" Nutrients 14, no. 3: 710. https://doi.org/10.3390/nu14030710
APA StyleSato, A., Sato, S., Omori, G., & Koshinaka, K. (2022). Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle. Nutrients, 14(3), 710. https://doi.org/10.3390/nu14030710




