Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
- –
- Lack of support to breastfeeding by healthcare professionals;
- –
- Use of growth curves based on formula-fed infants or cultural beliefs about child growth, which can lead to breastfed infants being considered underweight and to supplementation with additional foods to achieve what is wrongly thought to be an appropriate weight gain;
- –
- Belief that supplementation is an acceptable routine practice, not an intervention;
- –
- Early maternal return to work and unavailability of workplace breastfeeding facilities;
- –
- Social disapproval of breastfeeding in public.
Objectives
- –
- Does starting CF between 4 and 6 months of age lead to different short-term and long-term nutritional and metabolic outcomes compared with exclusive breastfeeding up to 6 months?
- –
- Does starting CF between 4 and 6 months of age lead to different short-term and long-term nutritional and metabolic outcomes compared with exclusive formula or mixed feeding (human milk and baby formula) up to 6 months?
- To compare the effects on growth at 12 months and the development of overweight/obesity at 3–6 years of EBF for less than 6 months and introduction of CF between 4 and 6 months, compared with EBF for 6 months with the introduction of CF at 6 months of age;
- To compare the effects on growth at 12 months and the development of overweight/obesity at 3–6 years of exclusive formula feeding (EFF) or mixed for less than 6 months and introduction of CF between 4 and 6 months, compared with EFF for 6 months with the introduction of CF at 6 months.
2. Materials and Methods
2.1. Design of the Studies Included
2.2. Population
2.3. Intervention(s), Exposure(s)
2.4. Comparator(s)/Control
2.5. Exclusion Criteria
2.6. Main Outcome(s)
- –
- General growth parameters: weight (W), length (L), W/L Z-score ratio, body mass index (BMI); BMI Z-score (zBMI);
- –
- Risk of non-communicable diseases (NCDs; overweight/obesity, diabetes mellitus type 2 (DM2), and hypertension).
2.7. Additional Outcome(s)
2.8. Keywords and Search Strategy
2.9. Measures of Effect
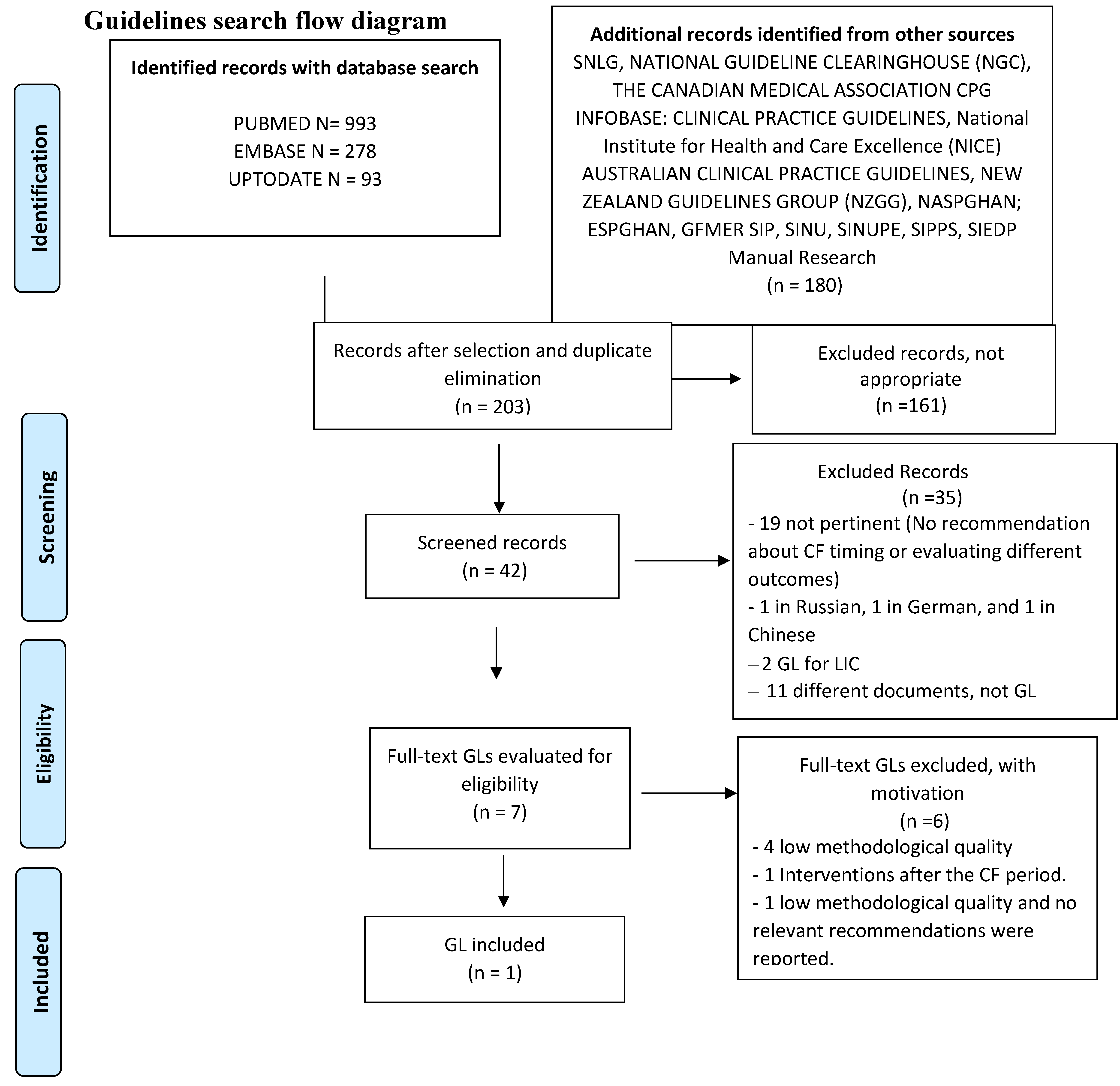
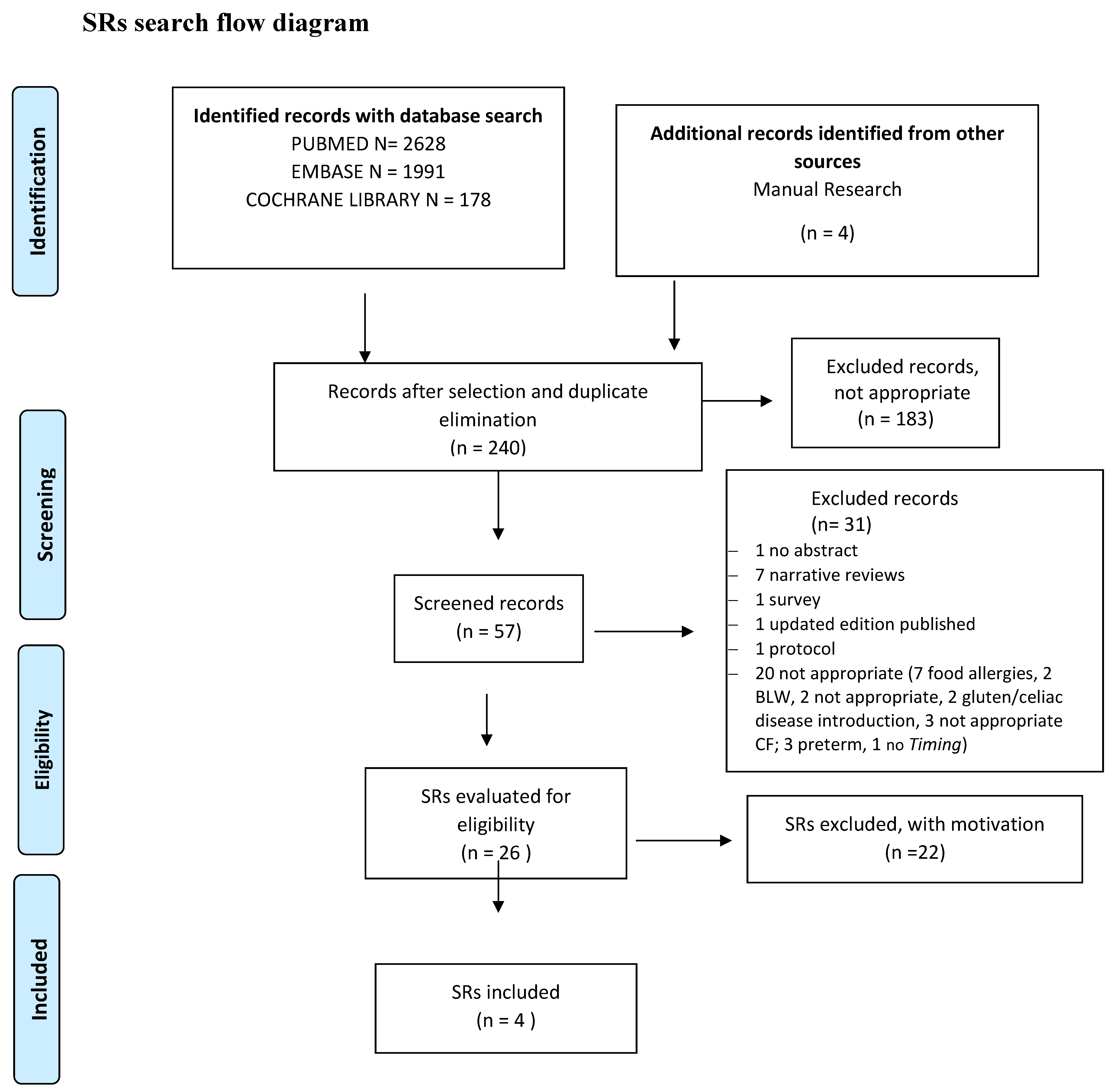
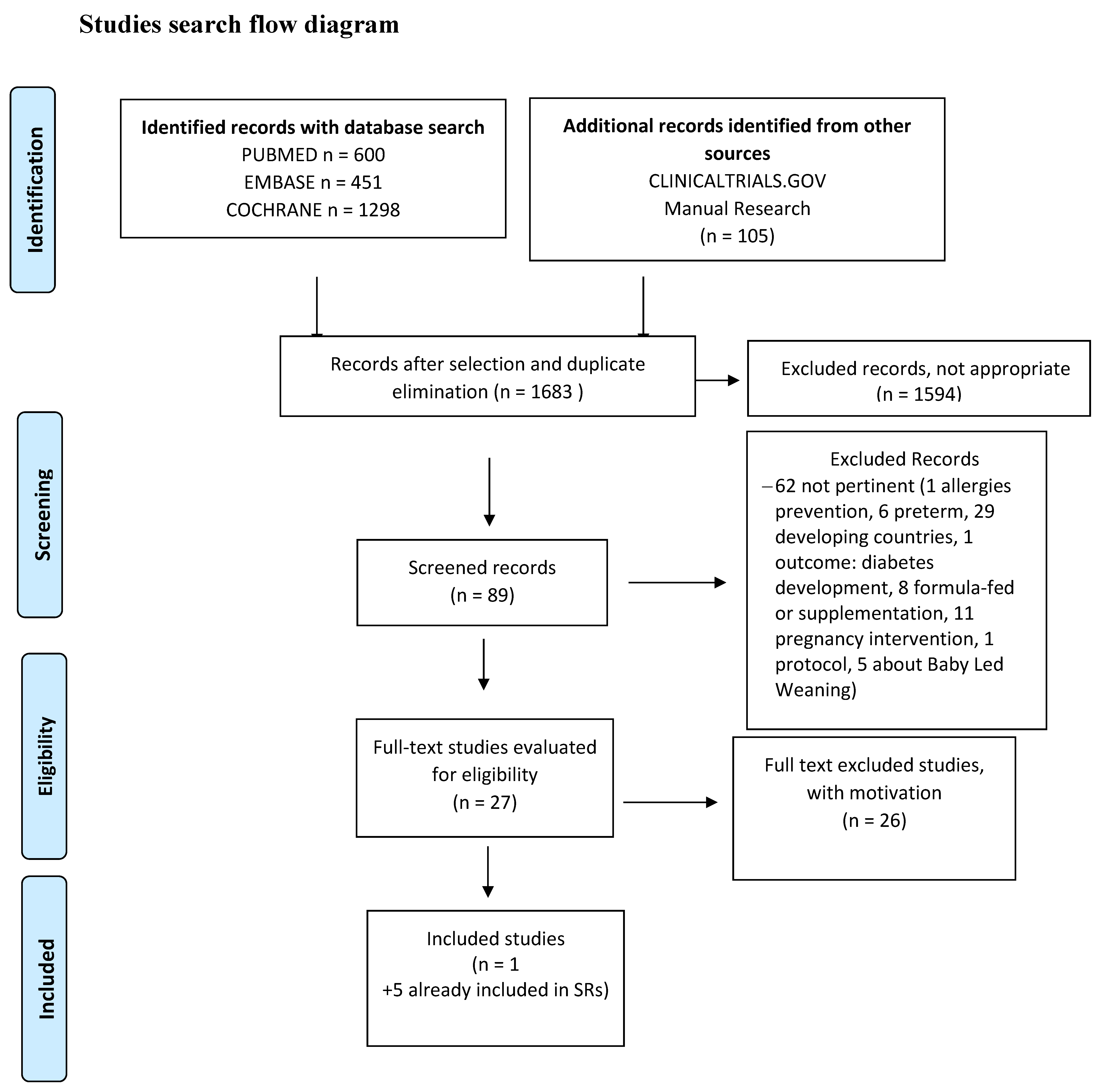
2.10. Studies Selection. Risk of Bias (Quality) Assessment. Missing Data. List of the Studies Excluded with Relevant Reasons. Data Extraction (Selection and Coding)
2.11. Assessment of Heterogeneity
2.12. Strategy for Data Synthesis
2.13. Reporting Bias Assessment
2.14. Softwares
3. Results
3.1. Exclusively Breastfed (EBF) Infant
3.1.1. Growth at 6 and 12 Months
- A total of 165 EBF infants were weaned at 4.0–4.9 and 5.0–5.9 months;
- A total of 86 EBF infants were weaned at 6.0–6.9 months;
- A total of 103 EFF/mixed infants were weaned at 4.0–4.9 and 5.0–5.9 months;
- A total of 69 EFF/mixed infants were weaned at 6.0–6.9 months.
3.1.2. Overweight/Obesity at 3 Years of Age
3.1.3. Iron status
| Research string: (Introduction CF at 4–6 months) than (introduction CF at 6 months) in order to (blood iron level at 6 months) | ||||||
| Patient or population: (Blood iron level at 6 months) Setting: Outpatient Intervention: (Introduction CF at 4–6 months) Comparator: (Introduction CF at 6 months) | ||||||
| Outcomes | Anticipated absolute outcome * (95% CI) | Relative outcome (95% CI) | № of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with (Introduction CF at 4–6 months) | Risk with (Introduction CF at 6 months) | |||||
| Serum Hb (Hb) evaluated with: gr/L follow-up: 6 months | The average serum hb was = 0.2 | MD = 0.2 (2.44 inferior to 2.48 major) | - | 100 (1 RCT) [17]. | ⨁⨁⨁◯ MODERATE a | |
| Serum ferritin (SF) evaluated with: ug/L follow-up: 6 months | The average serum ferritin was = 26 | MD = 26 (0.1 inferior to 52.1 major) | - | 100 (1 RCT) [17]. | ⨁⨁⨁◯ MODERATE a | |
3.2. Exclusively/Predominantly Formula-Fed Infant
3.2.1. Growth at 6 and 12 Months
3.2.2. Overweight/Obesity at 3 Years of Age
| Research string: GROWTH | ||||||
| (Introduction CF at 4–6 months) than (introduction CF at 6 months) in order to (ensure adequate growth at 6–12–18–24 months) | ||||||
| Patient or population: (Ensure adequate growth at 6–12–18–24 months) Setting: Outpatient Intervention: (Introduction CF at 4–6 months) Comparator: (Introduction CF at 6 months) | ||||||
| Outcomes | Anticipated absolute outcome * (95% CI) | Relative outcome (95% CI) | № of participants (studies) | Certainty of evidences (GRADE) | Comments | |
| Risk with (introduction CF at 4–6 months) | Risk with (introduction CF at 6 months) | |||||
| Weight gain Z-score (WGZ) follow-up: average 6 months | The average weight gain Z-score was = −0.01 | MD = −0.01 (0.15 inferior to 0.13 major) | - | 141 (2 RCT) [17,20]. a | ⨁⨁⨁◯ MODERATE b | |
| Length gain Z-score (LGZ) follow-up: 6 months | The average length gain Z-score was = −0.01 | MD = −0.01 (0.21 inferior to 0.19 major) | - | 141 (2 RCT) [17,20]. a | ⨁⨁⨁◯ MODERATE b | |
| Weight Z-score (WZ) follow-up: 12 months | N of patients introduction 4–6 n = 372. WZS at 12 months = 0.58 (0.99)–0.39 (0.95). N of patients introduction at 6 months = 155. WZ at 12 months = 0.25 (0.92) p = 0.01. Association with CF introduction age, fixed for age, sex, maternal age, parity, deprivation score, milk feeding at 3 months, and growth at precedent cut point (Model 3) = 0.01 (−0.06 to 0.07), p = 0.88 | 527 (1 observational study) [22]. | ⨁⨁⨁◯ MODERATE | |||
| Length Z-score (LZ) follow-up: 12 months | Patient CF introduction 4–6 months. LZS at 12 months = 0.48 (1.05), 0.23 (1.04). Patient CF introduction at 6 months. LZS at 12 months = 0.00 (1.04) p < 0.01. Association with CF introduction age fixed for confounding factors (Model 3) 0.04 (−0.01 to 0.11) p = 0.20 | (1 observational study) [22]. | ⨁⨁⨁◯ MODERATE | |||
| BMI Z-score (BZ) follow-up: 12 months | Introduction CF 4–6 months. BMIZ at 12 months 0.42 (0.94)–0.36 (0.83). CF introduction 6 months. BMIZ at 12 months 0.33 (0.84) p = 0.33. Association with CF introduction age fixed for confounding factors (Model 3) −0.02 (−0.08 to 0.05) p = 0.64 | (1 observational study) [22]. | ⨁⨁⨁◯ MODERATE | |||
| Research string: [RISK OF OVERWEIGHT/OBESITY] | ||||||
| (Introduction CF at 4–6 months) than (introduction CF at 6 months) in order to develop overweight/obesity at 3–6 years | ||||||
| Patient or population: Development of overweight/obesity at 3–6 years Setting: Outpatient Intervention: (Introduction CF at 4–6 months) Comparator: (Introduction CF at 6 months) | ||||||
| Outcomes | Anticipated absolute outcome * (95% CI) | Relative outcome (95% CI) | № of participants (Studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with (Introduction CF at 6 months) | Risk with (introduction CF at 4–6 months) | |||||
| Overweight/obesity at 18 months (S/O 24–36) evaluated with: BMI Z-score follow-up: 18 months | 109 per 1.000 | 141 per 1.000 (40 at 496) | RR 1.30 (0.37 at 4.56) | 94 (1 RCT) [17]. | ⨁⨁⨁◯ MODERATE a | |
| Overweight/obesity at 3 years (S/O 6a) evaluated with: RR (95% IC) follow-up: 3 years | 2. A total of 463 children, of which 28 (6.1%) were in an overweight/obesity condition. The linear regression analysis did not show a statistically significant correlation with the age of introduction of fruit and cereals: coefficient β, respectively = 0.020 (p = 0.743) and 0.011 (p = 0.828). 3. Starting CF at 4–6 months (n = 427) or at 6 months (n = 98). There is no difference in the probability of developing overweight/obesity at 3 years (RR = 0.80; 95%IC = 0.51–1.23) | 525 (2 observational studies) [20,22]. | ⨁⨁◯◯ LOW b | |||
4. Discussion
4.1. Quality of Evidence
4.2. Agreements and Disagreements with Other Studies or Reviews
4.3. Limitations of the SR and Potential Bias in the Review Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; UNICEF. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003; Available online: www.who.int/nutrition/publications/infantfeeding/9241562218/en/index.html (accessed on 7 August 2020).
- Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; Pentieva, K.; et al. EFSA Panel on NDA—Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, 5780. [Google Scholar]
- Smith, H.A.; Becker, G.E. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database Syst. Rev. 2016, 8, CD006462. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guiding Principles for Feeding Non-Breastfed Children 6–24 Months of Age; WHO: Geneva, Switzerland, 2005; Available online: http://apps.who.int/iris/bitstream/10665/43281/1/9241593431.pdf?ua=1&ua=1 (accessed on 7 August 2020).
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domello, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- AAP. Complementary feeding. In Pediatric Nutrition; AAP: Chicago, IL, USA, 2014; pp. 123–142. [Google Scholar]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [PubMed]
- United Nations Children’s Fund (UNICEF). Prevention of Overweight and Obesity in Children and Adolescents: UNICEF Programming Guidance; UNICEF: New York, NY, USA, 2019; Available online: https://www.unicef.org/media/92336/file/Programming-Guidance-Overweight-Prevention.pdf (accessed on 23 January 2022).
- Caroli, M.; Vania, A.; Verga, M.C.; Di Mauro, G.; Bergamini, M.; Cuomo, B.; D’Anna, R.; D’Antonio, G.; Dello Iacono, I.; Dessì, A.; et al. Recommendations on complementary feeding as a tool for prevention of non-communicable diseases (NCDs)—Paper co-drafted by the SIPPS, FIMP, SI-DOHaD, SINUPE Joint Working Group. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 27 December 2021).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919e. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5); Version 5.3; Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- McMaster University (Developed by Evidence Prime). GRADEpro GDT; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2021; Available online: https://gradepro.org/ (accessed on 2 June 2019).
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasem, W.; Fenton, T.; Friel, J. Age of introduction of first complementary feeding for infants: A systematic review. BMC Pediatr. 2015, 15, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA, Nutrition Evidence Systematic Review Team and Complementary Feeding Technical Expert Collaborative. Timing of Introduction of Complementary Foods and Beverages and Growth, Size, and Body Composition: A Systematic Review. Pregnancy and Birth to 24 Months Project; Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion: Alexandria, VA, USA, 2019. Available online: https://nesr.usda.gov/project-specific-overview-pb-24-0 (accessed on 27 December 2021).
- Jonsdottir, O.H.; Thorsdottir, I.; Hibberd, P.L.; Fewtrell, M.S.; Wells, J.C.; Palsson, G.I.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Timing of the introduction of complementary foods in infancy: A randomized controlled trial. Pediatrics 2012, 130, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C.; Jonsdottir, O.H.; Hibberd, P.L.; Fewtrell, M.S.; Thorsdottir, I.; Eaton, S.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Randomized controlled trial of 4 compared with 6 mo of exclusive breastfeeding in Iceland: Differences in breast-milk intake by stable-isotope probe. Am. J. Clin. Nutr. 2012, 96, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsdottir, O.H.; Kleinman, R.E.; Wells, J.C.; Fewtrell, M.S.; Hibberd, P.L.; Gunnlaugsson, G.; Thorsdottir, I. Exclusive breastfeeding for 4 versus 6 months and growth in early childhood. Acta Paediatr. 2014, 103, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, R.R.; Mimouni, F.B.; Landi, T.; Crossman, M.; Harris, L.; Tsang, R.C. Effect of rice cereal feedings on bone mineralization and calcium homeostasis in cow milk formula fed infants. J. Am. Coll. Nutr. 1996, 15, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Rifas-Shiman, S.L.; Taveras, E.M.; Oken, E.; Gillman, M.W. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011, 127, e544–e551. [Google Scholar] [CrossRef] [PubMed]
- Vail, B.; Prentice, P.; Dunger, B.; Hughes, I.A.; Acerini, C.L.; Ong, K.K. Age at Weaning and Infant Growth: Primary Analysis and Systematic Review. J. Pediatr. 2015, 167, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, A.F.; Rocha, E.M.B.; Costa da Silva, J.P.; Nascimento, V.G.; Bertoli, C.; Leone, C. Breastfeeding, complementary food introduction and overweight in preschool children. Arch. Lat. Nutr. 2016, 66, 195–200. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verga, M.C.; Scotese, I.; Bergamini, M.; Simeone, G.; Cuomo, B.; D’Antonio, G.; Dello Iacono, I.; Di Mauro, G.; Leonardi, L.; Miniello, V.L.; et al. Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis. Nutrients 2022, 14, 702. https://doi.org/10.3390/nu14030702
Verga MC, Scotese I, Bergamini M, Simeone G, Cuomo B, D’Antonio G, Dello Iacono I, Di Mauro G, Leonardi L, Miniello VL, et al. Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis. Nutrients. 2022; 14(3):702. https://doi.org/10.3390/nu14030702
Chicago/Turabian StyleVerga, Maria Carmen, Immacolata Scotese, Marcello Bergamini, Giovanni Simeone, Barbara Cuomo, Giuseppe D’Antonio, Iride Dello Iacono, Giuseppe Di Mauro, Lucia Leonardi, Vito Leonardo Miniello, and et al. 2022. "Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis" Nutrients 14, no. 3: 702. https://doi.org/10.3390/nu14030702
APA StyleVerga, M. C., Scotese, I., Bergamini, M., Simeone, G., Cuomo, B., D’Antonio, G., Dello Iacono, I., Di Mauro, G., Leonardi, L., Miniello, V. L., Palma, F., Tezza, G., Vania, A., & Caroli, M. (2022). Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis. Nutrients, 14(3), 702. https://doi.org/10.3390/nu14030702






