Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization
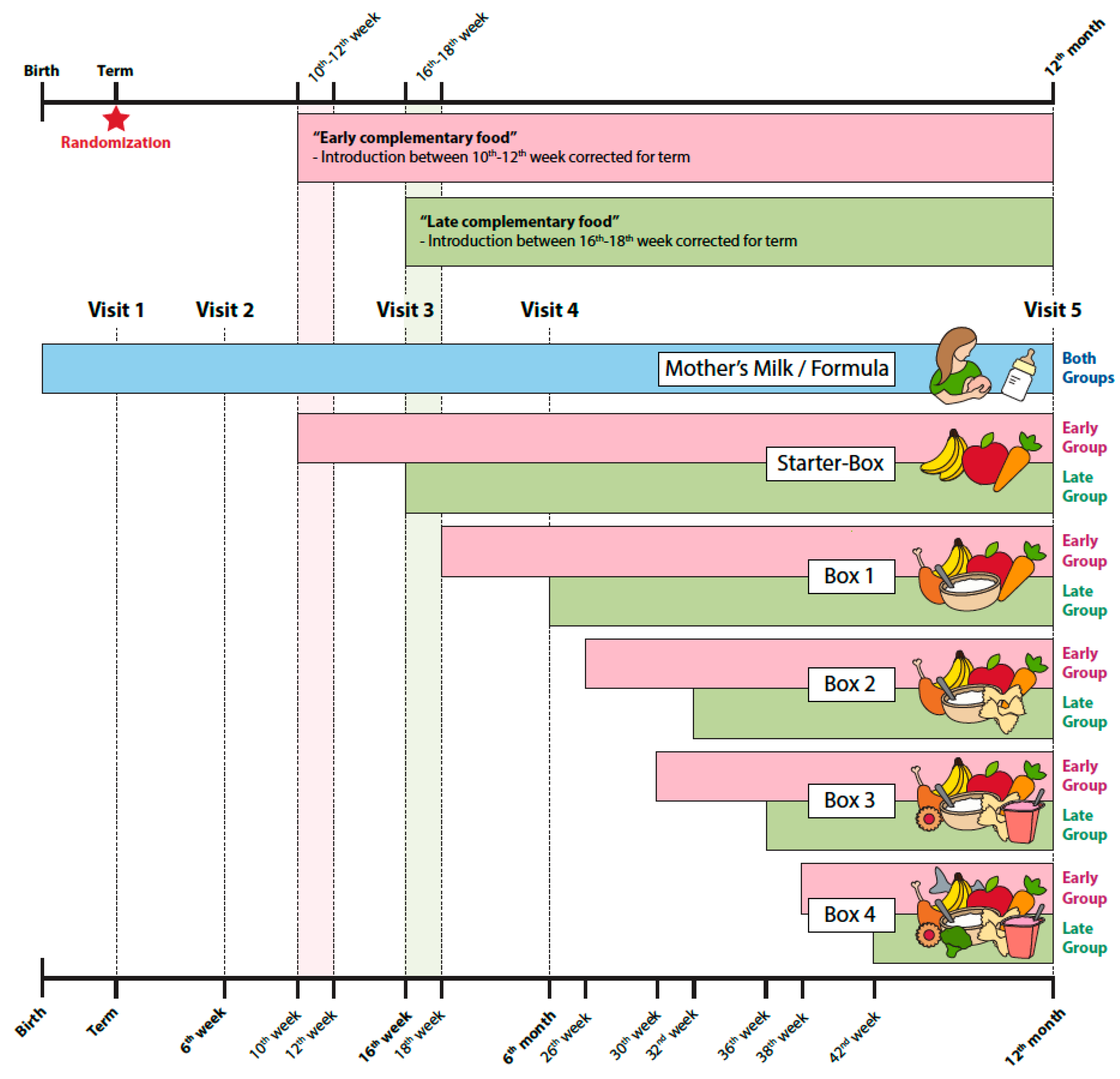
2.4. Procedures and Diet
2.5. Primary and Secondary Outcomes
2.6. Analysis Sets
2.7. Sample Size Planning
2.8. Statistical Analysis
3. Results
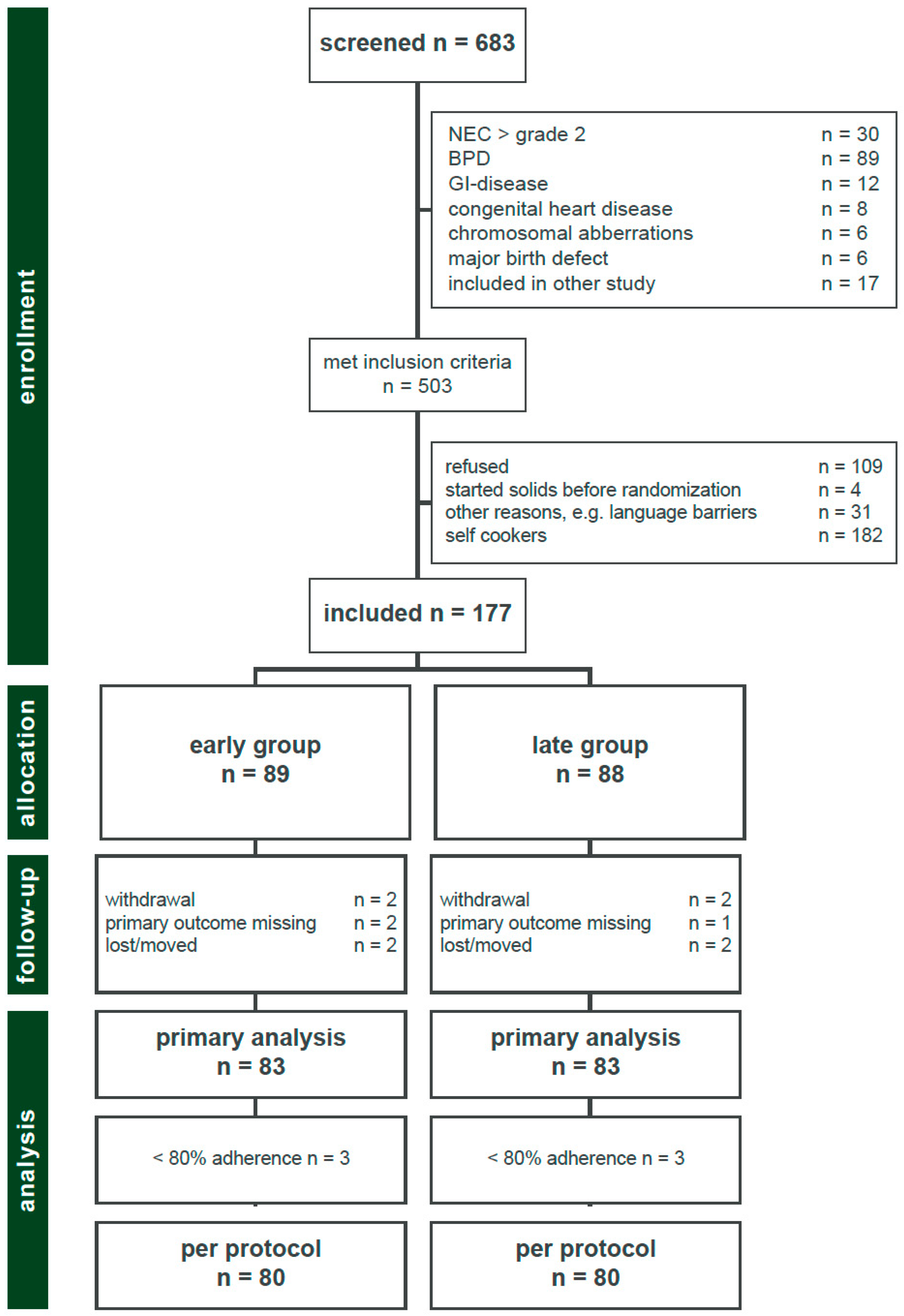
3.1. Participants
3.2. Baseline Characteristics, Morbidity and Diet
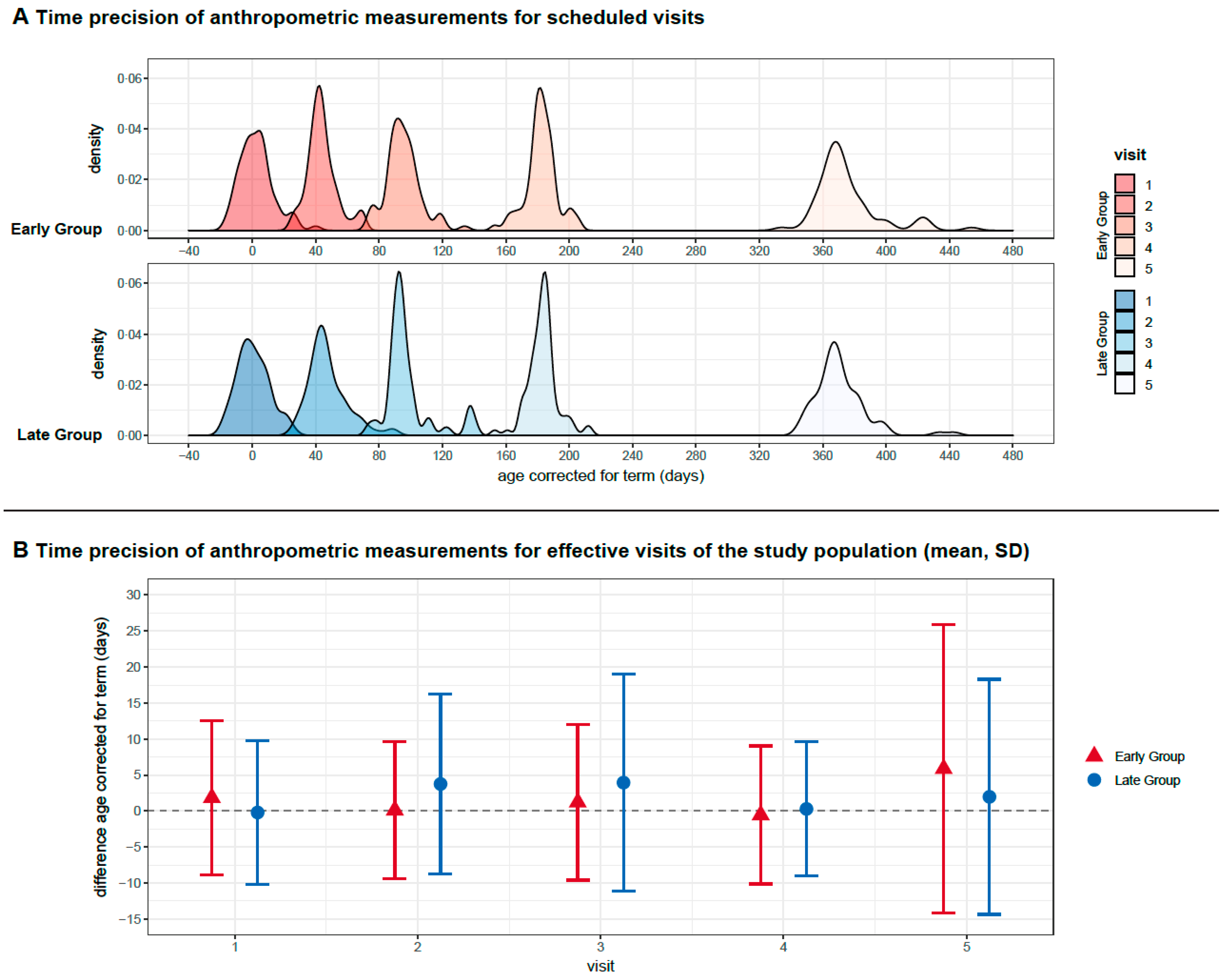
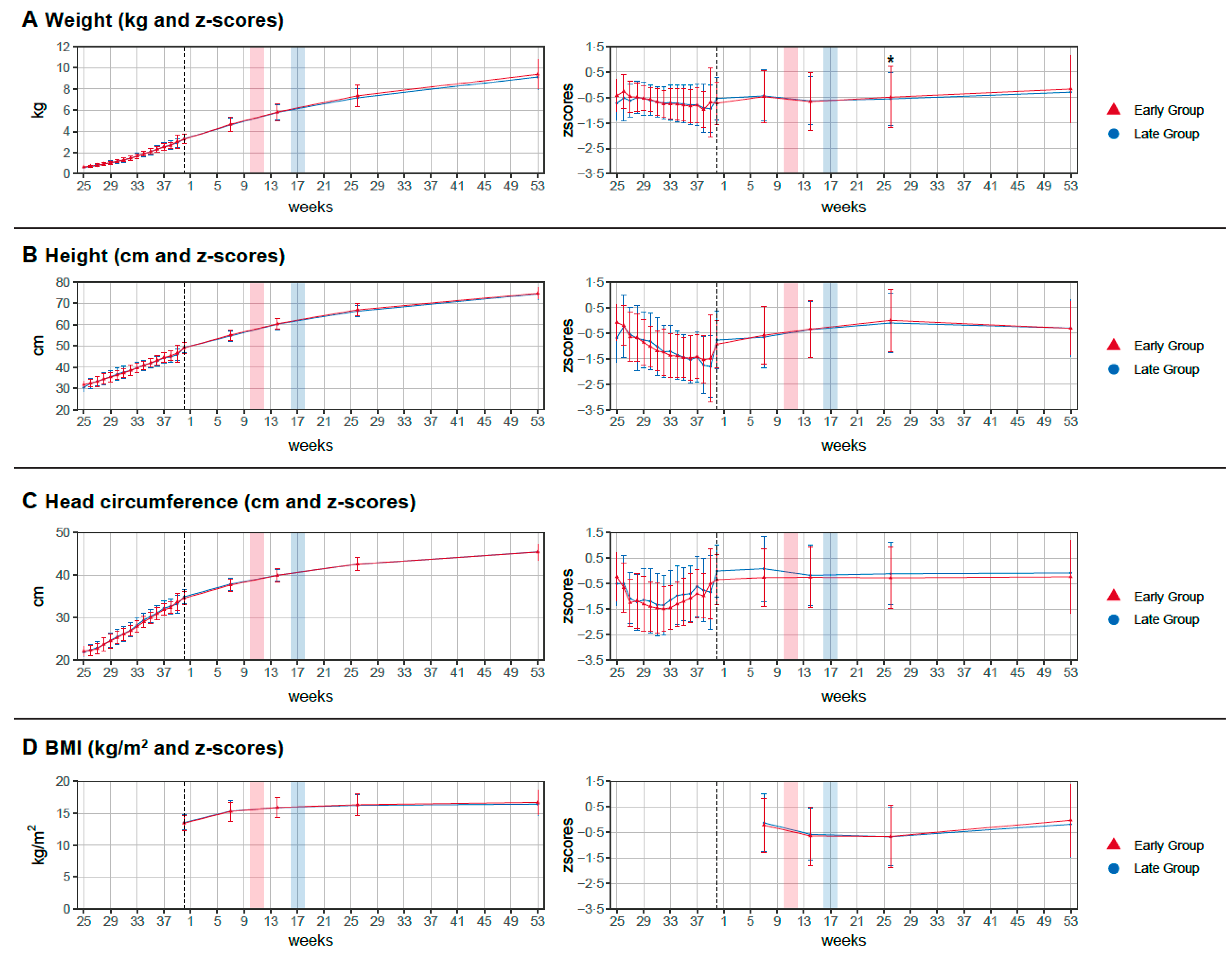
3.3. Primary Outcome and Secondary Outcomes
4. Discussion
4.1. Growth
4.2. Diet
4.3. Considerations for Weaning from Exclusive Breast or Bottle Feeding
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Liotto, N.; Cresi, F.; Beghetti, I.; Roggero, P.; Menis, C.; Corvaglia, L.; Mosca, F.; Aceti, A.; on behalf of the Study Group on Neonatal Nutrition and Gastroenterology. Italian Society of Neonatology Complementary Feeding in Preterm Infants: A Systematic Review. Nutrients 2020, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary Feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S.; Borsari, G.; Vigi, V. Complementary Feeding Practices in Preterm Infants: An Observational Study in a Cohort of Italian Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S210–S214. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S.; Vigi, V. Weaning Preterm Infants: An Open Issue. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S204–S209. [Google Scholar] [CrossRef]
- Marriott, L.D.; Foote, K.D.; Bishop, J.A.; Kimber, A.C.; Morgan, J.B. Weaning preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal. 2003, 88, F302–F307. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Agarwal, R.; Aggarwal, K.C.; Chellani, H.; Duggal, A.; Arya, S.; Bhatia, S.; Sankar, M.J.; Sreenivas, V.; Jain, V.; et al. Complementary feeding at 4 versus 6 months of age for preterm infants born at less than 34 weeks of gestation: A randomised, open-label, multicentre trial. Lancet Glob. Health 2017, 5, e501–e511. [Google Scholar] [CrossRef] [Green Version]
- Neu, J. NECROTIZING ENTEROCOLITIS: The Search for a Unifying Pathogenic Theory Leading to Prevention. Pediatr. Clin. N. Am. 1996, 43, 409–432. [Google Scholar] [CrossRef]
- Jobe, A.H. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2011, 23, 167–172. [Google Scholar] [CrossRef] [Green Version]
- WHOMGRS Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, A.N.; Grabich, S.C.; Olsen, I.E.; Cantrell, R.; Clark, R.H.; Ballew, W.N.; Chou, J.; Lawson, M.L. BMI Is a Better Body Proportionality Measure than the Ponderal Index and Weight-for-Length for Preterm Infants. Neonatology 2018, 113, 108–116. [Google Scholar] [CrossRef]
- Toeller, M.; The Eurodiab Iddm Complications Study Group; Buyken, A.; Heitkamp, G.; Milne, R.; Klischan, A.; Gries, F.A. Repeatability of three-day dietary records in the EURODIAB IDDM Complications Study. Eur. J. Clin. Nutr. 1997, 51, 74–80. [Google Scholar]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pr. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations inR. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Norris, F.; Larkin, M.; Williams, C.; Hampton, S.; Morgan, J. Factors affecting the introduction of complementary foods in the preterm infant. Eur. J. Clin. Nutr. 2002, 56, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Cleary, J.; MC Dalton, S.; Harman, A.; Wright, I.M. Current practice in the introduction of solid foods for preterm infants. Public Health Nutr. 2019, 23, 94–101. [Google Scholar] [CrossRef]
- Morgan, J.B.; Williams, P.; Foote, K.D.; Marriott, L.D. Do mothers understand healthy eating principles for low-birth-weight infants? Public Health Nutr. 2006, 9, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Boscarino, G.; Conti, M.G.; Pagano, F.; Di Chiara, M.; Pannucci, C.; Onestà, E.; Prota, R.; Deli, G.; Dito, L.; Regoli, D.; et al. Complementary Feeding and Growth in Infants Born Preterm: A 12 Months Follow-Up Study. Children 2021, 8, 1085. [Google Scholar] [CrossRef]
- Pereira-Da-Silva, L.; Virella, D.; Fusch, C. Nutritional Assessment in Preterm Infants: A Practical Approach in the NICU. Nutrients 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Ramel, S.E.; Zhang, L.; Misra, S.; Anderson, C.G.; Demerath, E.W. Do anthropometric measures accurately reflect body composition in preterm infants? Pediatr. Obes. 2017, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- El Rafei, R.; Jarreau, P.-H.; Norman, M.; Maier, R.F.; Barros, H.; Van Reempts, P.; Pedersen, P.; Cuttini, M.; Zeitlin, J. Variation in very preterm extrauterine growth in a European multicountry cohort. Arch. Dis. Child. Fetal Neonatal 2021, 106, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K. Appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, e05780. [Google Scholar] [PubMed] [Green Version]
| Early Group (n = 89) | Late Group (n = 88) | |
|---|---|---|
| Birthweight in g | 941 (±253) | 932 (±256) |
| Birth height in cm | 34.8 (±3.1) | 34.9 (±3.6) |
| Head circumference at birth in cm | 24.8 (±2.2) | 24.7 (±2.3) |
| Gestational age (days) at birth | 190 (±16) | 190 (±14) |
| Mean gestational age at birth weeks/days | 27/1 | 27/1 |
| Gestational age (days) at discharge | 265 (±12) | 265 (±15) |
| Mean gestational age at discharge weeks/days | 37/6 | 37/6 |
| Male Sex | 56 (63%) | 42 (48%) |
| Multiples | 32 (36%) | 28 (32%) |
| C-section | 78 (88%) | 84 (95%) |
| Antenatal steroids completed | 47 (54%) | 57 (66%) |
| Antenatal steroids incomplete | 35 (40%) | 26 (30%) |
| SGA | 7 (8%) | 5 (6%) |
| NEC I + II | 4 (4%) | 0 (0%) |
| PDA | 34 (38%) | 33 (38%) |
| ROP Grade 3 and more | 5 (6%) | 5 (6%) |
| IVH Grade I-II | 9 (10%) | 4 (5%) |
| IVH Grade III-IV | 4 (4%) | 6 (7%) |
| PVL | 0 (0%) | 2 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haiden, N.; Thanhaeuser, M.; Eibensteiner, F.; Huber-Dangl, M.; Gsoellpointner, M.; Ristl, R.; Kroyer, B.; Brandstetter, S.; Kornsteiner-Krenn, M.; Binder, C.; et al. Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants. Nutrients 2022, 14, 697. https://doi.org/10.3390/nu14030697
Haiden N, Thanhaeuser M, Eibensteiner F, Huber-Dangl M, Gsoellpointner M, Ristl R, Kroyer B, Brandstetter S, Kornsteiner-Krenn M, Binder C, et al. Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants. Nutrients. 2022; 14(3):697. https://doi.org/10.3390/nu14030697
Chicago/Turabian StyleHaiden, Nadja, Margarita Thanhaeuser, Fabian Eibensteiner, Mercedes Huber-Dangl, Melanie Gsoellpointner, Robin Ristl, Bettina Kroyer, Sophia Brandstetter, Margit Kornsteiner-Krenn, Christoph Binder, and et al. 2022. "Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants" Nutrients 14, no. 3: 697. https://doi.org/10.3390/nu14030697
APA StyleHaiden, N., Thanhaeuser, M., Eibensteiner, F., Huber-Dangl, M., Gsoellpointner, M., Ristl, R., Kroyer, B., Brandstetter, S., Kornsteiner-Krenn, M., Binder, C., Thajer, A., & Jilma, B. (2022). Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants. Nutrients, 14(3), 697. https://doi.org/10.3390/nu14030697







