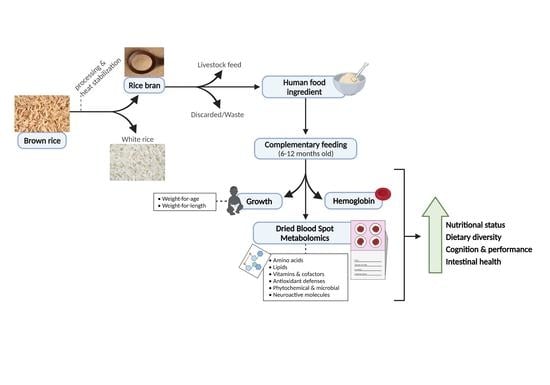
Non-Targeted Dried Blood Spot-Based Metabolomics Analysis Showed Rice Bran Supplementation Effects Multiple Metabolic Pathways during Infant Weaning and Growth in Mali
Abstract
1. Introduction
2. Materials and Methods
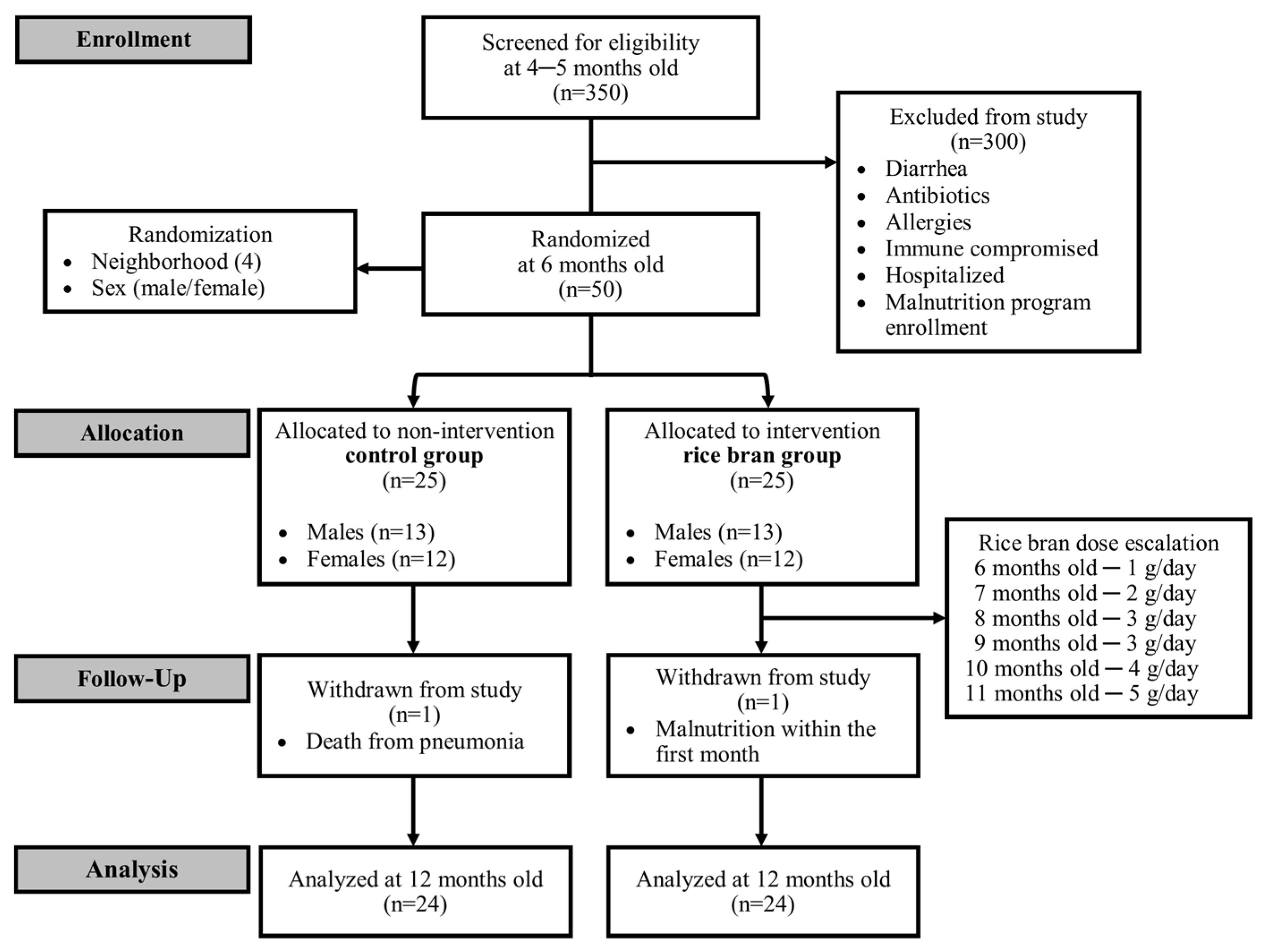
2.1. Study Population
2.2. Intervention Dosing and DBS Collection Procedures
2.3. DBS Metabolic Profiling
2.4. Statistical Analysis
3. Results
3.1. Anthropometric and Hemoglobin Measurements
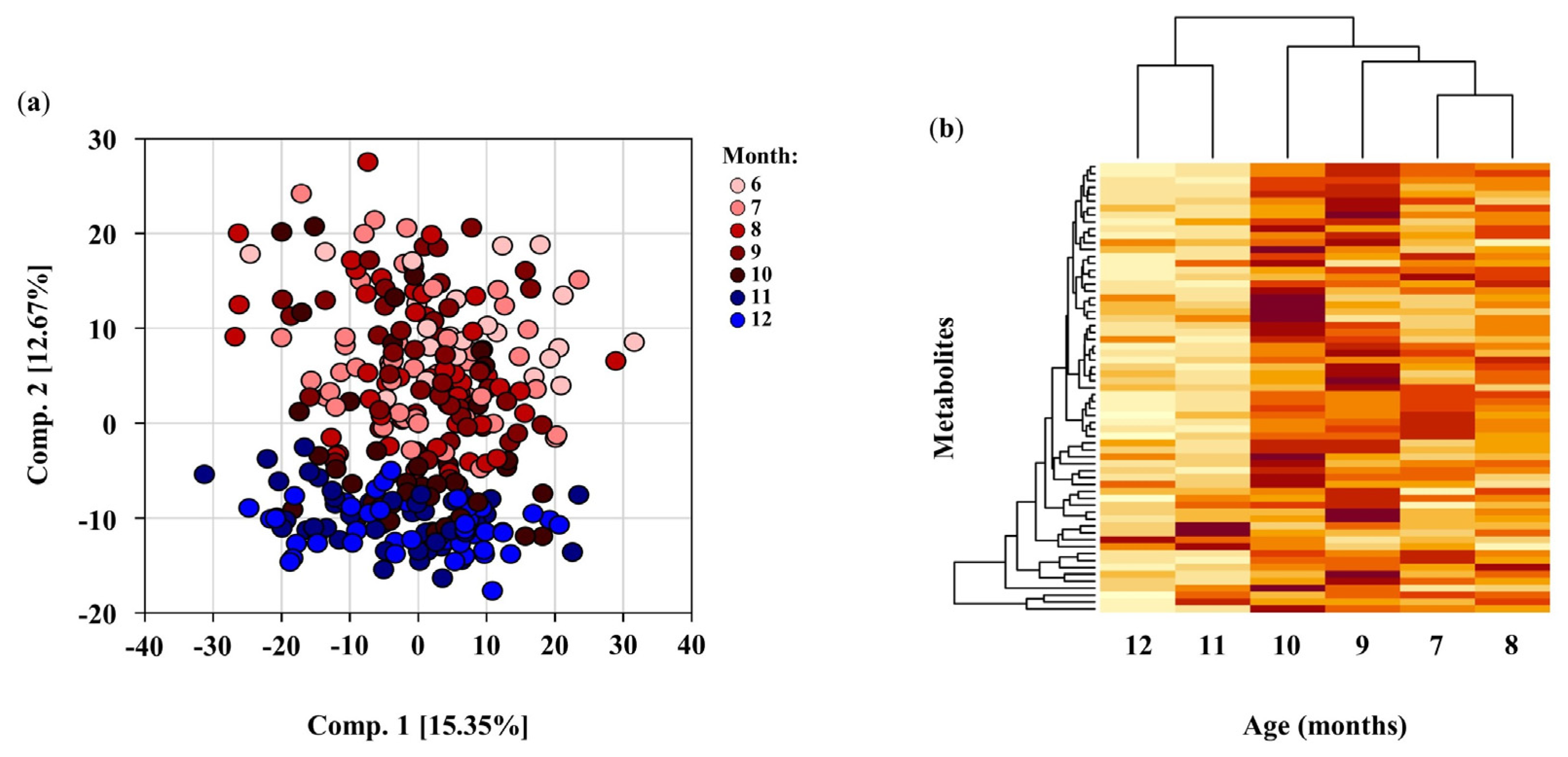
3.2. Principal Component Analysis
3.3. Infant DBS Metabolomics from 6–12 Months of Age
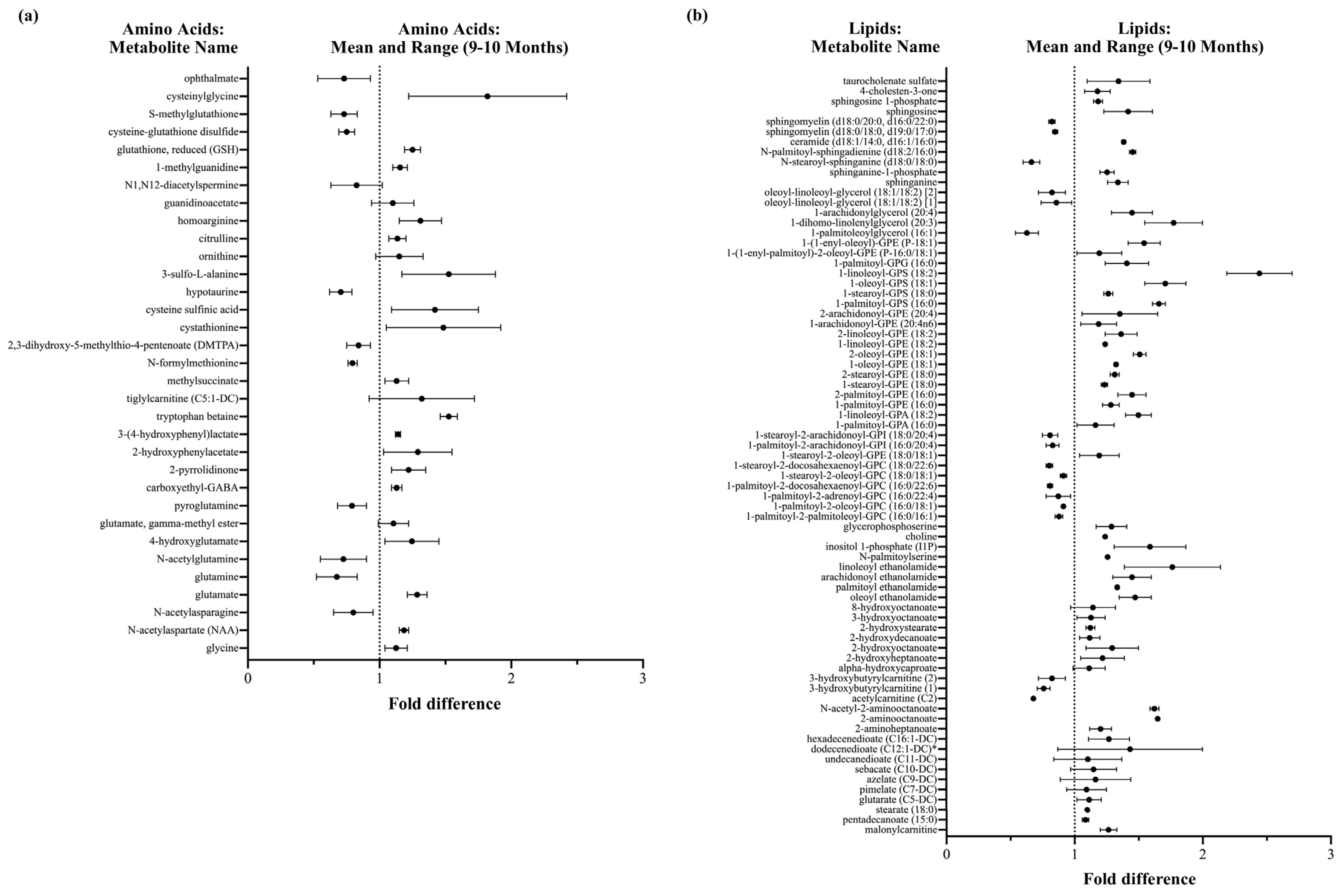
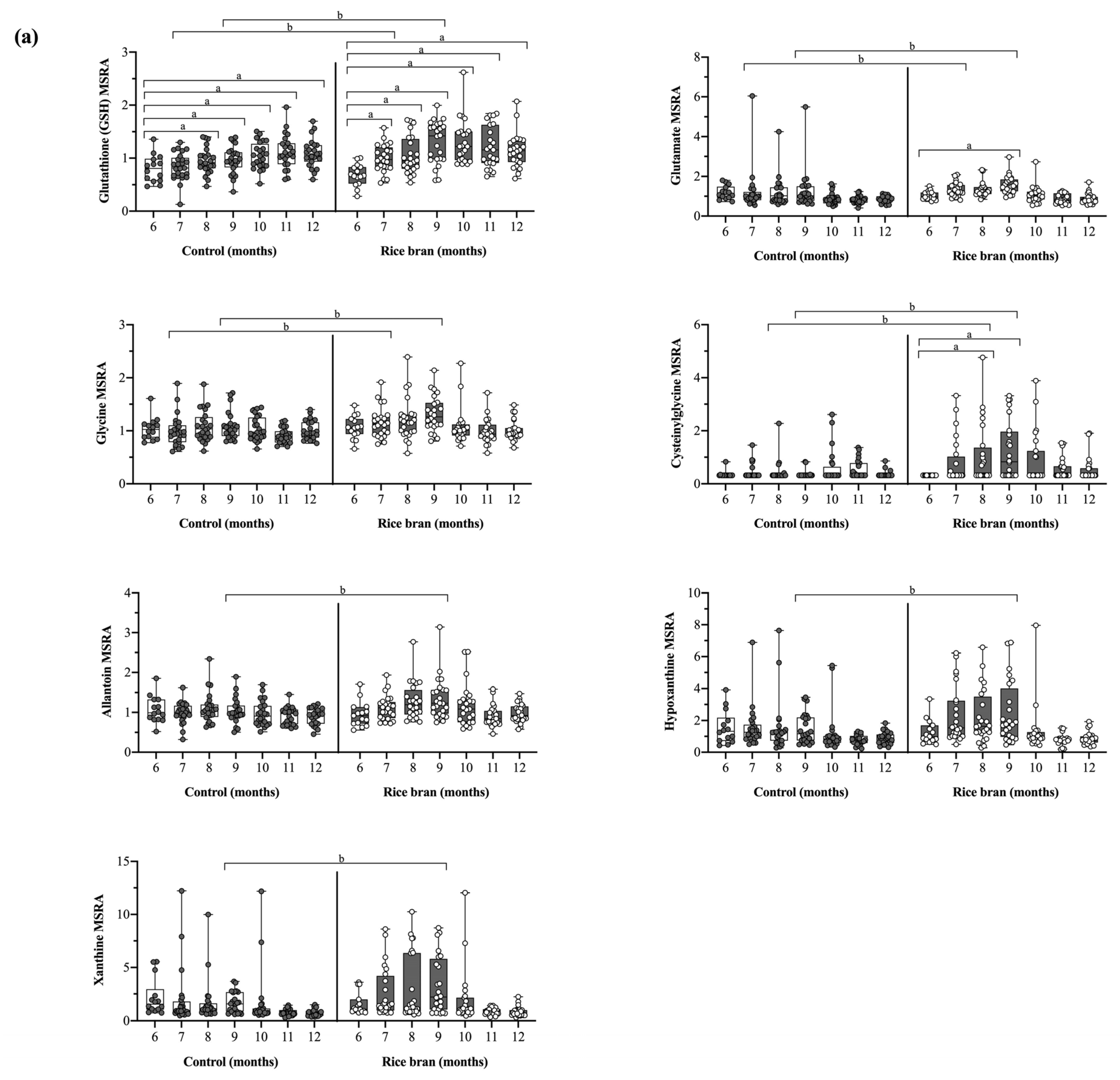
3.3.1. DBS Molecules Involved in Antioxidant Defenses
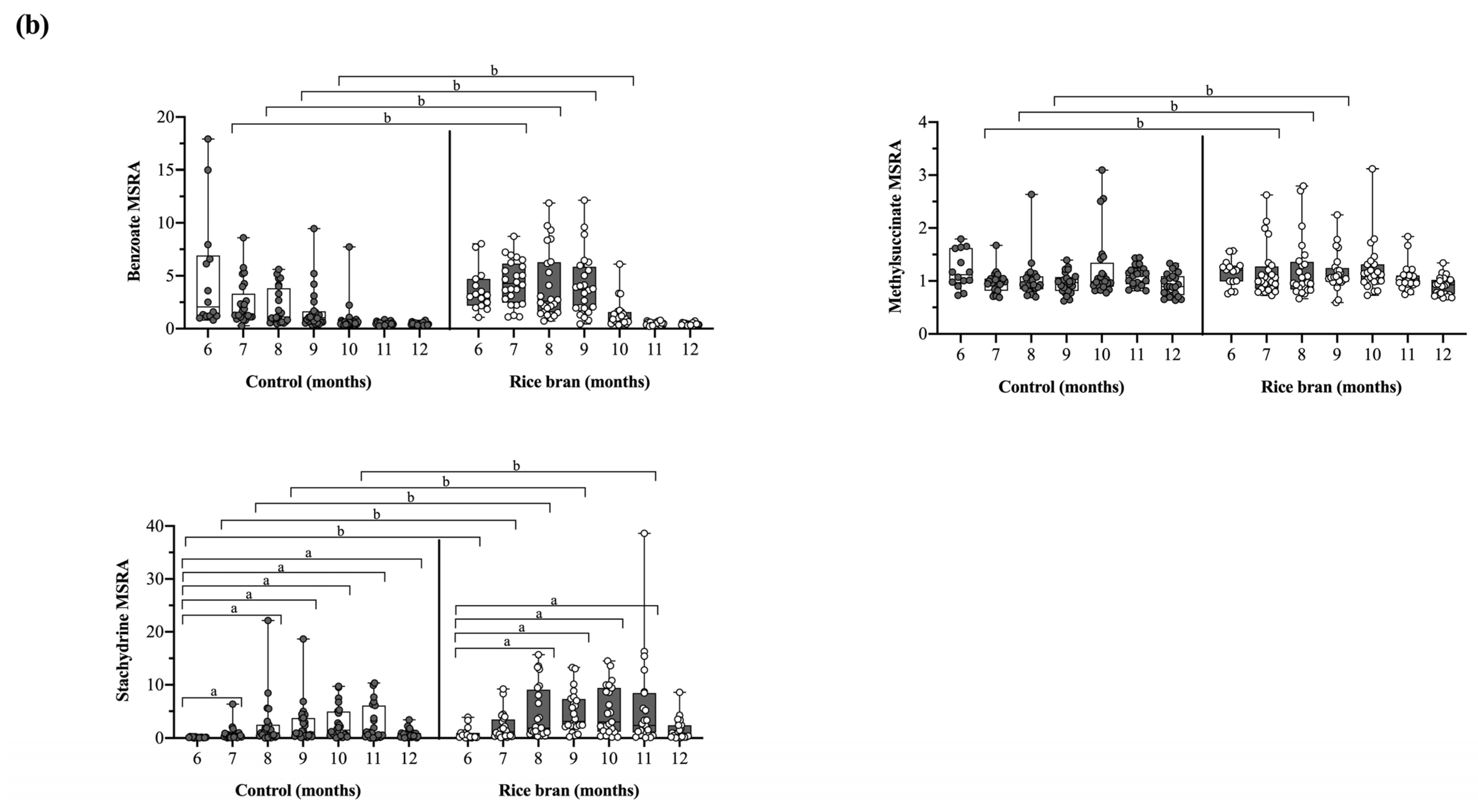
3.3.2. DBS Phytochemicals and Gut Microbial Derived Molecules
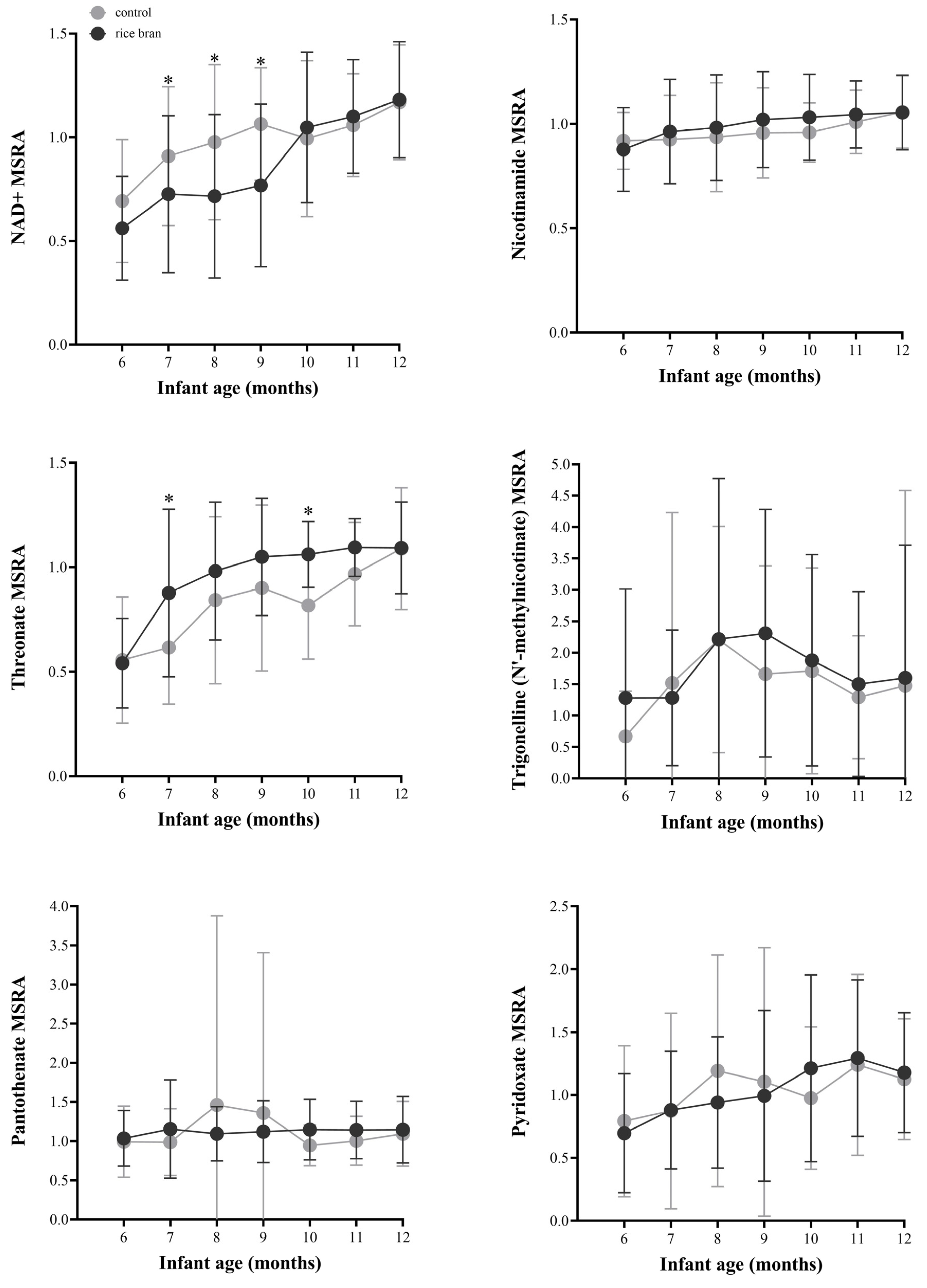
3.3.3. DBS Vitamin and Co-Factor Molecules
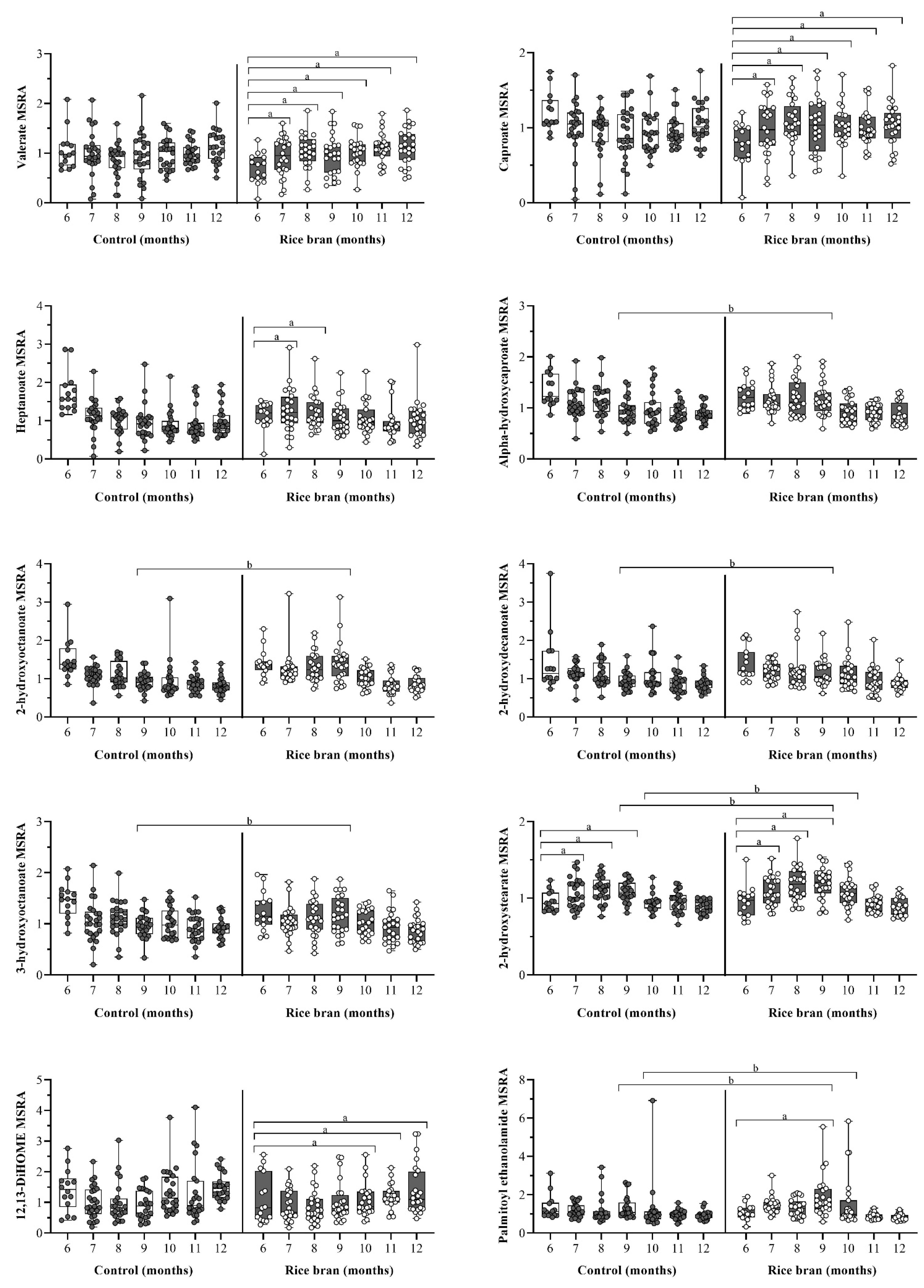
3.3.4. DBS Lipid Components and Metabolic Pathways
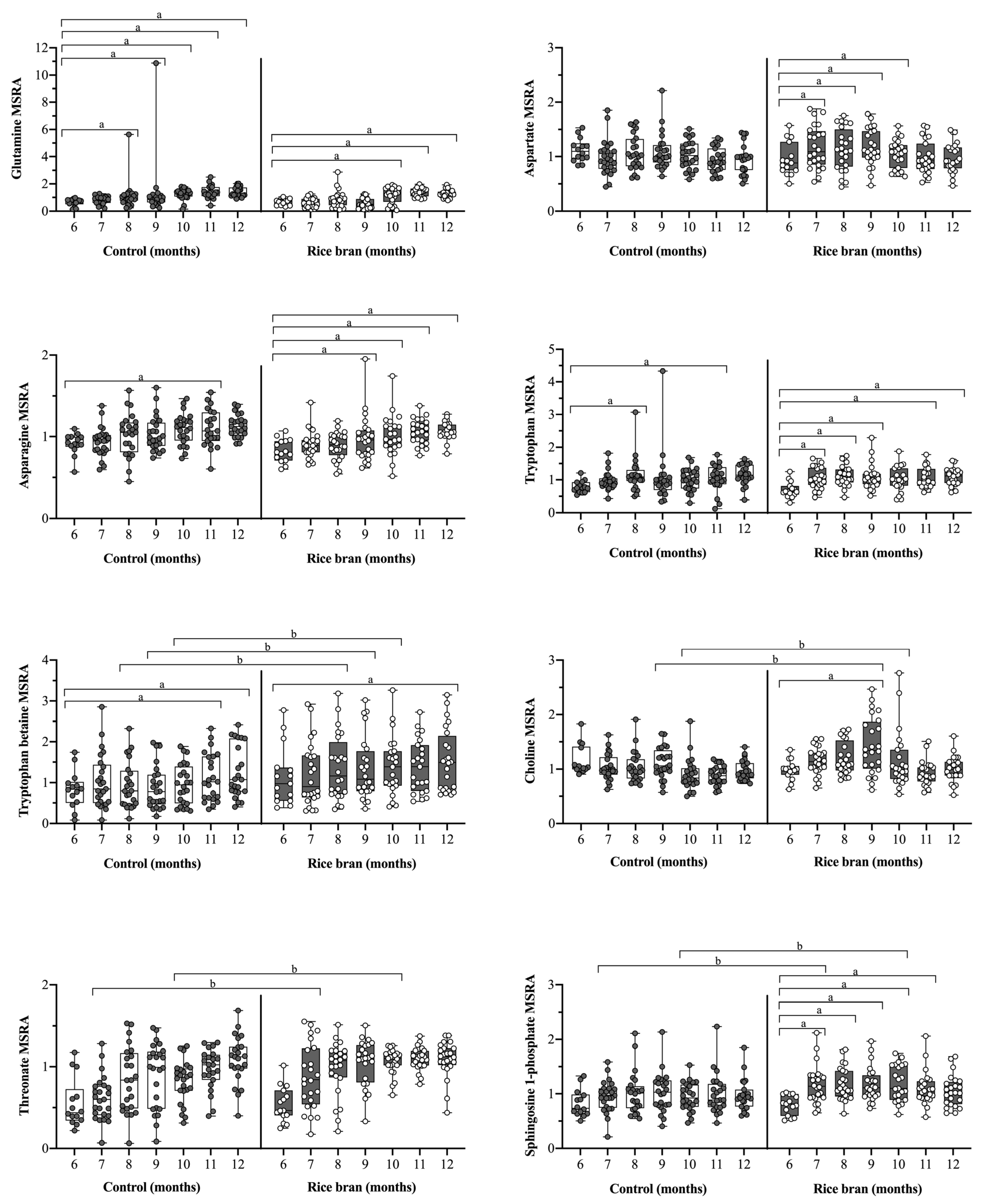
3.3.5. Rice Bran Supplementation and Neuroactive Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, S.M.; Walker, S.P.; Grantham-McGregor, S.; Powell, C.A. Early Childhood Stunting and Later Behaviour and School Achievement. J. Child Psychol. Psychiatry 2002, 43, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Wang, M.; Martorell, R.; Norris, S.A.; Adair, L.S.; Bas, I.; Sachdev, H.S.; Bhargava, S.K.; Fall, C.H.D.; Gigante, D.P.; et al. Growth Patterns in Early Childhood and Final Attained Stature: Data from Five Birth Cohorts from Low- and Middle-Income Countries. Am. J. Hum. Biol. 2010, 22, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; McCoy, D.C.; Danaei, G.; Fink, G.; Ezzati, M.; Andrews, K.G.; Fawzi, W.W. Linear Growth and Child Development in Low- and Middle-Income Countries: A Meta-Analysis. Pediatrics 2015, 135, e1266–e1275. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and Child Undernutrition Study Group Maternal and Child Undernutrition: Consequences for Adult Health and Human Capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- United Nation’s Children’s Fund (UNICEF). Improving Child Nutrition: The Achievable Imperative for Global Progress. 2013. Available online: https://data.unicef.org/resources/improving-child-nutrition-the-achievable-imperative-for-global-progress/ (accessed on 20 January 2022).
- United Nations Children’s Fund (UNICEF). World Health Organization, International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates. 2021. Available online: https://www.who.int/publications/i/item/9789240025257 (accessed on 22 January 2022).
- Allen, L.H. Global Dietary Patterns and Diets in Childhood: Implications for Health Outcomes. ANM 2012, 61, 29–37. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Global Strategy for Infant and Young Child Feeding. Available online: https://www.who.int/publications-detail-redirect/9241562218 (accessed on 20 January 2022).
- The World Health Organization (WHO). Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 20 January 2022).
- Bai, Y.; Alemu, R.; Block, S.A.; Headey, D.; Masters, W.A. Cost and Affordability of Nutritious Diets at Retail Prices: Evidence from 177 Countries. Food Policy 2021, 99, 101983. [Google Scholar] [CrossRef]
- Dewey, K.G.; Adu-Afarwuah, S. Systematic Review of the Efficacy and Effectiveness of Complementary Feeding Interventions in Developing Countries. Matern. Child Nutr. 2008, 4 (Suppl. S1), 24–85. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin Exposure and Associated Human Health Effects, a Review of Epidemiological Studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- United Nation’s Children’s Fund (UNICEF). The State of the World’s Children 2021. 2021. Available online: https://www.unicef.org/reports/state-worlds-children-2021 (accessed on 22 January 2022).
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal Source Foods: Sustainability Problem or Malnutrition and Sustainability Solution? Perspective Matters. Glob. Food Secur. 2020, 25, 100325. [Google Scholar] [CrossRef]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations (FAO). Mali: Country Fact Sheet on Food and Agriculture Policy Trends. 2017. Available online: http://www.fao.org/3/a-i7617e.pdf (accessed on 21 January 2022).
- The Food and Agriculture Organization of the United Nations (FAO). Rice Market Monitor (RMM). 2018. Available online: http://www.fao.org/economic/est/publications/rice-publications/rice-market-monitor-rmm/en/ (accessed on 20 January 2022).
- Borresen, E.C.; Ryan, E.P. Chapter 22—Rice Bran: A Food Ingredient with Global Public Health Opportunities. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 301–310. ISBN 9780124017160. [Google Scholar]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice Bran: A Novel Functional Ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Luna, E.; Leach, J.E.; McClung, A.; Vilchez, S.; Koita, O.; Ryan, E.P. Comparative Rice Bran Metabolomics across Diverse Cultivars and Functional Rice Gene–Bran Metabolite Relationships. Metabolites 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice Bran: Nutritional Values and Its Emerging Potential for Development of Functional Food—A Review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Dipti, S.S.; Bergman, C.; Indrasari, S.D.; Herath, T.; Hall, R.; Lee, H.; Habibi, F.; Bassinello, P.Z.; Graterol, E.; Ferraz, J.P.; et al. The Potential of Rice to Offer Solutions for Malnutrition and Chronic Diseases. Rice 2012, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Kurtys, E.; Eisel, U.L.M.; Hageman, R.J.J.; Verkuyl, J.M.; Broersen, L.M.; Dierckx, R.A.J.O.; de Vries, E.F.J. Anti-Inflammatory Effects of Rice Bran Components. Nutr. Rev. 2018, 76, 372–379. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. The Antioxidant and Anti-Inflammatory Properties of Rice Bran Phenolic Extracts. Foods 2020, 9, 829. [Google Scholar] [CrossRef]
- Henderson, A.J.; Kumar, A.; Barnett, B.; Dow, S.W.; Ryan, E.P. Consumption of Rice Bran Increases Mucosal Immunoglobulin A Concentrations and Numbers of Intestinal Lactobacillus Spp. J. Med. Food 2012, 15, 469–475. [Google Scholar] [CrossRef]
- Kumar, A.; Henderson, A.; Forster, G.M.; Goodyear, A.W.; Weir, T.L.; Leach, J.E.; Dow, S.W.; Ryan, E.P. Dietary Rice Bran Promotes Resistance to Salmonella Enterica Serovar Typhimurium Colonization in Mice. BMC Microbiol. 2012, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wen, K.; Tin, C.; Li, G.; Wang, H.; Kocher, J.; Pelzer, K.; Ryan, E.; Yuan, L. Dietary Rice Bran Protects against Rotavirus Diarrhea and Promotes Th1-Type Immune Responses to Human Rotavirus Vaccine in Gnotobiotic Pigs. Clin. Vaccine Immunol. 2014, 21, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High Protective Efficacy of Rice Bran against Human Rotavirus Diarrhea via Enhancing Probiotic Growth, Gut Barrier Function and Innate Immunity. Sci. Rep. 2015, 5, 15004. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.D. Dried Blood Spots for Global Health Diagnostics and Surveillance: Opportunities and Challenges. Am. J. Trop. Med. Hyg. 2018, 99, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, L.E.; McKeen, S.; Ibrahim, H.; Zarei, I.; Borresen, E.C.; Doumbia, L.; Boré, A.; Cissoko, A.; Douyon, S.; Koné, K.; et al. Rice Bran Supplementation Modulates Growth, Microbiota and Metabolome in Weaning Infants: A Clinical Trial in Nicaragua and Mali. Sci. Rep. 2019, 9, 13919. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Baxter, B.; Li, K.; Wolfe, L.; Yao, L.; Broecling, C.; Borreson, E.; Zhang, L.; Zarei, I.; Beale, M.; et al. Developing Biomarkers of Rice Bran and Navy Bean Intake via Integrated Metabolomics from Infants, Children and Adults for Association with Gut Health Properties. Curr. Dev. Nutr. 2020, 4, 463. [Google Scholar] [CrossRef]
- Hesham, M.S. Intestinal Parasitic Infections and Micronutrient Deficiency: A Review. Med. J. Malays. 2004, 59, 10. [Google Scholar]
- Ford, L.; Kennedy, A.D.; Goodman, K.D.; Pappan, K.L.; Evans, A.M.; Miller, L.A.D.; Wulff, J.E.; Wiggs, B.R., III; Lennon, J.J.; Elsea, S.; et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J. Appl. Lab. Med. 2020, 5, 342–356. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical Significance for Genomewide Studies. Proc. Natl. Aacd. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Martinez-Moral, M.-P.; Kannan, K. Allantoin as a Marker of Oxidative Stress: Inter- and Intraindividual Variability in Urinary Concentrations in Healthy Individuals. Environ. Sci. Technol. Lett. 2019, 6, 283–288. [Google Scholar] [CrossRef]
- Dingledine, R.; McBain, C.J. Glutamate and Aspartate Are the Major Excitatory Transmitters in the Brain. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Gonzalez-Riano, C.; Garcia, A.; Barbas, C. Metabolomics Studies in Brain Tissue: A Review. J. Pharm. Biomed. Anal. 2016, 130, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Moreau, G.B.; Ramakrishnan, G.; Cook, H.L.; Fox, T.E.; Nayak, U.; Ma, J.Z.; Colgate, E.R.; Kirkpatrick, B.D.; Haque, R.; Petri, W.A. Childhood Growth and Neurocognition Are Associated with Distinct Sets of Metabolites. eBioMedicine 2019, 44, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the Potential Role of Tryptophan as the Precursor of Serotonin and Melatonin for the Aged Sleep-Wake Cycle and Immune Function: Streptopelia Risoria as a Model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary Requirements and Role in Brain Development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Thompson, R.A.; Nelson, C.A. Developmental Science and the Media. Early Brain Development. Am. Psychol. 2001, 56, 5–15. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P.; et al. The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino Acid Metabolism in Intestinal Bacteria: Links between Gut Ecology and Host Health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Pollitt, E. A Developmental View of the Undernourished Child: Background and Purpose of the Study in Pangalengan, Indonesia. Eur. J. Clin. Nutr. 2000, 54, S2–S10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Zavaleta, N.; León, Z.; Shankar, A.H.; Caulfield, L.E. Maternal Zinc Supplementation and Growth in Peruvian Infants. Am. J. Clin. Nutr. 2008, 88, 154–160. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tofail, F.; Wahed, M.A.; Fuchs, G.J.; Baqui, A.H.; Alvarez, J.O. Short-Term Supplementation with Zinc and Vitamin A Has No Significant Effect on the Growth of Undernourished Bangladeshi Children. Am. J. Clin. Nutr. 2002, 75, 87–91. [Google Scholar] [CrossRef]
- Stewart, C.P.; Caswell, B.; Iannotti, L.; Lutter, C.; Arnold, C.D.; Chipatala, R.; Prado, E.L.; Maleta, K. The Effect of Eggs on Early Child Growth in Rural Malawi: The Mazira Project Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, L.E.; Weber, A.M.; Borresen, E.C.; Zarei, I.; Perez, J.; Perez, C.; Rodríguez, I.; Becker-Dreps, S.; Yuan, L.; Vilchez, S.; et al. Daily Rice Bran Consumption for 6 Months Influences Serum Glucagon-Like Peptide 2 and Metabolite Profiles without Differences in Trace Elements and Heavy Metals in Weaning Nicaraguan Infants at 12 Months of Age. Curr. Dev. Nutr. 2021, 5, nzab101. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Naviaux, J.C.; Monk, J.M.; Wang, L.; Naviaux, R.K. Improved Dried Blood Spot-Based Metabolomics: A Targeted, Broad-Spectrum, Single-Injection Method. Metabolites 2020, 10, 82. [Google Scholar] [CrossRef]
- Moreno-Torres, M.; García-Llorens, G.; Moro, E.; Méndez, R.; Quintás, G.; Castell, J.V. Factors That Influence the Quality of Metabolomics Data in in Vitro Cell Toxicity Studies: A Systematic Survey. Sci. Rep. 2021, 11, 22119. [Google Scholar] [CrossRef]
| Control (N = 24) | Rice Bran (N = 24) | |
|---|---|---|
| Sex (%) | ||
| Female | 12(50) | 12(50) |
| Male | 12(50) | 12(50) |
| Mother’s education (%) | ||
| None | 12(50) | 11(46) |
| Some primary | 4(17) | 7(29) |
| Completed primary | 6(25) | 1(4) |
| Some secondary | 1(4) | 2(8) |
| Completed secondary | 1(4) | 3(13) |
| University | 0(0) | 0(0) |
| Breastfeeding status (%) | ||
| 6 months | 24(100) | 24(100) |
| Sanitation systems (%) | ||
| Community latrine | 21(87.5) | 19(76) |
| Private latrine | 3(12.5) | 5(20.8) |
| House type (%) | ||
| Mud | 16(66) | 17(70) |
| Sheet metal | 5(20) | 5(21) |
| Cement | 3(14) | 2(8) |
| Water source (%) | ||
| Untreated ground water | 24(100) | 24(100) |
| Water risk assessment (%) | ||
| Safe | 7(44) | 3(13) |
| Low risk | 2(9) | 2(8) |
| Low-mid risk | 4(17) | 5(21) |
| Mid-high risk | 2(9) | 2(8) |
| High risk | 4(17) | 6(25) |
| Unsafe | 5(21) | 6(25) |
| Anthropometry (mean ± SD) | ||
| Weight (kg) | 7.02 ± 0.88 | 7.14 ± 0.99 |
| Length (cm) | 65.57 ± 2.56 | 66.56 ± 3.12 |
| Weight-for-age Z-score (WAZ) | −0.65 ± 1.29 | −0.64 ± 1.09 |
| Length-for-age Z-score (LAZ) | −0.30 ± 1.70 | −0.15 ± 1.46 |
| Weight-for-length Z-score (WLZ) | −0.51 ± 0.95 | −0.58 ± 1.05 |
| 6–7 Mos. | 6–8 Mos. | 6–9 Mos. | 6–10 Mos. | 6–11 Mos. | 6–12 Mos. | |
|---|---|---|---|---|---|---|
| Length-for-Age z-Score (LAZ) | ||||||
| Rice bran | 0.276 | 0.343 | 0.326 | 0.234 | 0.239 | 0.160 |
| Control | 0.049 | 0.098 | 0.137 | 0.028 | −0.132 | −0.284 |
| Difference | 0.227 | 0.245 | 0.189 | 0.205 | 0.371 | 0.444 |
| p-value | 0.342 | 0.336 | 0.442 | 0.414 | 0.122 | 0.085 |
| Weight-for-age z-score (WAZ) | ||||||
| Rice bran | 0.356 | 0.623 | 0.528 | 0.429 | 0.234 | 0.203 |
| Control | 0.125 | 0.207 | 0.095 | −0.132 | −0.383 | −0.457 |
| Difference | 0.231 | 0.416 | 0.433 | 0.561 | 0.618 | 0.660 |
| p-value | 0.190 | 0.029 | 0.024 | 0.011 | 0.009 | 0.003 |
| Weight-for-length z-score (WLZ) | ||||||
| Rice bran | 0.218 | 0.535 | 0.392 | 0.286 | 0.011 | −0.003 |
| Control | 0.052 | 0.145 | −0.070 | −0.323 | −0.598 | −0.670 |
| Difference | 0.166 | 0.390 | 0.462 | 0.609 | 0.609 | 0.667 |
| p-value | 0.544 | 0.105 | 0.069 | 0.023 | 0.013 | 0.008 |
| Hemoglobin (g/dL) | ||||||
| Rice bran | 0.201 | 0.218 | 0.396 | 0.405 | −0.095 | 0.404 |
| Control | −0.154 | −0.254 | −0.113 | −0.454 | −0.703 | −0.355 |
| Difference | 0.355 | 0.472 | 0.508 | 0.859 | 0.608 | 0.759 |
| p-value | 0.435 | 0.278 | 0.314 | 0.031 | 0.165 | 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfluger, B.A.; Smith, H.V.; Weber, A.M.; Ibrahim, H.; Doumbia, L.; Bore, A.; Cissoko, A.; Douyon, S.; Kone, K.; Sangare, L.; et al. Non-Targeted Dried Blood Spot-Based Metabolomics Analysis Showed Rice Bran Supplementation Effects Multiple Metabolic Pathways during Infant Weaning and Growth in Mali. Nutrients 2022, 14, 609. https://doi.org/10.3390/nu14030609
Pfluger BA, Smith HV, Weber AM, Ibrahim H, Doumbia L, Bore A, Cissoko A, Douyon S, Kone K, Sangare L, et al. Non-Targeted Dried Blood Spot-Based Metabolomics Analysis Showed Rice Bran Supplementation Effects Multiple Metabolic Pathways during Infant Weaning and Growth in Mali. Nutrients. 2022; 14(3):609. https://doi.org/10.3390/nu14030609
Chicago/Turabian StylePfluger, Brigitte A., Hillary V. Smith, Annika M. Weber, Hend Ibrahim, Lassina Doumbia, Abdoulaye Bore, Alima Cissoko, Seydou Douyon, Karim Kone, Lansana Sangare, and et al. 2022. "Non-Targeted Dried Blood Spot-Based Metabolomics Analysis Showed Rice Bran Supplementation Effects Multiple Metabolic Pathways during Infant Weaning and Growth in Mali" Nutrients 14, no. 3: 609. https://doi.org/10.3390/nu14030609
APA StylePfluger, B. A., Smith, H. V., Weber, A. M., Ibrahim, H., Doumbia, L., Bore, A., Cissoko, A., Douyon, S., Kone, K., Sangare, L., Maiga, A., Koita, O. A., Goodman, K., Evans, A. M., & Ryan, E. P. (2022). Non-Targeted Dried Blood Spot-Based Metabolomics Analysis Showed Rice Bran Supplementation Effects Multiple Metabolic Pathways during Infant Weaning and Growth in Mali. Nutrients, 14(3), 609. https://doi.org/10.3390/nu14030609