Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic
Abstract
:1. Introduction
Vitamin B12
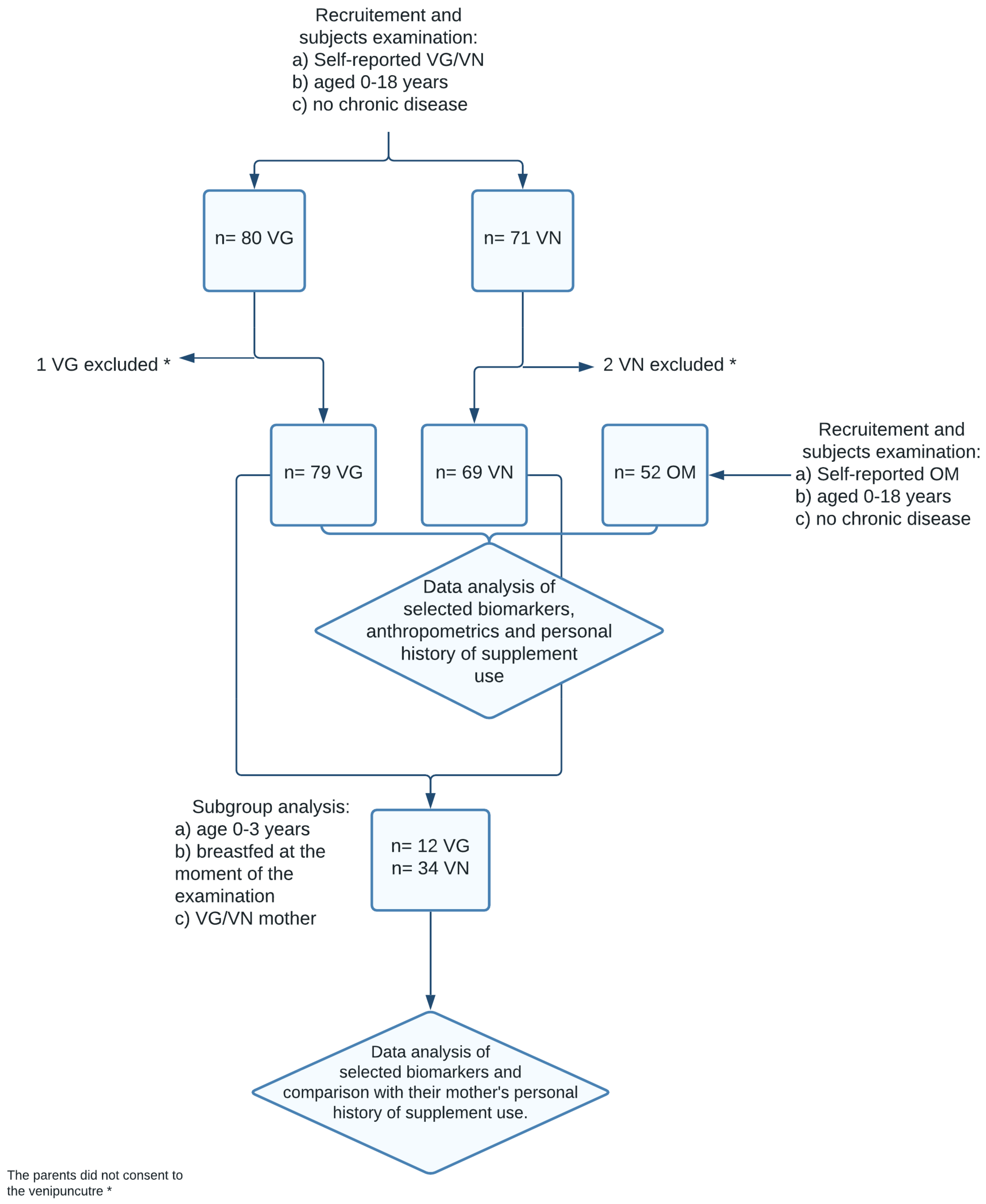
2. Materials and Methods
2.1. Study Design and Participants
2.2. Personal History and Examination
2.3. Biochemical Analysis
2.4. Nutritional Assessment
2.5. Anthropometrics
2.6. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. B12 Dietary and Supplement Intake Analysis
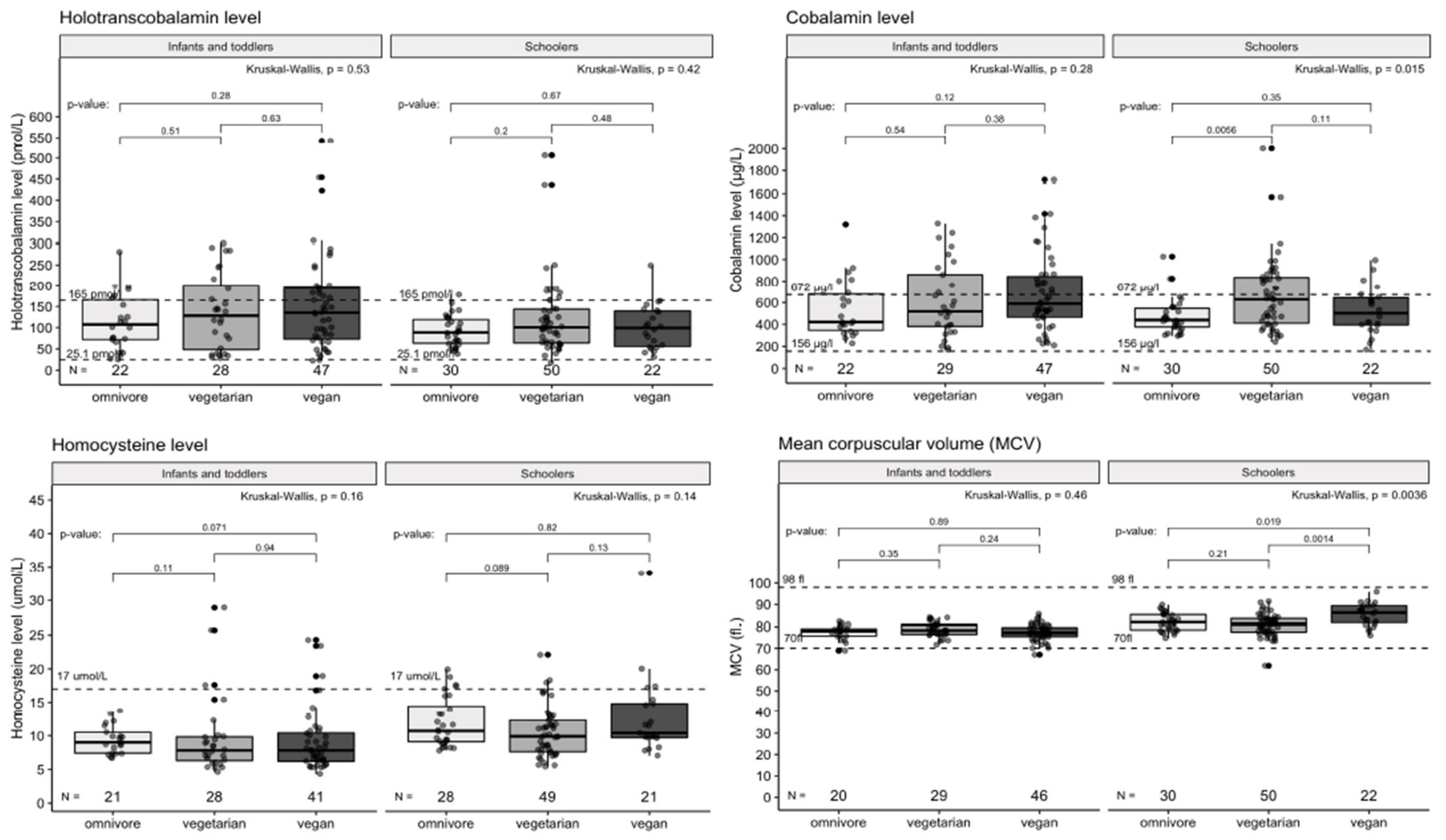
3.3. Differences between Dietary Groups in Selected Blood Markers Results
3.4. Subanalysis: B12 Status of Breastfed VG and VN Children Aged 0–3 Years According to B12 Supplementation Habits, Breastfeeding, and Mothers’ Supplementation Habits during Lactation and/or Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrara, P.; Corsello, G.; Quattrocchi, E.; Dell’Aquila, L.; Ehrich, J.; Giardino, I.; Pettoello-Mantovani, M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017, 187, 339–340.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, S.; Meier, V.; Patrick, H. Review of Vitamin B12 deficiency in pregnancy: A diagnosis not to miss as veganism and vegetarianism become more prevalent. Eur. J. Haematol. 2021, 106, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice: Vegetarian infant and child nutrition. Eur. J. Pediatr. 2011, 170, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, S.; Kersting, M.; Alexy, U. Vegetarian diets in children: A systematic review. Eur. J. Nutr. 2017, 56, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Müller, S. Vegetarische und vegane Ernährung bei Kindern-Stand der Forschung und Forschungsbedarf. Complement. Med. Res. 2016, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Pilar Vaquero, M. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Heyssel, R.M.; Bozian, R.C.; Darby, W.J.; Bell, M.C. Vitamin B12 turnover in man. The assimilation of vitamin B12 from natural foodstuff by man and estimates of minimal daily dietary requirements. Am. J. Clin. Nutr. 1966, 18, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Bousselamti, A.; El Hasbaoui, B.; Echahdi, H.; Krouile, Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr. Med. J. 2018, 30, 152. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Krajčovičová-Kudláčková, M.; Blažíček, P.; Kopčová, J.; Béderová, A.; Babinská, K. Homocysteine levels in vegetarians versus omnivores. Ann. Nutr. Metab. 2000, 44, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Honzik, T.; Adamovicova, M.; Smolka, V.; Magner, M.; Hruba, E.; Zeman, J. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency—What have we learned? Eur. J. Paediatr. Neurol. 2010, 14, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Chalies, S.; Jeziorski, E.; Ludwig, C.; Lalande, M.; Rodière, M. Consequences of exclusive breast-feeding in vegan mother newborn—Case report. Arch. Pediatr. 2009, 16, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Světnička, M.; Selinger, E.; Gojda, J.; El-Lababidi, E. Plant based diets: Breastfeeding and complementary feeding. Pediatr. Pro Praxi 2020, 21, 409–413. [Google Scholar]
- Snow, C.F. Laboratory diagnosis of vitamin B12 and folate deficiency: A guide for the primary care physician. Arch. Intern. Med. 1999, 159, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Obeid, R.; Schorr, H.; Geisel, J. The Usefulness of Holotranscobalamin in Predicting Vitamin B12 Status in Different Clinical Settings. Curr. Drug Metab. 2005, 6, 47–53. [Google Scholar] [CrossRef]
- Miller, J.W.; Garrod, M.G.; Rockwood, A.L.; Kushnir, M.M.; Allen, L.H.; Haan, M.N.; Green, R. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin. Chem. 2006, 52, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Světnička, M.; Selinger, E.; Gojda, J.; El-Lababidi, E. Health consequences of vegan diet in children and adolescents. Diabetol. Metab. Endokrinol. Vyziv. 2020, 23, 166–173. [Google Scholar]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 deficiency is prevalent among czech vegans who do not use vitamin B12 supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [Green Version]
- Webster-Gandy, J.; Madden, A.; Holdsworth, M. Oxford Handbook of Nutrition and Dietetics; Oxford University Press: Oxford, UK, 2020; p. 921. [Google Scholar]
- Vignerová, J.; Riedlová, J.; Bláha, P.; Kobzová, J.; Krejčovský, L.; Brabec, M. 6. Celostátní Antropologický Výzkum dětí a Mládeže 2001, Česká Republika: Souhrnné Výsledky = 6th Nation-Wide Anthropological Survey of Childern And Adolescents 2001; Univerzita Karlova: Prague, Czech Republic, 2006; ISBN 80-86561-30-5. [Google Scholar]
- Guez, S.; Chiarelli, G.; Menni, F.; Salera, S.; Principi, N.; Esposito, S. Severe vitamin B12 deficiency in an exclusively breastfed 5-month-old Italian infant born to a mother receiving multivitamin supplementation during pregnancy. BMC Pediatr. 2012, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiihne, T.; Bnbl, R.; Baumgartner, R. Maternal vegan diet causing a serious infantile neurological disorder due to vitamin B12 deficiency. Eur. J. Pediatrics 1991, 150, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, G.B.; Mittal, M.; Jain, R. Compromised Vitamin B12 Status of Indian Infants and Toddlers. Food Nutr. Bull. 2020, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the nutrinet-santé study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Lemale, J.; Mas, E.; Jung, C.; Bellaiche, M.; Tounian, P. Vegan diet in children and adolescents. Recommendations from the French-speaking Pediatric Hepatology, Gastroenterology and Nutrition Group (GFHGNP). Arch. Pediatr. 2019, 26, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, S.; Bührer, C.; Jochum, F.; Kauth, T.; Kersting, M.; Körner, A.; Koletzko, B.; Mihatsch, W.; Prell, C.; Reinehr, T.; et al. Vegetarian diets in childhood and adolescence: Position paper of the nutrition committee, German Society for Paediatric and Adolescent Medicine (DGKJ). Mol. Cell. Pediatr. 2019, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [Green Version]
- Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets. J. Am. Diet. Assoc. 2003, 103, 748–765. [CrossRef]
- Redecilla Ferreiro, S.; Moráis López, A.; Moreno Villares, J.M.; Leis Trabazo, R.; José Díaz, J.; Sáenz de Pipaón, M.; Blesa, L.; Campoy, C.; Ángel Sanjosé, M.; Gil Campos, M.; et al. Position paper on vegetarian diets in infants and children. Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association. An. Pediatr. 2020, 92, e1–e306. [Google Scholar] [CrossRef]
- Araghi, S.O.; Kiefte-De Jong, J.C.; Van Dijk, S.C.; Swart, K.M.A.; Van Laarhoven, H.W.; Van Schoor, N.M.; De Groot, L.C.P.G.M.; Lemmens, V.; Stricker, B.H.; Uitterlinden, A.G.; et al. Folic acid and Vitamin B12 supplementation and the risk of cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol. Biomark. Prev. 2019, 28, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Fanidi, A.; Carreras-Torres, R.; Larose, T.L.; Yuan, J.-M.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Pettinger, M.; Cai, Q.; et al. Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer 2019, 145, 1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weder, S.; Keller, M.; Fischer, M.; Becker, K.; Alexy, U. Intake of micronutrients and fatty acids of vegetarian, vegan, and omnivorous children (1–3 years) in Germany (VeChi Diet Study). Eur. J. Nutr. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Fischer, M.; Weder, S.; Längler, A.; Michalsen, A.; Sputtek, A.; Keller, M. Nutrient intake and status of german children and adolescents consuming vegetarian, vegan or omnivore diets: Results of the vechi youth study. Nutrients 2021, 13, 1707. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; De Stavola, B.; et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef]
- Karcz, K.; Królak-Olejnik, B. Vegan or vegetarian diet and breast milk composition—A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1081–1098. [Google Scholar] [CrossRef]
- Hebbelinck, M.; Clarys, P.; De Malsche, A. Growth, development, and physical fitness of Flemish vegetarian children, adolescents, and young adults. Am. J. Clin. Nutr. 1999, 70, 579s–585s. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, J.M.; Dibley, M.J.; Sierra, J.; Wallace, B.; Marks, J.S.; Yip, R. Growth of vegetarian children: The Farm Study. Pediatrics 1989, 84, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Weder, S.; Hoffmann, M.; Becker, K.; Alexy, U.; Keller, M. Energy, macronutrient intake, and anthropometrics of vegetarian, vegan, and omnivorous children (1-3 years) in Germany (VeChi diet study). Nutrients 2019, 11, 832. [Google Scholar] [CrossRef] [Green Version]
- Burrows, T.; Goldman, S.; Rollo, M. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labelled water. Eur. J. Clin. Nutr. 2020, 74, 669–681. [Google Scholar] [CrossRef]
- Scolamiero, E.; Villani, G.R.D.; Ingenito, L.; Pecce, R.; Albano, L.; Caterino, M.; di Girolamo, M.G.; Di Stefano, C.; Franzese, I.; Gallo, G.; et al. Maternal vitamin B12 deficiency detected in expanded newborn screening. Clin. Biochem. 2014, 47, 312–317. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalay, Z.; Islek, A.; Parlak, M.; Kirecci, A.; Guney, O.; Koklu, E.; Kalay, S. Reliable and powerful laboratory markers of cobalamin deficiency in the newborn: Plasma and urinary methylmalonic acid. J. Matern. Neonatal Med. 2016, 29, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan nutrition for mothers and children: Practical tools for healthcare providers. Nutrients 2019, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Age | B12 [μg] |
|---|---|---|
| nursling | 0–12 months | 0.4 |
| children | 1–3 years | 0.5 |
| 4–6 years | 0.8 | |
| 7–10 years | 1.0 | |
| 11–14 years | 1.2 | |
| adolescents | 13–18 years | 1.5 |
| Lower Reference Limit | Upper Reference Limit | |
|---|---|---|
| holotranscobalamin | 25.1 pmol/L | 165 pmol/L |
| cyanocobalamin | 156 µg/L | 672 µg/L |
| folate | 5.38 µg/L | 40 µg/L |
| homocystein | 3.0 µmol/L | 17.0 µmol/L |
| hemoglobin | ||
| 6 months–2 years | 105 g/L | 135 g/L |
| 2 years–6 years | 115 g/L | 135 g/L |
| 6 years–12 years | 115 g/L | 155 g/L |
| 12 years–15 years female | 120 g/L | 160 g/L |
| 12 years–15 years male | 130 g/L | 160 g/L |
| mean corpuscular volume | ||
| 6 months–2 years | 70 fl | 86 fl |
| 2 years–6 years | 75 fl | 87 fl |
| 6 years–12 years | 77 fl | 95 fl |
| 12 years–15 years female | 78 fl | 102 fl |
| 12 years–15 years male | 78 fl | 98 fl |
| 15 years + Female and male | 82 fl | 98 fl |
| (a) | ||||||||
| Group | n | Sex | Median Age | IQR | p-Value | Min Age | Max Age | |
| Female | Male | |||||||
| VN | 69 | 31 | 38 | 2.0 | 1.0–6.0 | 0.003 | 0.5 | 18 |
| VG | 79 | 44 | 35 | 4.5 | 2.6–8.0 | 0.5 | 18 | |
| OM | 52 | 25 | 27 | 4.5 | 2.0–10.9 | 0.75 | 18.5 | |
| (b) | ||||||||
| Variable | Omnivore | Vegetarian | Vegan | p-Value | ||||
| Med | IQR | Med | IQR | Med | IQR | |||
| height percentile | 45.0 | 23.0–70.0 | 48.0 | 24.7–70.0 | 48.0 | 28.0–73.0 | 0.883 | |
| weight percentile | 44.0 | 16.0–65.5 | 47.0 | 20.5–70.0 | 40.0 | 11.0–65.0 | 0.386 | |
| BMI percentile | 40.0 | 19.5–55:0 | 42.0 | 25.0–67.0 | 35.0 | 14.0–65.0 | 0.529 | |
| n (total) | n (total) | n (total) | p-Value | |||||
| height ≤3 perc. | 2 (52) | 3 (69) | 2 (79) | 1.000 | ||||
| weight ≤3 perc. | 5 (52) | 4 (69) | 6 (79) | 0.588 | ||||
| BMI ≤3 perc. | 1 (52) | 0 (69) | 7 (79) | 0.003 | ||||
| (a) | ||||||||
| Group | n | Sex | Median Age | IQR | p-Value Age | |||
| Female | Male | |||||||
| VN | 47 | 19 | 28 | 1.5 | 0.8–2.0 | 0.076 | ||
| VG | 29 | 17 | 12 | 2.0 | 1.5–2.8 | |||
| OM | 22 | 8 | 14 | 1.8 | 1.1–2.2 | |||
| (b) | ||||||||
| Variable | Omnivore | Vegetarian | Vegan | p-Value | ||||
| Med | IQR | Med | IQR | Med | IQR | |||
| height percentile | 27.5 | 17.85–6.2 | 62.0 | 31.0–79.0 | 42.0 | 20.0–72.0 | 0.054 | |
| weight percentile | 18.0 | 5.8–45.0 | 50.0 | 35.0–70.0 | 40.0 | 9.5–59.5 | 0.017 | |
| BMI percentile | 30.0 | 14.5–48.8 | 40.0 | 28.0–71.0 | 35.0 | 14.5–59.5 | 0.204 | |
| n (Total) | n (Total) | n (Total) | p-Value | |||||
| height ≤3 perc. | 2 (22) | 1 (47) | 1 (29) | 0.342 | ||||
| weight ≤3 perc. | 5 (22) | 2 (47) | 4 (29) | 0.197 | ||||
| BMI ≤3 perc. | 0 (22) | 0 (47) | 5 (29) | 0.079 | ||||
| (a) | ||||||||
| Group | n | Sex | Median Age | IQR | p-Value Age | |||
| Female | Male | |||||||
| VN | 22 | 12 | 10 | 14.0 | 6.2–17.0 | 0.012 | ||
| VG | 50 | 27 | 23 | 7.5 | 5.0–9.9 | |||
| OM | 30 | 17 | 13 | 10.2 | 5.6–14.4 | |||
| (b) | ||||||||
| Variable | Omnivore | Vegetarian | Vegan | p-Value | ||||
| Med | IQR | Med | IQR | Med | IQR | |||
| height percentile | 55.0 | 36.2–74.2 | 42.5 | 19.0–59.5 | 53.0 | 33.2–74.2 | 0.138 | |
| weight percentile | 52.5 | 23.5–78.8 | 45.0 | 19.0–68.8 | 47.5 | 21.2–80.0 | 0.524 | |
| BMI percentile | 45.0 | 20.5–74.5 | 44.0 | 20.0–65.0 | 35.5 | 13.0–65.8 | 0.781 | |
| n (Total) | n (Total) | n (Total) | p-Value | |||||
| height ≤3 perc. | 0 (30) | 2 (22) | 1 (50) | 0.594 | ||||
| weight ≤3 perc. | 0 (30) | 2 (22) | 2 (50) | 0.250 | ||||
| BMI ≤3 perc. | 1 (30) | 0 (22) | 2 (50) | 0.075 | ||||
| VG (79) | VN (69) | p-Value | ||
|---|---|---|---|---|
| supplement use | yes | 54 | 59 | 0.019 |
| no | 25 | 10 | ||
| regularity | not supplementing | 25 | 10 | 0.014 |
| irregular | 2 | 2 | ||
| every day | 37 | 44 | ||
| once a week | 6 | 2 | ||
| twice a week | 7 | 3 | ||
| three times a week | 2 | 8 | ||
| chemical form | not supplementing | 25 | 10 | 0.009 |
| methylcobalamin | 23 | 35 | ||
| cyanocobalamin | 30 | 21 | ||
| adenosylcobalamin | 1 | 1 | ||
| hydroxymethylcobalamin | 0 | 2 | ||
| medical form | not supplementing | 25 | 10 | 0.048 |
| drops | 28 | 33 | ||
| pills | 26 | 26 | ||
| Group | Median | IQR | p-Value | |
|---|---|---|---|---|
| aB12 [pmol/L] | VN | 116.6 | 66.2–170.3 | 0.257 |
| VG | 108.2 | 63.4–162.7 | ||
| OM | 91.4 | 66.3–125.1 | ||
| B12 [µg/L] | VN | 545.9 | 410.0–789.0 | 0.019 |
| VG | 572.0 | 397.0–849.0 | ||
| OM | 432.5 | 370.5–576.2 | ||
| folate [µg/L] | VN | 18.1 | 13.7–21.4 | 0.057 |
| VG | 15.9 | 13.5–20.3 | ||
| OM | 14.4 | 11.1–20.1 | ||
| Hcys [µmol/L] | VN | 9.1 | 6.7–11.5 | 0.098 |
| VG | 9.1 | 7.0–11.7 | ||
| OM | 9.8 | 8.6–12.2 | ||
| Group | Mean | SD | p-Value | |
| MCV [fl] | VN | 79.9 | 5.7 | 0.929 |
| VG | 79.8 | 4.7 | ||
| OM | 80.2 | 4.4 |
| Group | Median | IQR | p-Value | |
|---|---|---|---|---|
| aB12 [pmol/L] | VN | 135.2 | 73.1–192.6 | 0.530 |
| VG | 128.1 | 48.5–199.0 | ||
| OM | 107.5 | 72.1–165.9 | ||
| B12 [µg/L] | VN | 590.0 | 466.0–843.5 | 0.278 |
| VG | 519.0 | 381.0–860.0 | ||
| OM | 422.5 | 346.8–676.8 | ||
| folate [µg/L] | VN | 18.9 | 16.1–22.2 | 0.636 |
| VG | 18.2 | 15.7–20.8 | ||
| OM | 20.0 | 14.9–23.6 | ||
| Hcys [µmol/L] | VN | 7.8 | 6.2–10.4 | 0.157 |
| VG | 7.8 | 6.3–9.8 | ||
| OM | 9.0 | 7.4–10.5 | ||
| Group | Mean | SD | p-Value | |
| MCV [fl] | VN | 77.3 | 3.8 | 0.295 |
| VG | 78.5 | 3.3 | ||
| OM | 77.3 | 3.2 |
| Group | Median | IQR | p-Value | |
|---|---|---|---|---|
| aB12 [pmol/L] | VN | 99.2 | 55.9–139.3 | 0.420 |
| VG | 100.8 | 64.0–143.6 | ||
| OM | 88.7 | 63.5–119.0 | ||
| B12 [µg/L] | VN | 502.5 | 394.5–645.0 | 0.015 |
| VG | 629.0 | 411.8–835.2 | ||
| OM | 440.0 | 376.5–547.0 | ||
| folate [µg/L] | VN | 12.7 | 10.4–17.3 | 0.024 |
| VG | 14.8 | 12.4–18.4 | ||
| OM | 11.3 | 9.1–15.3 | ||
| Hcys [µmol/L] | VN | 10.4 | 9.7–14.8 | 0.138 |
| VG | 9.9 | 7.6–12.3 | ||
| OM | 10.7 | 9.1–14.4 | ||
| Group | Mean | SD | p-Value | |
| MCV [fl] | VN | 85.4 | 5.1 | 0.001 |
| VG | 80.6 | 5.2 | ||
| OM | 82.1 | 4.0 |
| Vitamin B12 Supplementation in Mothers | Total Number | Percent |
|---|---|---|
| none | 3 | 6.5% |
| only during pregnancy | 1 | 2.2% |
| only during breastfeeding | 6 | 13.0% |
| during pregnancy and breastfeeding | 36 | 78.3% |
| (a) | |||||
| Group | n | Sex | Median Age | IQR | |
| Female | Male | ||||
| supplementing | 33 | 15 | 18 | 1.5 | 0.9–2.0 |
| not supplementing | 13 | 7 | 6 | 0.8 | 0.5–1.8 |
| (b) | |||||
| Variable | Supplementing | Not Supplementing | p-Value | ||
| Median | IQR | Median | IQR | ||
| aB12 [pmol/L] | 157.6 | 96.9–224.7 | 37.5 | 33.0–42.0 | <0.001 |
| B12 [µg/L] | 664.0 | 527.0–863.0 | 264.0 | 232.0–386.0 | <0.001 |
| folate [µg/L] | 18.6 | 16.2–22.2 | 19.2 | 18.2–20.3 | 0.942 |
| Hcys [µmol/L] | 7.0 | 6.0–8.3 | 14.2 | 10.4–20.5 | <0.001 |
| Mean | SD | Mean | SD | ||
| MCV [fl] | 77.3 | 3.5 | 77.8 | 0.665 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Světnička, M.; Sigal, A.; Selinger, E.; Heniková, M.; El-Lababidi, E.; Gojda, J. Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic. Nutrients 2022, 14, 535. https://doi.org/10.3390/nu14030535
Světnička M, Sigal A, Selinger E, Heniková M, El-Lababidi E, Gojda J. Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic. Nutrients. 2022; 14(3):535. https://doi.org/10.3390/nu14030535
Chicago/Turabian StyleSvětnička, Martin, Anat Sigal, Eliška Selinger, Marina Heniková, Eva El-Lababidi, and Jan Gojda. 2022. "Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic" Nutrients 14, no. 3: 535. https://doi.org/10.3390/nu14030535
APA StyleSvětnička, M., Sigal, A., Selinger, E., Heniková, M., El-Lababidi, E., & Gojda, J. (2022). Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic. Nutrients, 14(3), 535. https://doi.org/10.3390/nu14030535









