Colonic Microbiota Profile Characterization of the Responsiveness to Dietary Fibre Treatment in Hypercholesterolemia
Abstract
:1. Introduction
2. Materials and Methods
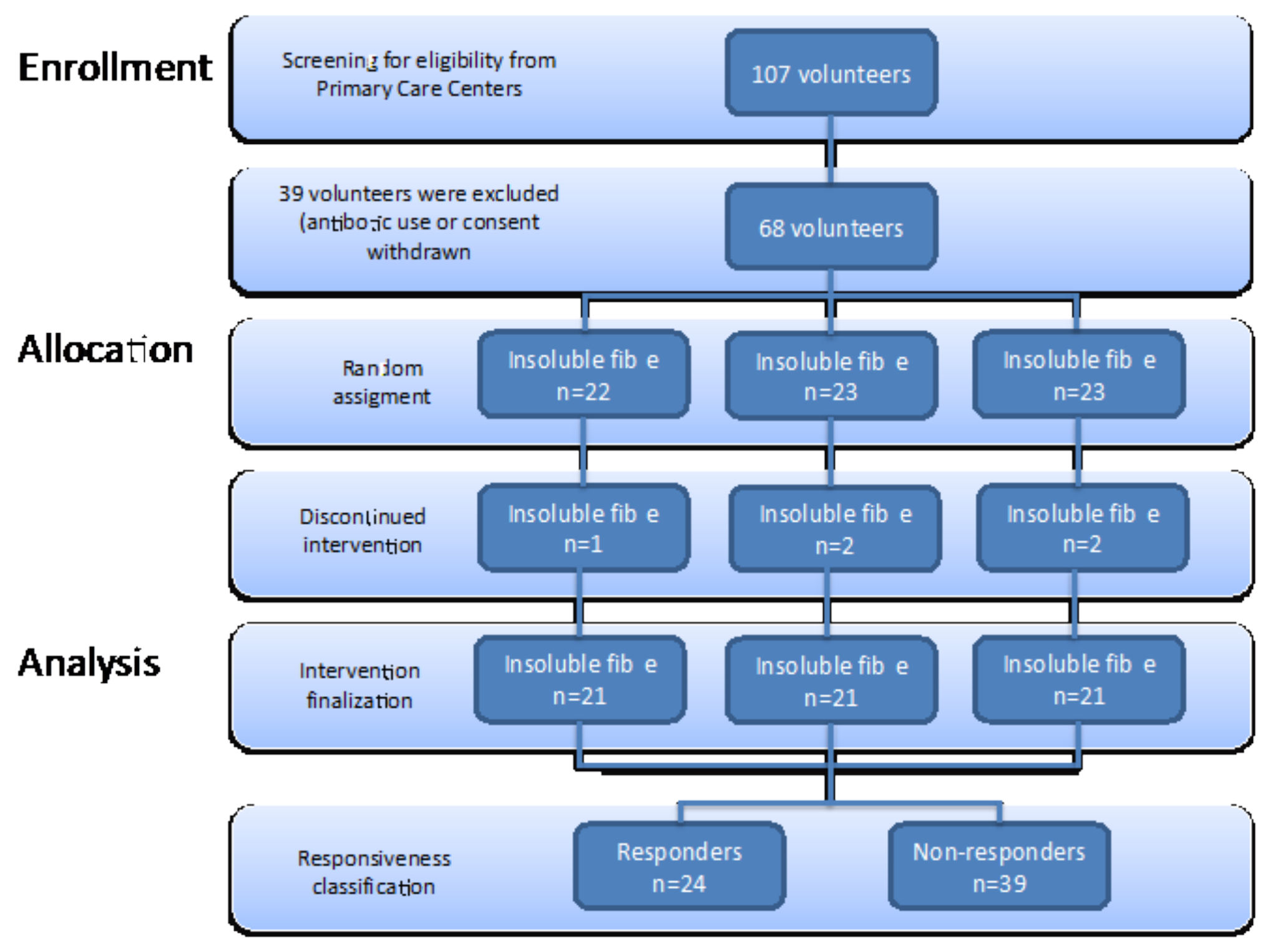
2.1. Study Design
2.2. Sample Size Calculation
2.3. Subjects
2.4. Blood Biochemistry
2.5. Microbial DNA Purification, 16S Amplicon Preparation, Sequencing, and Processing
2.6. Bacterial Analysis from 16S rRNA Data
2.6.1. Species Diversity
2.6.2. Taxonomic Composition of Fecal Microbiota Profile
2.7. Prediction of Functional Metagenome (PICRUSt Analysis)
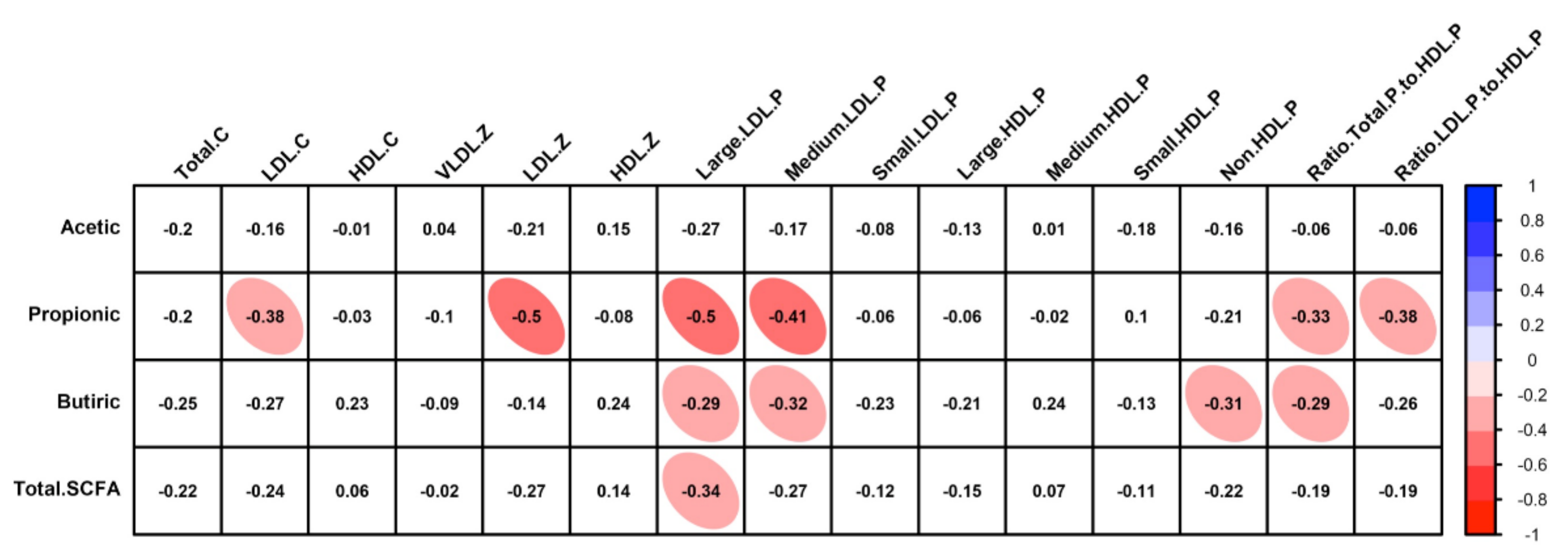
2.8. Faecal Short-Chain Fatty Acids Profile
2.9. Dietary and Nutritional Parameters
2.10. Statistical Analysis
3. Results
3.1. Study Population Characteristics at Baseline
3.2. Global Effects of Dietary Fibre Supplementation in Lipid Profile
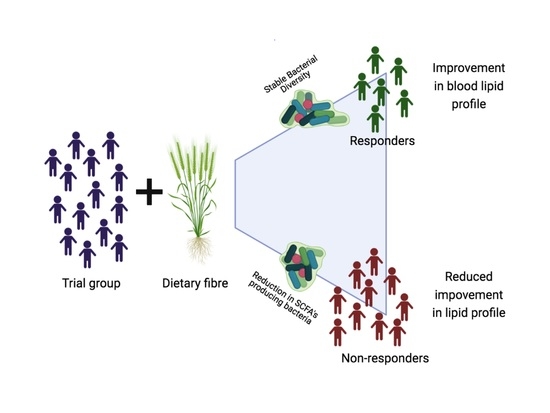
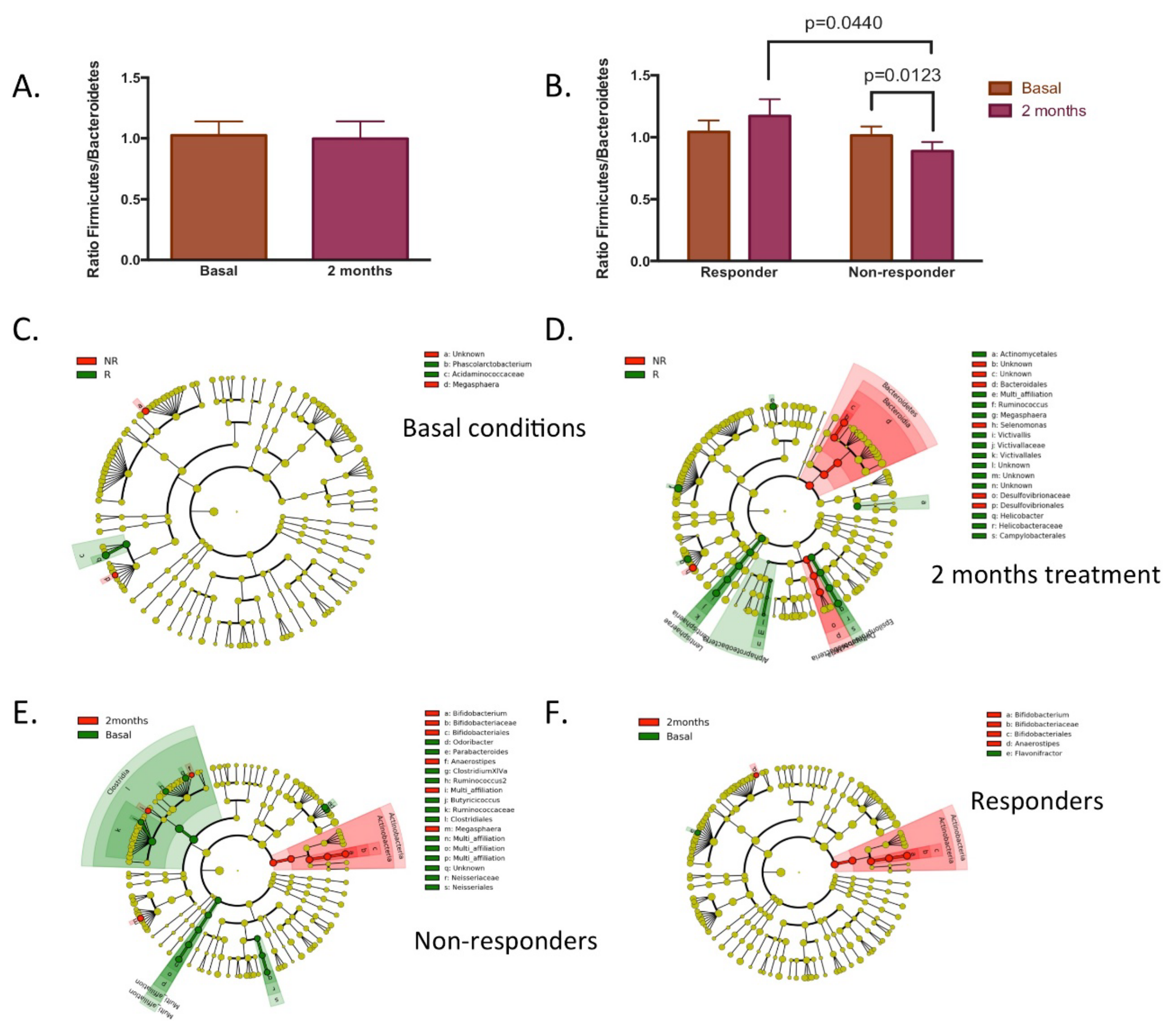
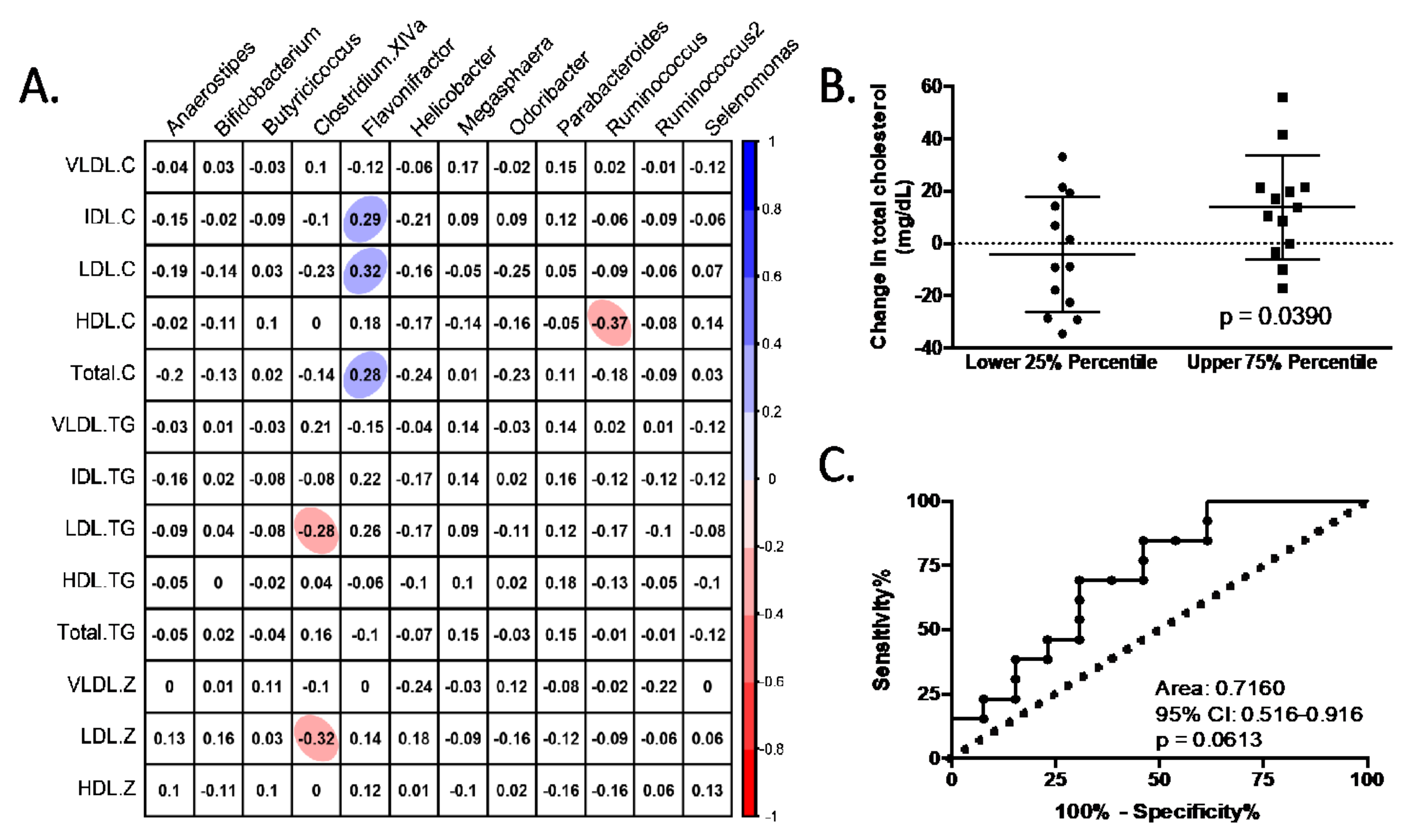
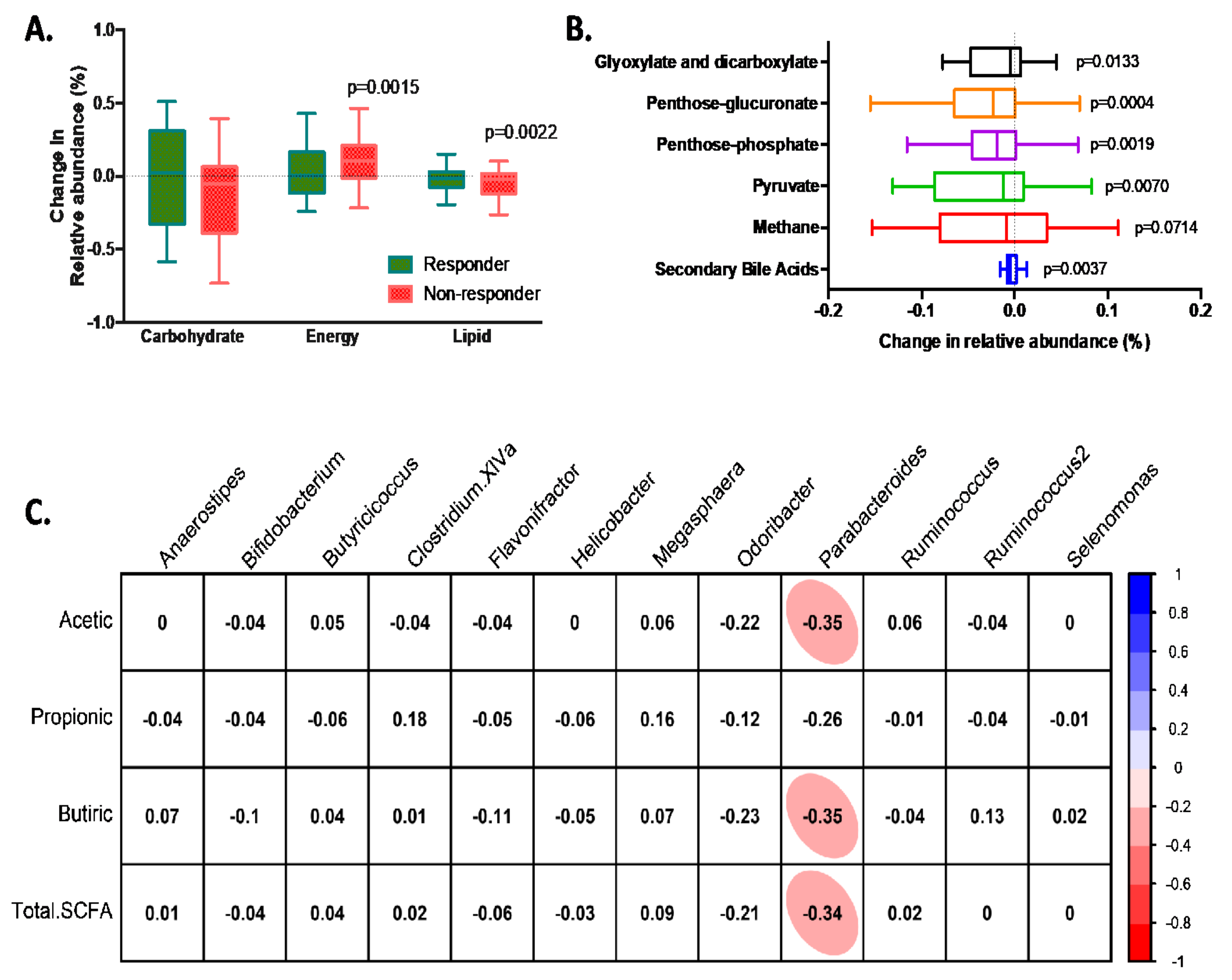
3.3. Responsiveness to Dietary Fibre Treatment Based on Initial Microbiota Profile
3.4. Characteristics of Responders to Dietary Fibre Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozzetto, L.; Costabile, G.; Della Pepa, G.; Ciciola, P.; Vetrani, C.; Vitale, M.; Rivellese, A.A.; Annuzzi, G. Dietary Fibre as a Unifying Remedy for the Whole Spectrum of Obesity-Associated Cardiovascular Risk. Nutrients 2018, 10, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Je, Y. Dietary Fibre Intake and Mortality from Cardiovascular Disease and All Cancers: A Meta-Analysis of Prospective Cohort Studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Hartley, L.; May, M.D.; Loveman, E.; Colquitt, J.L.; Rees, K. Dietary Fibre for the Primary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2016, 2016, CD011472. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.M.; Hartley, L.; Loveman, E.; Colquitt, J.L.; Jones, H.M.; Al-Khudairy, L.; Clar, C.; Germanò, R.; Lunn, H.R.; Frost, G.; et al. Whole Grain Cereals for the Primary or Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2017, 2017, CD005051. [Google Scholar]
- Wang, Z.; Koonen, D.; Hofker, M.; Fu, J. Gut Microbiome and Lipid Metabolism: From Associations to Mechanisms. Curr. Opin. Lipidol. 2016, 27, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal Bacterial and Short-Chain Fatty Acids Signature in Hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.E.; Kim, J.; Kim, M.; Park, E.; Kim, H.-L.; Chang, Y.; Ryu, S.; Kim, H.-N. Major Lipids, Apolipoproteins, and Alterations of Gut Microbiota. J. Clin. Med. 2020, 9, 1589. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual Dietary Fibre Intake Influences Gut Microbiota Response to an Inulin-Type Fructan Prebiotic: A Randomised, Double-Blind, Placebo-Controlled, Cross-over, Human Intervention Study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Buerlein, M.; Frohberg, C.; Landschtze, V.; Gibson, G.R. A Double-Blind, Placebo-Controlled, Cross-over Study to Establish the Bifidogenic Effect of a Very-Long-Chain Inulin Extracted from Globe Artichoke (Cynara scolymus) in Healthy Human Subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Graham, M.; Van Domselaar, G.; Forbes, J.D.; Laminman, V.; Olson, N.; DeGagne, P.; Bray, D.; et al. A Randomized Trial to Determine the Impact of a Digestion Resistant Starch Composition on the Gut Microbiome in Older and Mid-Age Adults. Clin. Nutr. 2018, 37, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.A.; Weggemans, R.M.; Jacobs, D.R.; Hannan, P.J.; Zock, P.L.; Ordovas, J.M.; Katan, M.B. Within-Person Variation in Serum Lipids: Implications for Clinical Trials. Int. J. Epidemiol. 2004, 33, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A Novel Advanced Lipoprotein Test Based on 2D Diffusion-Ordered 1H NMR Spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lluch, J.; Servant, F.; Païssé, S.; Valle, C.; Valière, S.; Kuchly, C.; Vilchez, G.; Donnadieu, C.; Courtney, M.; Burcelin, R.; et al. The Characterization of Novel Tissue Microbiota Using an Optimized 16S Metagenomic Sequencing Pipeline. PLoS ONE 2015, 10, e0142334. [Google Scholar] [CrossRef]
- Saeb, A.T.M.; Al-Rubeaan, K.A.; Aldosary, K.; Udaya Raja, G.K.; Mani, B.; Abouelhoda, M.; Tayeb, H.T. Relative Reduction of Biological and Phylogenetic Diversity of the Oral Microbiota of Diabetes and Pre-Diabetes Patients. Microb. Pathog. 2019, 128, 215–229. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of Short-Chain Fatty Acids in Human Faeces Using High-Performance Liquid Chromatography: Specimen Stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef]
- Mora, S.; Szklo, M.; Otvos, J.D.; Greenland, P.; Psaty, B.M.; Goff, D.C.; O’Leary, D.H.; Saad, M.F.; Tsai, M.Y.; Sharrett, A.R. LDL Particle Subclasses, LDL Particle Size, and Carotid Atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007, 192, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, I.; Turroni, F.; Piemontese, A.; Mancabelli, L.; Milani, C.; Viappiani, A.; Prevedini, G.; Sanchez, B.; Margolles, A.; Elviri, L.; et al. Evidence for Cholesterol-Lowering Activity by Bifidobacterium Bifidum PRL2010 through Gut Microbiota Modulation. Appl. Microbiol. Biotechnol. 2015, 99, 6813–6829. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical Approach for the Management of Cardiovascular Risk—A Combination Containing the Probiotic Bifidobacterium Longum BB536 and Red Yeast Rice Extract: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Xiao, S.; Fei, N.; Pang, X.; Shen, J.; Wang, L.; Zhang, B.; Zhang, M.; Zhang, X.; Zhang, C.; Li, M.; et al. A Gut Microbiota-Targeted Dietary Intervention for Amelioration of Chronic Inflammation Underlying Metabolic Syndrome. FEMS Microbiol. Ecol. 2014, 87, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.K.; Brunius, C.; Mazidi, M.; Hellström, P.M.; Risérus, U.; Iversen, K.N.; Fristedt, R.; Sun, L.; Huang, Y.; Nørskov, N.P.; et al. Effects of Whole-Grain Wheat, Rye, and Lignan Supplementation on Cardiometabolic Risk Factors in Men with Metabolic Syndrome: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 864–876. [Google Scholar] [CrossRef]
- Lappi, J.; Salojärvi, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K.; de Vos, W.M.; Salonen, A. Intake of Whole-Grain and Fiber-Rich Rye Bread versus Refined Wheat Bread Does Not Differentiate Intestinal Microbiota Composition in Finnish Adults with Metabolic Syndrome. J. Nutr. 2013, 143, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Ampatzoglou, A.; Atwal, K.K.; Maidens, C.M.; Williams, C.L.; Ross, A.B.; Thielecke, F.; Jonnalagadda, S.S.; Kennedy, O.B.; Yaqoob, P. Increased Whole Grain Consumption Does Not Affect Blood Biochemistry, Body Composition, or Gut Microbiology in Healthy, Low-Habitual Whole Grain Consumers. J. Nutr. 2015, 145, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting Whole Grains for Refined Grains in a 6-WK Randomized Trial Has a Modest Effect on Gut Microbiota and Immune and Inflammatory Markers of Healthy Adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [Green Version]
- De Preter, V.; Vanhoutte, T.; Huys, G.; Swings, J.; Rutgeerts, P.; Verbeke, K. Baseline Microbiota Activity and Initial Bifidobacteria Counts Influence Responses to Prebiotic Dosing in Healthy Subjects. Aliment. Pharmacol. Ther. 2008, 27, 504–513. [Google Scholar] [CrossRef]
- Griffin, N.W.; Ahern, P.P.; Cheng, J.; Heath, A.C.; Ilkayeva, O.; Newgard, C.B.; Fontana, L.; Gordon, J.I. Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe 2017, 21, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Zhang, C.; Wu, H.; Wang, R.; Shen, J.; Wang, L.; Zhao, Y.; Pang, X.; Zhang, X.; Zhao, L.; et al. Genomic Microdiversity of Bifidobacterium Pseudocatenulatum Underlying Differential Strain-Level Responses to Dietary Carbohydrate Intervention. MBio 2017, 8, e02348-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of pH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host Variables Confound Gut Microbiota Studies of Human Disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Bacteroides (n = 16) | Prevotella (n = 7) | Difference between Microbiota Profile | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Two-months | |||||||
| Baseline | Two-months | p-value | Baseline | Two-months | p-value | p-value | p-value | |
| Total cholesterol (mg/dL) | 212.5 (195.8–229.3) | 210.0 (195.2–224.7) | 0.6656 | 209.6 (194.5–224.6) | 212.1 (189.6–234.6) | 0.7605 | 0.8072 | 0.0749 |
| VLDL-Cholesterol (mg/dL) | 14.0 (8.8–19.2) | 12.8 (8.3–17.3) | 0.5313 | 11.3 (4.9–17.8) | 17.0 (6.2–27.8) | 0.3040 | 0.5131 | 0.3436 |
| IDL-Cholesterol (mg/dL) | 11.8 (9.8–13.8) | 11.1 (9.0–13.3) | 0.2762 | 10.6 (6.9–14.3) | 12.9 (9.5–16.3) | 0.6639 | 0.5068 | 0.3211 |
| LDL-Cholesterol (mg/dL) | 128.3 (115.7–140.9) | 127.7 (116.7–138.7) | 0.8778 | 132.9 (123.9–141.9) | 131.2 (115.6–146.8) | 0.7630 | 0.6106 | 0.6916 |
| HDL-Cholesterol (mg/dL) | 58.4 (53.0–63.9) | 58.3 (53.1–63.6) | 0.9530 | 54.7 (49.0–60.3) | 51.0 (44.9–57.0) | 0.0012 | 0.3607 | 0.0749 |
| Total Triacylglycerides (mg/dL) | 116.6 (88.3–144.9) | 110.8 (87.1–134.4) | 0.5136 | 96.8 (69.0–124.6) | 130.0 (73.8–186.1) | 0.2240 | 0.3487 | 0.4095 |
| VLDL-Triacylglycerides (mg/dL) | 72.1 (48.1–96.0) | 68.7 (49.7–87.7) | 0.6597 | 55.9 (33.0–78.7) | 84.3 (34.3–134.3) | 0.2416 | 0.3621 | 0.4288 |
| IDL-Triacylglycerides (mg/dL) | 12.3 (10.7–14.0) | 11.6 (9.9–13.4) | 0.1627 | 10.9 (8.1–13.6) | 12.4 (10.1–14.7) | 0.1828 | 0.2948 | 0.6018 |
| LDL-Triacylglycerides (mg/dL) | 16.3 (13.7–19.0) | 15.7 (13.4–18.0) | 0.3857 | 17.1 (14.4–19.8) | 17.7 (14.1–21.4) | 0.6015 | 0.6999 | 0.2804 |
| HDL-Triacylglycerides (mg/dL) | 15.8 (13.0–18.6) | 14.8 (12.5–17.0) | 0.1446 | 12.9 (10.2–15.6) | 15.5 (10.6–20.5) | 0.2854 | 0.1759 | 0.7149 |
| Particle size | ||||||||
| VLDLz | 42.77 (42.45–43.08) | 42.56 (42.30–42.81) | 0.0712 | 42.64 (42.17–43.11) | 42.65 (42.15–43.15) | 0.9707 | 0.6263 | 0.6704 |
| LDLz | 21.11 (21.03–21.20) | 21.08 (21.01–21.15) | 0.4079 | 21.09 (20.94–21.24) | 20.96 (20.81–21.11) | 0.0070 | 0.7213 | 0.0714 |
| HDLz | 8.19 (8.18–8.22) | 8.20 (8.18–8.22) | 0.8589 | 8.20 (8.16–8.24) | 8.18 (8.15–8.21) | 0.2073 | 0.7481 | 0.2833 |
| Faeces short-chain fatty acids | ||||||||
| Total (mmol/g faeces) | 40.7 (27.0–54.4) | 46.1 (26.8–65.4) | 0.4804 | 94.5 (56.9–132.1) | 106.4 (70.8–142.0) | 0.8718 | 0.0008 | 0.0015 |
| % Acetic | 60.9 (57.4–64.5) | 59.5 (56.2–62.8) | 0.3210 | 58.9 (51.2–66.5) | 54.9 (50.4–59.4) | 0.0908 | 0.5302 | 0.0852 |
| % Propionic | 20.1 (17.1–23.0) | 20.2 (16.9–23.4) | 0.9082 | 19.8 (16.6–23.0) | 24.4 (21.7–27.2) | 0.0013 | 0.9123 | 0.0806 |
| % Butyric | 16.7 (13.4–20.0) | 18.4 (15.4–21.5) | 0.3836 | 19.0 (13.8–24.1) | 18.9 (15.5–22.4) | 0.8801 | 0.4134 | 0.8342 |
| Diversity Index | Basal | 2 Months | p-Value |
|---|---|---|---|
| All volunteers | |||
| OTUs observed | 392.2 (373.9–410.5) | 384.6 (367.5–401.7) | 0.3062 |
| Chao1 | 442.1 (423.1–461.1) | 432.8 (413.8–451.7) | 0.2395 |
| Se.Chao1 | 16.4 (15.1–17.6) | 15.9 (14.9–16.8) | 0.5761 |
| Shannon | 4.01 (3.89–4.12) | 3.83 (3.68–3.98) | 0.0112 |
| Simpson | 0.944 (0.936–0.953) | 0.924 (0.907–0.942) | 0.0109 |
| Inv.Simpson | 23.5 (20.2–26.8) | 20.4 (17.4–23.3) | 0.1280 |
| Responders | |||
| OTUs observed | 391.9 (361.6–422.1) | 387.4 (357.7–417.1) | 0.6844 |
| Chao1 | 448.3 (417.2–479.5) | 434.0 (400.4–467.6) | 0.2748 |
| Se.Chao1 | 18.4 (16.1–20.6) | 15.5 (13.8–17.2) | 0.0684 |
| Shannon | 3.98 (3.79–4.16) | 3.92 (3.67–4.16) | 0.5135 |
| Simpson | 0.943 (0.929–0.957) | 0.930 (0.901–0.960) | 0.2363 |
| Inv.Simpson | 22.2 (16.7–27.7) | 20.9 (16.1–25.76) | 0.6644 |
| Non-responders | |||
| OTUs observed | 392.5 (368.1–416.8) | 382.8 (360.9–404.8) | 0.3437 |
| Chao1 | 438.1 (412.8–463.3) | 432.0 (408.8–455.9) | 0.5456 |
| Se.Chao1 | 15.1 (13.6–16.6) | 16.1 (15.0–17.2) | 0.2752 |
| Shannon | 4.03 (3.88–4.18) | 3.77 (3.57–3.97) | 0.0110 |
| Simpson | 0.945 (0.933–0.957) | 0.921 (0.898–0.943) | 0.0255 |
| Inv.Simpson | 24.3 (20.0–28.6) | 20.0 (16.0–23.9) | 0.1269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Lafarga Giribets, A.; Berdún, R.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Colonic Microbiota Profile Characterization of the Responsiveness to Dietary Fibre Treatment in Hypercholesterolemia. Nutrients 2022, 14, 525. https://doi.org/10.3390/nu14030525
Granado-Serrano AB, Martín-Garí M, Sánchez V, Riart Solans M, Lafarga Giribets A, Berdún R, Vilaprinyó E, Portero-Otín M, Serrano JCE. Colonic Microbiota Profile Characterization of the Responsiveness to Dietary Fibre Treatment in Hypercholesterolemia. Nutrients. 2022; 14(3):525. https://doi.org/10.3390/nu14030525
Chicago/Turabian StyleGranado-Serrano, Ana Belen, Meritxell Martín-Garí, Virginia Sánchez, Marissa Riart Solans, Antonia Lafarga Giribets, Rebeca Berdún, Ester Vilaprinyó, Manuel Portero-Otín, and José C. E. Serrano. 2022. "Colonic Microbiota Profile Characterization of the Responsiveness to Dietary Fibre Treatment in Hypercholesterolemia" Nutrients 14, no. 3: 525. https://doi.org/10.3390/nu14030525
APA StyleGranado-Serrano, A. B., Martín-Garí, M., Sánchez, V., Riart Solans, M., Lafarga Giribets, A., Berdún, R., Vilaprinyó, E., Portero-Otín, M., & Serrano, J. C. E. (2022). Colonic Microbiota Profile Characterization of the Responsiveness to Dietary Fibre Treatment in Hypercholesterolemia. Nutrients, 14(3), 525. https://doi.org/10.3390/nu14030525