Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Study Design
2.4. Vitamin D Measurement
2.5. COVID-19 Lockdown and Home-Based Training
2.6. Procedures
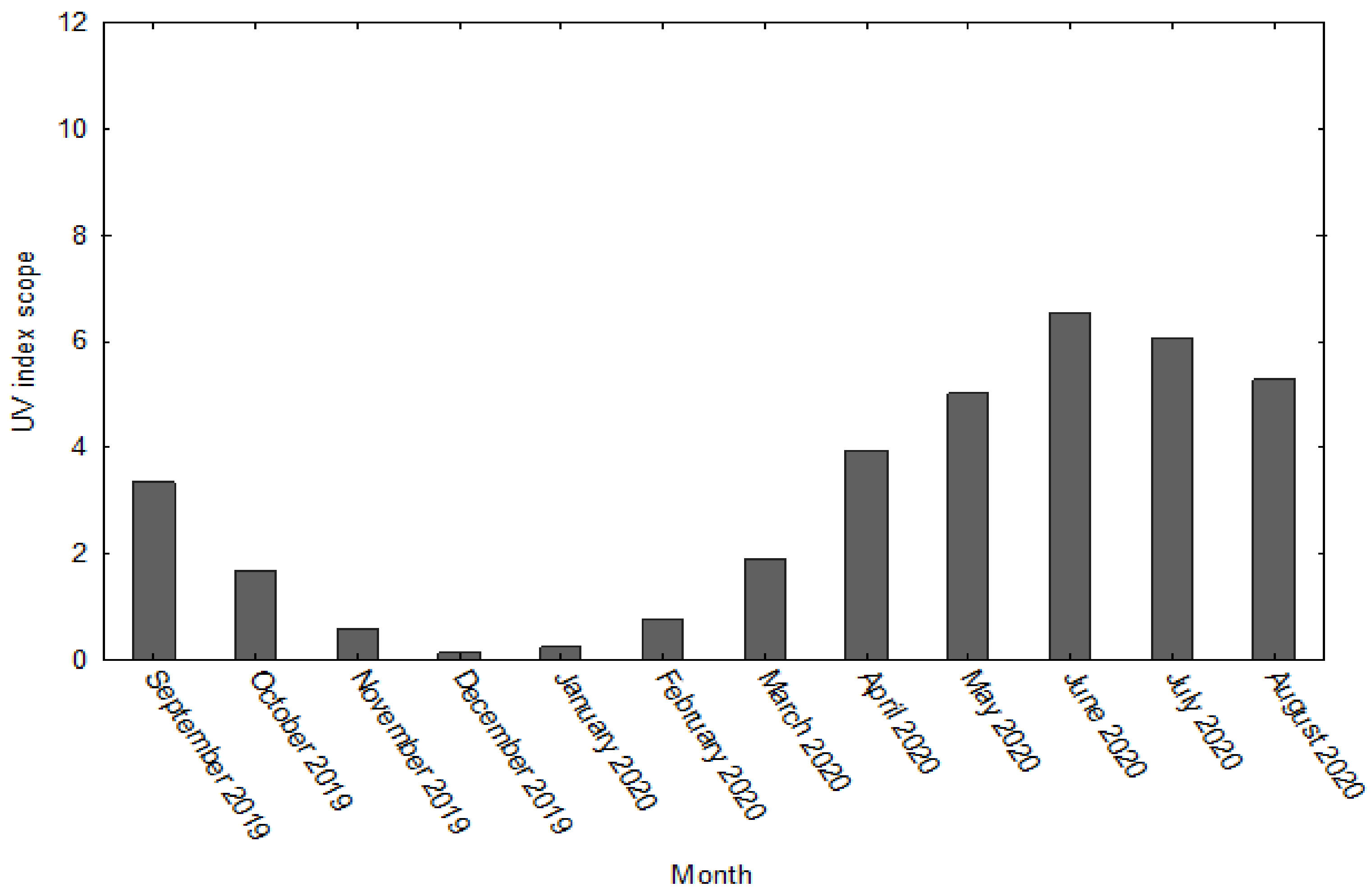
2.6.1. Degree of UV Radiation
2.6.2. Determination of 25(OH)D, Calcium (Ca), Phosphorus (P), and Parathyroid Hormone (PTH) Concentration in Blood Plasma
2.6.3. Calculation of Average Vitamin D Intake
2.6.4. Supplementation of Vitamin D
2.6.5. PACER (Progressive Aerobic Cardiovascular Endurance Run) Test
2.6.6. Sprint Test
2.6.7. Explosive Power Measurement
2.7. Statistical Analyses
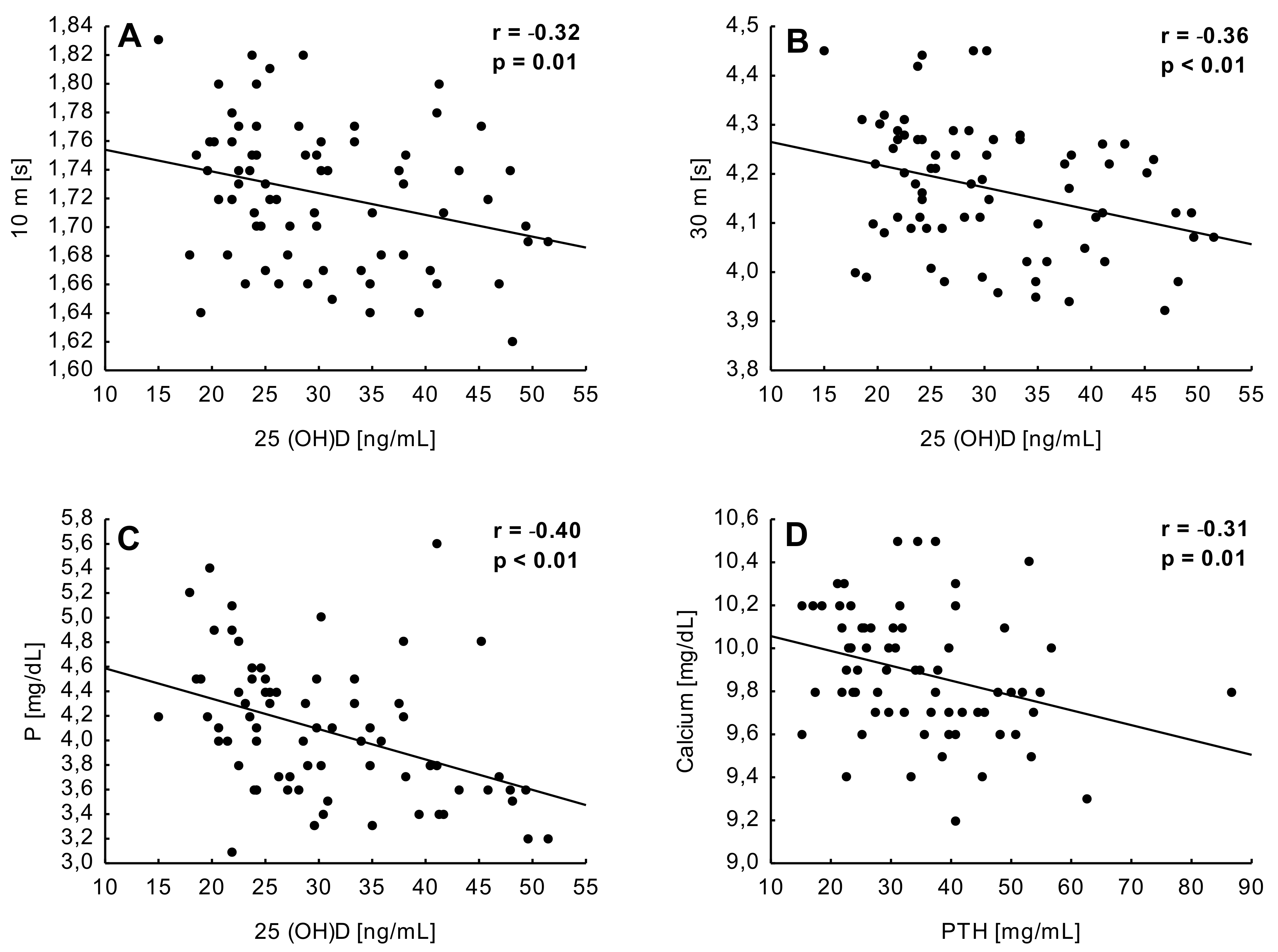
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F.; Binkley, N.C.; Bischof-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes-A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiciński, M.; Adamkiewicz, D.; Adamkiewicz, M.; Śniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of Vitamin D on Physical Efficiency and Exercise Performance-A Review. Nutrients 2019, 11, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trybek, G.; Aniko-Włodarczyk, M.; Kwiatek, J.; Preuss, O.; Brodkiewicz, A.; Sinicyn, A.; Grzywacz, A. The effect of vitamin D3 on the osteointegration of dental implant. Balt. J. Health Phys. Activ. 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Lai, Y.H.; Fang, T.C. The pleiotropic effect of vitamin D. ISRN Nephrol. 2013, 898125. [Google Scholar] [CrossRef]
- Grants, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast. Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020, 40, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopeć, A.; Solarz, K.; Majda, F.; Słowińska-Lisowska, M.; Mędraś, M. An evaluation of the levels of vitamin d and bone turnover markers after the summer and winter periods in polish professional soccer players. J. Hum. Kinet. 2013, 38, 135–140. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Gołaś, A.; Maszczyk, A.; Kaczka, P.; Zając, A. Influence of Sunlight and Oral D3 Supplementation on Serum 25(OH)D Concentration and Exercise Performance in Elite Soccer Players. Nutrients 2020, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Solarz, K.; Kopeć, A.; Pietraszewska, J.; Majda, F.; Słowińska-Lisowska, M.; Mędraś, M. An evaluation of the levels of 25-hydroxyvitamin D3 and bone turnover markers in professional football players and in physically inactive men. Physiol. Res. 2014, 63, 237–243. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Lyubushkina, A.; Achkasov, E.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Nikolaidis, P.T.; et al. The Dependence of Running Speed and Muscle Strength on the Serum Concentration of Vitamin D in Young Male Professional Football Players Residing in the Russian Federation. Nutrients 2019, 11, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Rosemann, T.; Nikolaidis, P.; Knechtle, B. Prevalence and Treatment of Vitamin D Deficiency in Young Male Russian Soccer Players in Winter. Nutrients 2019, 11, 2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson-Barnes, S.L.; Hunt, J.E.A.; Williams, E.L.; Allison, S.J.; Wild, J.J.; Wainwright, J.; Lanham-New, S.A.; Manders, R.J.F. Seasonal variation in vitamin D status, bone health and athletic performance in competitive university student athletes: A longitudinal study. J. Nutr. Sci. 2020, 9, e8. [Google Scholar] [CrossRef] [Green Version]
- Quadri, A.; Gojanovic, B.; Noack, P.; Fuhrer, C.; Steuer, C.; Huber, A.; Kriemler, S. Seasonal variation of vitamin D levels in Swiss athletes. SEMS-J. 2019. [Google Scholar] [CrossRef]
- Maruyama-Nagao, A.; Sakuraba, K.; Suzuki, Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed. Rep. 2016, 5, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Skalska, M.; Nikolaidis, P.T.; Knechtle, B.; Rosemann, T.J.; Radzimiński, Ł.; Jastrzębska, J.; Kaczmarczyk, M.; Myśliwiec, A.; Dragos, P.; López-Sánchez, G.F.; et al. Vitamin D Supplementation and Physical Activity of Young Soccer Players during High-Intensity Training. Nutrients 2019, 11, 349. [Google Scholar] [CrossRef] [Green Version]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Girgis, C.M.; Clifton-Bligh, R.J.; Turner, N.; Lau, S.L.; Gunton, J.E. Effects of vitamin D in skeletal muscle: Falls, strength, athletic performance and insulin sensitivity. Clin. Endocrinol. 2014, 80, 169–181. [Google Scholar] [CrossRef]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef]
- Schuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports Health Benefits of Vitamin, D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Bartoszewska, M.; Kamboj, M.; Patel, D.R. Vitamin D, muscle function, and exercise performance. Pediatr. Clin. N. Am. 2010, 57, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeani, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of vitamin D inadequacy in athletes: A systematic review and meta-analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrzębska, M.; Kaczmarczyk, M.; Michalczyk, M.; Radzimiński, Ł.; Stępień, P.; Jastrzębska, J.; Wakuluk, D.; Suárez, A.D.; López Sánchez, G.F.; Cięszczyk, P.; et al. Can Supplementation of Vitamin D Improve Aerobic Capacity in Well Trained Youth Soccer Players? J. Hum. Kinet. 2018, 61, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and exercise performance in professional soccer players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef]
- Valtuena, J.; Gracia-Marco, L.; Huybrechts, I.; Breidenassel, C.; Ferrari, M.; Gottrand, F.; Dallongeville, J.; Sioen, I.; Gutierrez, A.; Kersting, M.; et al. Cardiorespiratory fitness in males, and upper limbs muscular strength in females, are positively related with 25-hydroxyvitamin D plasma concentrations in European adolescents: The HELENA study. QJM. 2013, 106, 809–821. [Google Scholar] [CrossRef] [Green Version]
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The effects of vitamin D(3) supplementation on serum total 25[OH]D concentration and physical performance: A randomized dose-response study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef]
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J. Sci. Med. Sport. 2014, 17, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Dziubek, W.; Pietraszewski, B.; Ochmann, B.; Słowińska-Lisowka, M. 25(OH)D3 Levels Relative to Muscle Strength and Maximum Oxygen Uptake in Athletes. J. Hum. Kinet. 2016, 50, 71–77. [Google Scholar] [CrossRef]
- Montenegro, K.R.; Cruzat, V.; Melder, H.; Jacques, A.; Newsholme, P.; Ducker, K.J. Vitamin D Supplementation Does Not Impact Resting Metabolic Rate, Body Composition and Strength in Vitamin D Sufficient Physically Active Adults. Nutrients 2020, 12, 3111. [Google Scholar] [CrossRef]
- Radzimiński, Ł.; Jastrzębski, Z.; López-Sanchez, G.F.; Szwarc, A.; Duda, H.; Stuła, A.; Paszulewicz, J.; Dragos, P. Relationships between training loads and selected blood parameters in professional soccer players during a 12-day sports camp. Int. J. Environ. Res. Public Health 2020, 17, 8580. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Tubagi Polito, L.F.; Clemente, F.M.; Pérez-Gómez, J.; Ahmadi, M.; Garcia-Gordillo, M.Á.; Silva, A.F.; Adsuar, J.C. Relationships between Training Workload Parameters with Variations in Anaerobic Power and Change of Direction Status in Elite Youth Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 7934. [Google Scholar] [CrossRef]
- Fessi, M.S.; Zarrouk, N.; Filetti, C.; Rebai, H.; Elloumi, M.; Moalla, W. Physical and anthropometric changes during pre- and in season in professional soccer players. J. Sports Med. Phys. Fit. 2016, 56, 1163–1170. [Google Scholar]
- Radzimiński, Ł.; Padrón-Cabo, A.; Konefał, M.; Chmura, P.; Szwarc, A.; Jastrzębski, Z. The influence of COVID-19 lockdown on the physical match performance of the professional soccer players: An example of German and Polish leagues. Int. J. Environ. Res. Public Health. 2021, 18, 8796. [Google Scholar] [CrossRef] [PubMed]
- Rampinini, E.; Donghi, F.; Martin, M.; Bosio, A.; Riggio, M.; Maffiuletti, N.A. Impact of COVID-19 Lockdown on Serie A Soccer Players’ Physical Qualities. Int. J. Sports Med. 2021, 42, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, R.; Loturco, I.; Baroni, B.M.; Oliveira, G.S.; Saciura, V.; Vanoni, E.; Dias, R.; Veeck, F.; Pinto, R.S.; Cadore, E.L. Coronavirus Disease-19 Quarantine Is More Detrimental Than Traditional Off-Season on Physical Conditioning of Professional Soccer Players. J. Strength Cond. Res. 2020, 34, 3316–3320. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2021, 79, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef] [PubMed]
- Weather Online. Available online: https://www.weatheronline.co.uk/Poland/Gdynia/UVindex.htm (accessed on 20 January 2022).
- Leger, L.A.; Lambert, J. A maximal multistage 20m shuttle run test to predict VO2 max. Eur. J. Appl. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe-Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin, D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydın, C.G.; Dinçel, Y.M.; Arıkan, Y.; Taş, S.K.; Deniz, S. The effects of indoor and outdoor sports participation and seasonal changes on vitamin D levels in athletes. SAGE Open Med. 2019, 7, 2050312119837480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, L.Y.; Wortsman, J.; Dannenberg, M.J.; Hollis, B.W.; Lu, Z.; Holick, M.F. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J. Clin. Endocrinol. Metab. 1992, 75, 1099–1103. [Google Scholar] [CrossRef]
- Tuchendler, D.; Bolanowski, M. Sezonowość zmian stężeń witaminy D w organizmie człowieka (Seasonal variations in serum vitamin D concentrations in human). Endokrynol. Otyłość I Zaburzenia Przemiany Materii. 2010, 6, 36–41. [Google Scholar]
- Valtueña, J.; Dominguez, D.; Til, L.; González-Gross, M.; Drobnic, F. High prevalence of vitamin D insufficiency among elite Spanish athletes the importance of outdoor training adaptation. Nutr. Hosp. 2014, 30, 124–131. [Google Scholar] [CrossRef]
- Kuchuk, N.O.; van Schoor, N.M.; Pluijm, S.M.; Chines, A.; Lips, P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: Global perspective. J. Bone Miner. Res. 2009, 24, 693–701. [Google Scholar] [CrossRef]
- Napiórkowska, L.; Budlewski, T.; Jakubas-Kwiatkowska, W.; Hamzy, V.; Gozdowski, D.; Franek, E. Prevalence of low serum vitamin D concentration in an urban population of elderly women in Poland. Pol. Arch. Med. Wewn. 2009, 119, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Ritz, C.; Kiely, M. Odin Collaborators. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients. 2017, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Brustad, M.; Edvardsen, K.; Wilsgaard, T.; Engelsen, O.; Aksnes, L.; Lund, E. Seasonality of UV-radiation and vitamin D status at 69 degrees north. Photochem. Photobiol. Sci. 2007, 6, 903–908. [Google Scholar] [CrossRef]
- Jurek, J.M.; Brzeziański, M.; Brzeziańska-Lasota, E. Rola witaminy D u sportowców (A role of vitamin D in athletes). Medycyna Sportowa / Polish J. Sport Med. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front. Endocrinol. (Lausanne) 2018, 9, 246. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; Rockwell, M.S. The Benefits of Vitamin D Supplementation for Athletes: Better Performance and Reduced Risk of COVID-19. Nutrients 2020, 12, 3741. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. The effect of vitamin d supplementation on training adaptation in well trained soccer players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Mack, D.; Donde, K.; Harzer, O.; Krutsch, W.; Rössler, A.; Kimpel, J.; Von Laer, D.; Gärtner, B.C. Successful return to professional men’s football (soccer) competition after the COVID-19 shutdown: A cohort study in the German Bundesliga. Br. J. Sports Med. 2021, 55, 62–66. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D epidemic and its health consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Reichrath, J. The challenge resulting from positive and negative effects of sunlight: How much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog. Biophys. Mol. Biol. 2006, 92, 9–16. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [Green Version]
- Gilic, B.; Kosor, J.; Jimenez-Pavon, D.; Markic, J.; Karin, Z.; Domic, D.S.; Sekulic, D. Associations of Vitamin D Levels with Physical Fitness and Motor Performance; A Cross-Sectional Study in Youth Soccer Players from Southern Croatia. Biology 2021, 10, 751. [Google Scholar] [CrossRef]
- Fitzgerald, J.S.; Peterson, B.J.; Warpeha, J.M.; Wilson, P.B.; Rhodes, G.S.; Ingraham, S.J. Vitamin D status and VO2peak during a skate treadmill graded exercise test in competitive ice hockey players. J. Strength Cond. Res. 2014, 28, 3200–3205. [Google Scholar] [CrossRef]
- Forney, L.A.; Earnest, C.P.; Henagan, T.M.; Johnson, L.E.; Castleberry, T.J.; Stewart, L.K. Vitamin D status, body composition, and fitness measures in college-aged students. J. Strength Cond. Res. 2014, 28, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Galan, F.; Ribas, J.; Sánchez-Martinez, P.M.; Calero, T.; Sánchez, A.B.; Muñoz, A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin. Nutr. 2012, 31, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lorenzo, V. Parathyroid hormone, a uremic toxin. Semin. Dial. 2009, 22, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Sultan, C. Effects of physical activity on bone remodeling. Metabolism 2011, 60, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Colombini, A.; Freschi, M.; Tavana, R.; Banfi, G. Seasonal variation of bone turnover markers in top-level female skiers. Eur. J. Appl. Physiol. 2011, 111, 433–440. [Google Scholar] [CrossRef]

| Competition Period | ||
|---|---|---|
| Day of the Week | Training Drills | |
| Morning | Afternoon | |
| Monday | endurance, technical, tactical | Free |
| Tuesday | speed, technical, small-sided games | individual training (formation) |
| Wednesday | stretching, regeneration | individual training (formation) |
| Thursday | plyometric and speed, technical, tactical | individual training (formation) |
| Friday | coordination, technical, tactical | Free |
| Saturday | Competition game | |
| Sunday | Free day | |
| Preparation period | ||
| Day of the week | Training drills | |
| Morning | Afternoon | |
| Monday | endurance, technical, tactical | fitness, strength |
| Thursday | speed, technical, small-sided games | individual training (formation) |
| Wednesday | coordination, strength | individual training (formation) |
| Thursday | endurance, technical, tactical | technical, small-sided games |
| Friday | coordination, tactical | free |
| Saturday | Friendly game | |
| Sunday | Free day (regeneration) | |
| Time-Point | T1 | T2 | T3 | T4 | ICC | α |
|---|---|---|---|---|---|---|
| 25(OH)D (ng/mL) | 35.0 ± 6.26 | 24.5 ± 4.89 * | 26.4 ± 15.32 * | 40.5 ± 6.86 *,#,† | 0.38 | 0.81 |
| Ca (mg/dL) | 9.5 ± 0.28 | 10.0 ± 0.24 * | 9.8 ± 0.29 *,# | 9.8 ± 0.30 * | 0.62 | 0.82 |
| P (mg/dL) | 3.8 ± 0.47 | 4.3 ± 0.53 * | 4.1 ± 0.52 | 3.9 ± 0.58 | 0.73 | 0.79 |
| PTH (mg/mL) | 39.3 ± 15.99 | 32.4 ± 15.86 * | 39.8 ± 10.89 # | 32.8 ± 11.06 † | 0.81 | 0.85 |
| PACER (m) | 2379 ± 174.9 | 2473 ± 201.1 | 2473 ± 241.6 | 2423 ± 173.6 | 0.92 | 0.94 |
| VO2max (ml/kg/min) | 57.6 ± 2.62 | 58.7 ± 2.89 | 58.7 ± 3.35 | 58.1 ± 2.26 | 0.92 | 0.94 |
| 10m (s) | 1.75 ± 0.05 | 1.73 ± 0.05 | 1.73 ± 0.05 | 1.71 ± 0.06 * | 0.81 | 0.84 |
| 30m (s) | 4.24 ± 0.13 | 4.21 ± 0.15 | 4.19 ± 0.11 | 4.12 ± 0.12 * | 0.89 | 0.95 |
| SJ (cm) | 38.0 ± 3.68 | 37.9 ± 4.30 | 37.9 ± 3.47 | 38.0 ± 3.92 | 0.88 | 0.87 |
| CMJ (cm) | 45.9 ± 3.30 | 44.2 ± 4.85 | 44.6 ± 3.93 | 45.9 ± 3.65 | 0.88 | 0.92 |
| 10 jumps (cm) | 40.7 ± 3.24 | 39.4 ± 3.89 | 39.4 ± 3.29 | 40.4 ± 2.78 | 0.85 | 0.86 |
| Group | GS | ICC | α | GP | ICC | α | Inter-Actions | p | pη2 | OP | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time-Point | T2 | T3 | T4 | T2 | T3 | T4 | ||||||||
| 25(OH)D (ng/mL) | 24.1 ± 5.23 | 26.6 ± 6.35 | 41.7 ± 6.95 *,† | 0.18 | 0.76 | 24.8 ± 4.77 | 26.2 ± 4.52 | 39.5 ± 6.91 *,† | 0.11 | 0.69 | time | <0.001 | 0.82 | 1.00 |
| Ca (mg/dL) | 10.0 ± 0.32 | 9.8 ± 0.30 * | 9.9 ± 0.31 | 0.74 | 0.79 | 10.0 ± 0.15 | 9.8 ± 0.28 * | 9.7 ± 0.28 * | 0.59 | 0.75 | time | 0.001 | 0.27 | 0.93 |
| P (mg/dL) | 4.31 ± 0.52 | 4.13 ± 0.57 | 4.03 ± 0.79 | 0.78 | 0.81 | 4.23 ± 0.55 | 3.99 ± 0.49 | 3.83 ± 0.32 * | 0.58 | 0.68 | time | 0.012 | 0.18 | 0.78 |
| PTH (ng/mL) | 33.9 ± 11.64 | 41.0 ± 11.83 * | 31.8 ± 7.74 † | 0.71 | 0.81 | 31.0 ± 19.11 | 38.8 ± 10.41 * | 33.5 ± 13.54 | 0.77 | 0.83 | time | 0.003 | 0.23 | 0.88 |
| PACER (m) | 2518 ± 145.5 | 2491 ± 188.8 | 2380 ± 138.9 * | 0.84 | 0.94 | 2434 ± 237.4 | 2459 ± 285.8 | 2456 ± 196.6 | 0.90 | 0.92 | group × time | 0.011 | 0.19 | 0.79 |
| VO2max (ml/kg/min) | 59.2 ± 1.91 | 59.0 ± 2.60 | 57.7 ± 1.57 *,† | 0.78 | 0.91 | 58.4 ± 3.54 | 58.5 ± 3.98 | 58.4 ± 2.73 | 0.89 | 0.91 | - | - | - | - |
| 10m (s) | 1.75 ± 0.04 | 1.76 ± 0.04 | 1.72 ± 0.06 *,† | 0.56 | 0.66 | 1.71 ± 0.05 | 1.71 ± 0.04 | 1.71 ± 0.06 | 0.74 | 0.78 | - | - | - | - |
| 30m (s) | 4.24 ± 0.17 | 4.24 ± 0.11 | 4.15 ± 0.12 *,† | 0.76 | 0.89 | 4.18 ± 0.13 | 4.14 ± 0.10 | 4.08 ± 0.11 *,† | 0.82 | 0.93 | time | <0.001 | 0.42 | 0.99 |
| SJ (cm) | 36.9 ± 3.59 | 37.9 ± 3.39 | 37.3 ± 3.95 | 0.72 | 0.80 | 38.9 ± 4.76 | 37.9 ± 3.68 | 38.5 ± 3.95 | 0.82 | 0.86 | - | - | - | - |
| CMJ (cm) | 43.7 ± 4.91 | 44.2 ± 3.66 | 45.6 ± 4.03 | 0.80 | 0.88 | 44.7 ± 4.95 | 44.9 ± 4.26 | 46.1 ± 3.44 | 0.86 | 0.90 | time | 0.031 | 0.15 | 0.65 |
| 10 jumps (cm) | 38.8 ± 4.24 | 39.3 ± 3.82 | 40.9 ± 3.44 * | 0.71 | 0.82 | 39.9 ± 3.67 | 39.4 ± 2.93 | 39.9 ± 2.10 | 0.82 | 0.84 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębska, J.; Skalska, M.; Radzimiński, Ł.; López-Sánchez, G.F.; Weiss, K.; Hill, L.; Knechtle, B. Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients 2022, 14, 521. https://doi.org/10.3390/nu14030521
Jastrzębska J, Skalska M, Radzimiński Ł, López-Sánchez GF, Weiss K, Hill L, Knechtle B. Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients. 2022; 14(3):521. https://doi.org/10.3390/nu14030521
Chicago/Turabian StyleJastrzębska, Joanna, Maria Skalska, Łukasz Radzimiński, Guillermo F. López-Sánchez, Katja Weiss, Lee Hill, and Beat Knechtle. 2022. "Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season" Nutrients 14, no. 3: 521. https://doi.org/10.3390/nu14030521
APA StyleJastrzębska, J., Skalska, M., Radzimiński, Ł., López-Sánchez, G. F., Weiss, K., Hill, L., & Knechtle, B. (2022). Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients, 14(3), 521. https://doi.org/10.3390/nu14030521









