Postprandial Effects of Salmon Fishmeal and Whey on Metabolic Markers in Serum and Gene Expression in Liver Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Postprandial Study
2.2. Analyses in Serum and Plasma
2.3. Cell Culture Experiments
2.4. cDNA Synthesis and Quantitative RT-PCR
2.5. RNA Sequencing and Read Counting
2.6. Data Analysis
3. Results and Discussion
3.1. Nutritional Content of Fishmeal and Whey
3.2. Effects of Intake of Fishmeal and Whey on Serum Amino Acids
3.3. Effects of Intake of Fishmeal and Whey on Metabolic Markers in Serum
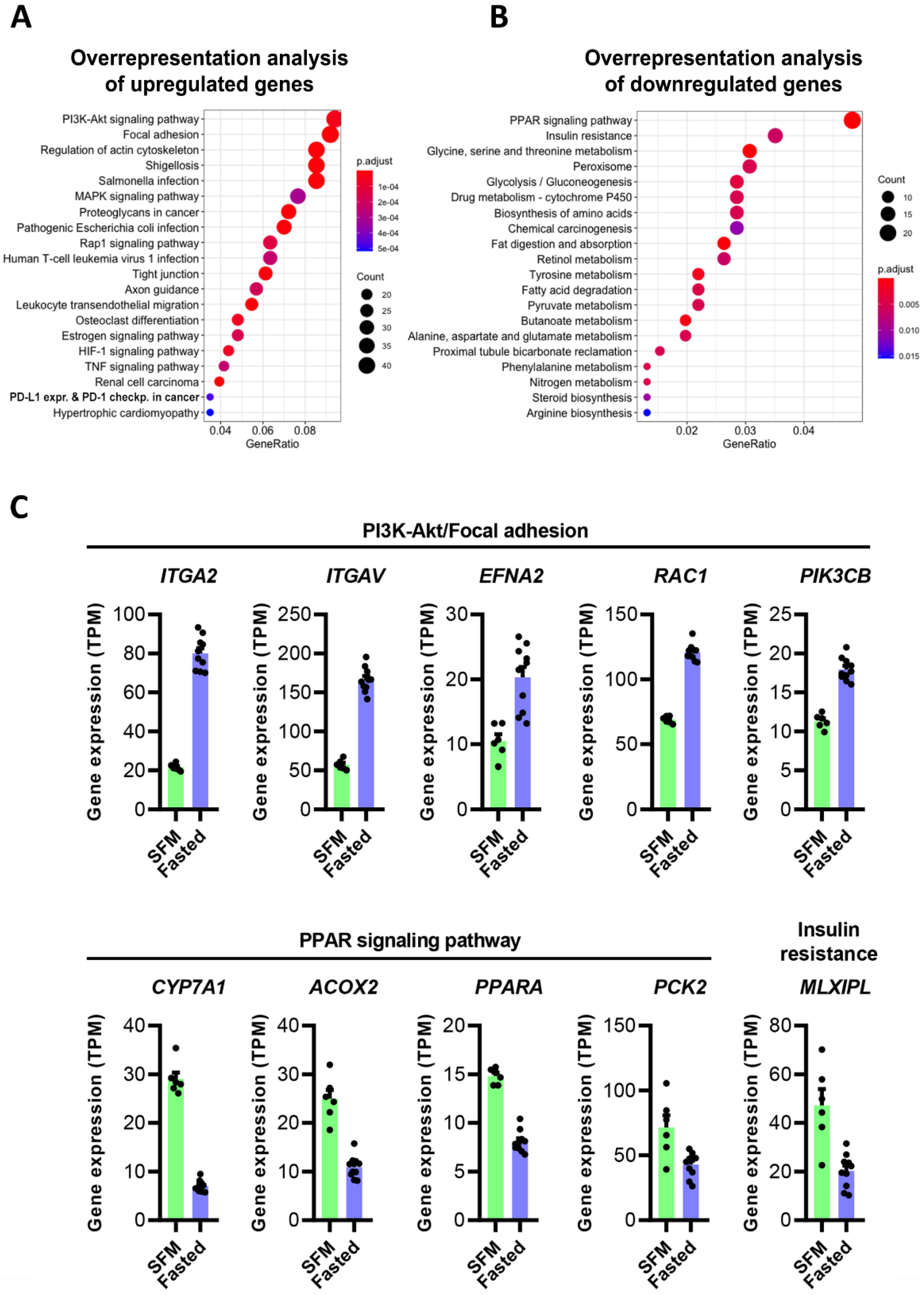
3.4. Intake of Fishmeal or Whey Did Not Influence Gene Expression in Liver Cells In Vitro
3.5. Potential Nutritional Value of Fish Protein or By-Products
3.6. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization on the United Nations. The Future of Food and Agriculture—Trends and Challenges; Food and Agriculture Organization on the United Nations: Rome, Italy, 2017. [Google Scholar]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Myhre, M.S.; Richardsen, R.; Nystøyl, R.; Strandheim, G. Analyse Marint Restråstoff 2019; SINTEF Ocean AS: Tromsø, Norway, 2020; p. 00904. [Google Scholar]
- Ramírez, A. Salmon by-Product Proteins; Fisheries Circular No. 1027; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar] [CrossRef]
- Norwegian Directorate of Health. The Norwegian Dietary Guidelines; Norwegian Directorate of Health: Oslo, Norway, 2015; p. 28.
- Torris, C.; Smastuen, M.C.; Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Madsen, L.; Lied, G.A. Fish–derived proteins and their potential to improve human health. Nutr. Rev. 2019, 77, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: A randomized controlled trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef] [Green Version]
- Vikøren, L.A.; Nygård, O.K.; Lied, E.; Rostrup, E.; Gudbrandsen, O.A. A randomised study on the effects of fish protein supplement on glucose tolerance, lipids and body composition in overweight adults. Br. J. Nutr. 2013, 109, 648–657. [Google Scholar] [CrossRef]
- Hovland, I.H.; Leikanger, I.S.; Stokkeland, O.; Waage, K.H.; Mjos, S.A.; Brokstad, K.A.; McCann, A.; Ueland, P.M.; Slizyte, R.; Carvajal, A.; et al. Effects of low doses of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: A randomised, double blind study. Eur. J. Nutr. 2020, 59, 1013–1029. [Google Scholar] [CrossRef]
- Hustad, K.S.; Ottestad, I.; Hjorth, M.; Dalen, K.T.; Sæther, T.; Sheikh, N.A.; Strømnes, M.; Ulven, S.M.; Holven, K.B. No effect of salmon fish protein on 2-h glucose in adults with increased risk of type 2 diabetes: A randomised controlled trial. Br. J. Nutr. 2021, 126, 1304–1313. [Google Scholar] [CrossRef]
- Pilon, G.; Ruzzin, J.; Rioux, L.-E.; Lavigne, C.; White, P.J.; Frøyland, L.; Jacques, H.; Bryl, P.; Beaulieu, L.; Marette, A.J.M. Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat–fed rats. Metabolism 2011, 60, 1122–1130. [Google Scholar] [CrossRef]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mørk, S.; Berge, R.K. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of hdl cholesterol, and lowers acyl-coa: Cholesterol acyltransferase activity in liver of zucker rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef] [Green Version]
- Drotningsvik, A.; Mjøs, S.A.; Pampanin, D.M.; Slizyte, R.; Carvajal, A.; Remman, T.; Høgøy, I.; Gudbrandsen, O.A. Dietary fish protein hydrolysates containing bioactive motifs affect serum and adipose tissue fatty acid compositions, serum lipids, postprandial glucose regulation and growth in obese zucker fa/fa rats. Br. J. Nutr. 2016, 116, 1336–1345. [Google Scholar] [CrossRef]
- Olsen, T.; Øvrebø, B.; Turner, C.; Bastani, N.E.; Refsum, H.; Vinknes, K.J. Combining dietary sulfur amino acid restriction with polyunsaturated fatty acid intake in humans: A randomized controlled pilot trial. Nutrients 2018, 10, 1822. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-blast: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, B. Bbmap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 13 December 2020).
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with hisat2 and hisat-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. Clusterprofiler: An r package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Walzem, R.L.; Dillard, C.J.; German, J.B. Whey components: Millennia of evolution create functionalities for mammalian nutrition: What we know and what we may be overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Margolis, L.M.; Orr, J.S. Optimized dietary strategies to protect skeletal muscle mass during periods of unavoidable energy deficit. FASEB J. 2015, 29, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- WHO. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thogersen, R.; Bertram, H.C.; Vangsoe, M.T.; Hansen, M. Krill protein hydrolysate provides high absorption rate for all essential amino acids-a randomized control cross-over trial. Nutrients 2021, 13, 3187. [Google Scholar] [CrossRef] [PubMed]
- Alcock, R.D.; Shaw, G.C.; Tee, N.; Burke, L.M. Plasma amino acid concentrations after the ingestion of dairy and collagen proteins, in healthy active males. Front. Nutr. 2019, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Guttormsen, A.B.; Solheim, E.; Refsum, H. Variation in plasma cystathionine and its relation to changes in plasma concentrations of homocysteine and methionine in healthy subjects during a 24-h observation period. Am. J. Clin. Nutr. 2004, 79, 76–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irino, Y.; Toh, R.; Nagao, M.; Mori, T.; Honjo, T.; Shinohara, M.; Tsuda, S.; Nakajima, H.; Satomi-Kobayashi, S.; Shinke, T.; et al. 2-aminobutyric acid modulates glutathione homeostasis in the myocardium. Sci. Rep. 2016, 6, 36749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Björck, I.M. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef]
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274–1284. [Google Scholar] [CrossRef]
- Koopman, R.; Walrand, S.p.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.M.; van Loon, L.J.C. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Östman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and gip on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Siddhu, A.; Sud, S.; Bijlani, R.L.; Karmarkar, M.G.; Nayar, U. Modulation of postprandial glycaemia and insulinaemia by cellulose in mixed nutrient combinations. Br. J. Nutr. 1989, 62, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballance, S.; Knutsen, S.H.; Fosvold, O.W.; Wickham, M.; Trenado, C.D.; Monro, J. Glyceamic and insulinaemic response to mashed potato alone, or with broccoli, broccoli fibre or cellulose in healthy adults. Eur. J. Nutr. 2018, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lees, M.J.; Nolan, D.; Amigo-Benavent, M.; Raleigh, C.J.; Khatib, N.; Harnedy-Rothwell, P.; FitzGerald, R.J.; Egan, B.; Carson, B.P. A fish-derived protein hydrolysate induces postprandial aminoacidaemia and skeletal muscle anabolism in an in vitro cell model using ex vivo human serum. Nutrients 2021, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Brennan, M.A.; Brennan, C.S. Effect of fortification with fish (pseudophycis bachus) powder on nutritional quality of durum wheat pasta. Foods 2018, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Bastos, S.C.; Tavares, T.; de Sousa Gomes Pimenta, M.E.; Leal, R.; Fabrício, L.F.; Pimenta, C.J.; Nunes, C.A.; Pinheiro, A.C.M. Fish filleting residues for enrichment of wheat bread: Chemical and sensory characteristics. J. Food Sci. Technol. 2014, 51, 2240–2245. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Jensen, C.; Hausken, T.; Lied, E.; Hatlebakk, J.G.; Brønstad, I.; Lihaug Hoff, D.A.; Lied, G.A. Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: A double-blind cross-over trial. J. Nutr. Sci. 2018, 7, e33. [Google Scholar] [CrossRef] [Green Version]
- Borzoei, S.; Neovius, M.; Barkeling, B.; Teixeira-Pinto, A.; Rossner, S. A comparison of effects of fish and beef protein on satiety in normal weight men. Eur. J. Clin. Nutr. 2006, 60, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Neis, E.P.; Bloemen, J.G.; Rensen, S.S.; van der Vorst, J.R.; van den Broek, M.A.; Venema, K.; Buurman, W.A.; Dejong, C.H. Effects of liver resection on hepatic short-chain fatty acid metabolism in humans. PLoS ONE 2016, 11, e0166161. [Google Scholar] [CrossRef] [Green Version]
- Song, B.G.; Kwon, W.; Kim, H.; Lee, E.M.; Han, Y.M.; Kim, H.; Byun, Y.; Lee, K.B.; Lee, K.H.; Lee, K.T.; et al. Detection of circulating tumor cells in resectable pancreatic ductal adenocarcinoma: A prospective evaluation as a prognostic marker. Front. Oncol. 2020, 10, 616440. [Google Scholar] [CrossRef]



| Macronutrients (g/100 g) | Fishmeal | Whey |
|---|---|---|
| Fat | 13.2 | 6.5 |
| Carbohydrates | - | 8.0 |
| Sugar | - | 8.0 |
| Protein | 69.7 | 77.4 |
| Amino Acids (g/100 g) | Fishmeal | Whey |
|---|---|---|
| Essential amino acids | ||
| Histidine | 1.52 | 1.65 |
| Isoleucine | 2.48 | 4.85 |
| Leucine | 4.57 | 8.50 |
| Lysine | 4.63 | 7.39 |
| Phenylalanine (total) | 2.50 | 2.56 |
| Threonine | 2.92 | 5.54 |
| Valine (total) | 3.19 | 4.55 |
| Tryptophan | 0.86 | 1.51 |
| Methionine | 1.84 | 1.64 |
| Total EAA * | 24.51 | 38.19 |
| Non-essential amino acids | ||
| Alanine | 3.93 | 3.88 |
| Arginine (total) | 4.01 | 1.98 |
| Aspartic acid | 5.84 | 8.44 |
| Glutamic acid (total) | 7.62 | 13.9 |
| Glycine | 5.08 | 1.45 |
| Hydroxyproline | 0.57 | <0.05 |
| Ornithine | 0.07 | <0.05 |
| Proline (Total) | 3.22 | 5.03 |
| Serine (Total) | 2.95 | 4.03 |
| Tyrosine (Total) | 2.04 | 2.32 |
| Cysteine + Cystine | 0.75 | 1.64 |
| Fishmeal 1 | Whey 1 | Fishmeal vs. Whey | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fasting | 30 min | 60 min | p-Value Time Eff 2 | Fasting | 30 min | 60 min | p-Value Time Eff 2 | p-Value 3 | |
| s-Glucose, mmol/L | 4.82 (0.49) | 4.58 (0.40) | 4.52 (0.44) | 0.01 * | 4.70 (0.62) | 4.38 (0.56) | 4.42 (0.69) | 0.04 * | 0.69 |
| s-Insulin, pmol/L | 36.0 (13.6) | 57.6 (32.4) | 37.4 (18.8) | 0.13 | 39.4 (24.3) | 37.8 (17.0) | 46.8 (32.2) | 0.45 | 0.87 |
| s-Triglyceride, mmol/L | 0.86 (0.25) | 0.79 (0.22) | 0.80 (0.25) | 0.02 * | 0.93 (0.22) | 0.90 (0.22) | 0.89 (0.21) | 0.03 * | 0.54 |
| s-Cholesterol, mmol/L | 4.60 (1.07) | 4.44 (1.00) | 4.46 (1.12) | 0.17 | 4.76 (1.36) | 4.66 (1.31) | 4.66 (1.27) | 0.30 | 0.80 |
| s-microCRP, mg/L | 0.38 (0.11) | 0.38 (0.08) | 0.36 (0.09) | 0.59 | 0.32 (0.08) | 0.34 (0.09) | 0.34 (0.09) | 0.37 | 0.49 |
| p-GLP-1, pmol/L | 3.37 (1.58) | 4.07 (1.02) | 3.67 (0.71) | 0.30 | 3.63 (1.58) | 3.36 (1.48) | 3.68 (1.79) | 0.30 | 0.74 |
| Gene Symbol | Gene Name | Log2FC | FDR | TPM * |
|---|---|---|---|---|
| Upregulated | ||||
| MT1G | metallothionein 1G | 8.5 | 5 × 10−11 | 133.0 |
| MT1M | metallothionein 1M | 8.3 | 4 × 10−8 | 5.0 |
| MT1E | metallothionein 1E | 7.8 | 2 × 10−14 | 110.7 |
| MT1X | metallothionein 1X | 7.7 | 7 × 10−13 | 29.7 |
| CYP24A1 | cytochrome P450 family 24 subfamily A member 1 | 6.0 | 9 × 10−20 | 12.4 |
| MT2A | metallothionein 2A | 6.0 | 3 × 10−8 | 173.8 |
| MT1F | metallothionein 1F | 5.6 | 4 × 10−13 | 21.3 |
| ACTA1 | actin alpha 1, skeletal muscle | 4.9 | 6 × 10−12 | 67.3 |
| TAGLN | transgelin | 4.4 | 2 × 10−15 | 87.7 |
| FSTL3 | follistatin like 3 | 4.0 | 4 × 10−14 | 121.0 |
| Downregulated | ||||
| CYP1A1 | cytochrome P450 family 1 subfamily A member 1 | −3.5 | 5 × 10−19 | 12.3 |
| CYP7A1 | cytochrome P450 family 7 subfamily A member 1 | −2.1 | 2 × 10−13 | 10.3 |
| SLC39A10 | solute carrier family 39 member 10 | −1.9 | 7 × 10−13 | 22.9 |
| CYP4F3 | cytochrome P450 family 4 subfamily F member 3 | −1.9 | 3 × 10−13 | 9.5 |
| TMEM140 | transmembrane protein 140 | −1.7 | 8 × 10−10 | 8.9 |
| SPTLC3 | serine palmitoyltransferase, long chain base subunit 3 | −1.7 | 3 × 10−11 | 24.6 |
| PLPPR1 | phospholipid phosphatase related 1 | −1.7 | 1 × 10−11 | 12.4 |
| LIPG | lipase G, endothelial type | −1.6 | 8 × 10−14 | 5.9 |
| ITIH1 | inter-alpha-trypsin inhibitor heavy chain 1 | −1.5 | 5 × 10−7 | 6.0 |
| CLDN19 | claudin 19 | −1.5 | 9 × 10−7 | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hjorth, M.; Galigniana, N.M.; Ween, O.; Ulven, S.M.; Holven, K.B.; Dalen, K.T.; Sæther, T. Postprandial Effects of Salmon Fishmeal and Whey on Metabolic Markers in Serum and Gene Expression in Liver Cells. Nutrients 2022, 14, 1593. https://doi.org/10.3390/nu14081593
Hjorth M, Galigniana NM, Ween O, Ulven SM, Holven KB, Dalen KT, Sæther T. Postprandial Effects of Salmon Fishmeal and Whey on Metabolic Markers in Serum and Gene Expression in Liver Cells. Nutrients. 2022; 14(8):1593. https://doi.org/10.3390/nu14081593
Chicago/Turabian StyleHjorth, Marit, Natalia M. Galigniana, Ola Ween, Stine M. Ulven, Kirsten B. Holven, Knut Tomas Dalen, and Thomas Sæther. 2022. "Postprandial Effects of Salmon Fishmeal and Whey on Metabolic Markers in Serum and Gene Expression in Liver Cells" Nutrients 14, no. 8: 1593. https://doi.org/10.3390/nu14081593
APA StyleHjorth, M., Galigniana, N. M., Ween, O., Ulven, S. M., Holven, K. B., Dalen, K. T., & Sæther, T. (2022). Postprandial Effects of Salmon Fishmeal and Whey on Metabolic Markers in Serum and Gene Expression in Liver Cells. Nutrients, 14(8), 1593. https://doi.org/10.3390/nu14081593







