The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis
Abstract
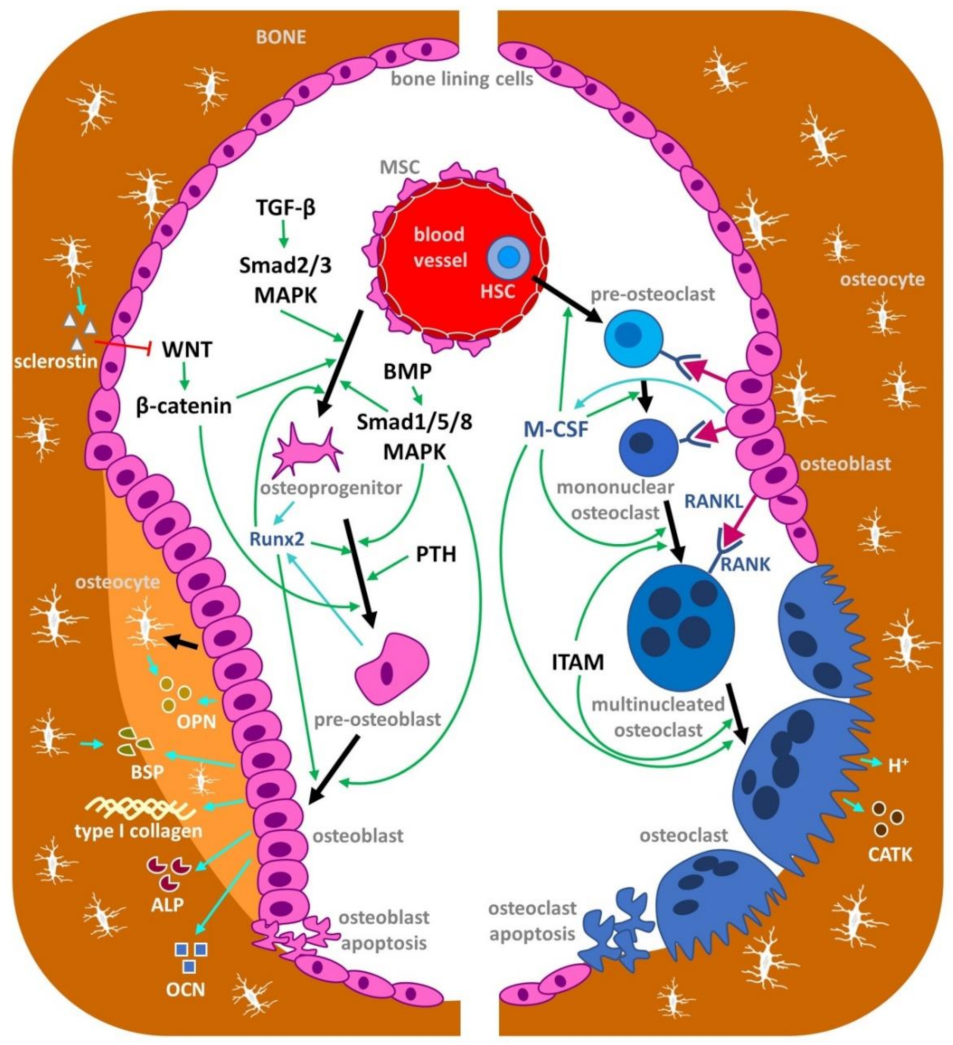
1. Introduction
2. Macronutrients and Osteoporosis
2.1. Proteins
2.2. Lipids
2.3. Carbohydrates
3. Micronutrients and Osteoporosis
3.1. Minerals
3.1.1. Calcium
3.1.2. Phosphorus
3.1.3. Magnesium
3.2. Vitamins
3.2.1. Vitamin D
3.2.2. Vitamin C
3.2.3. Vitamin K
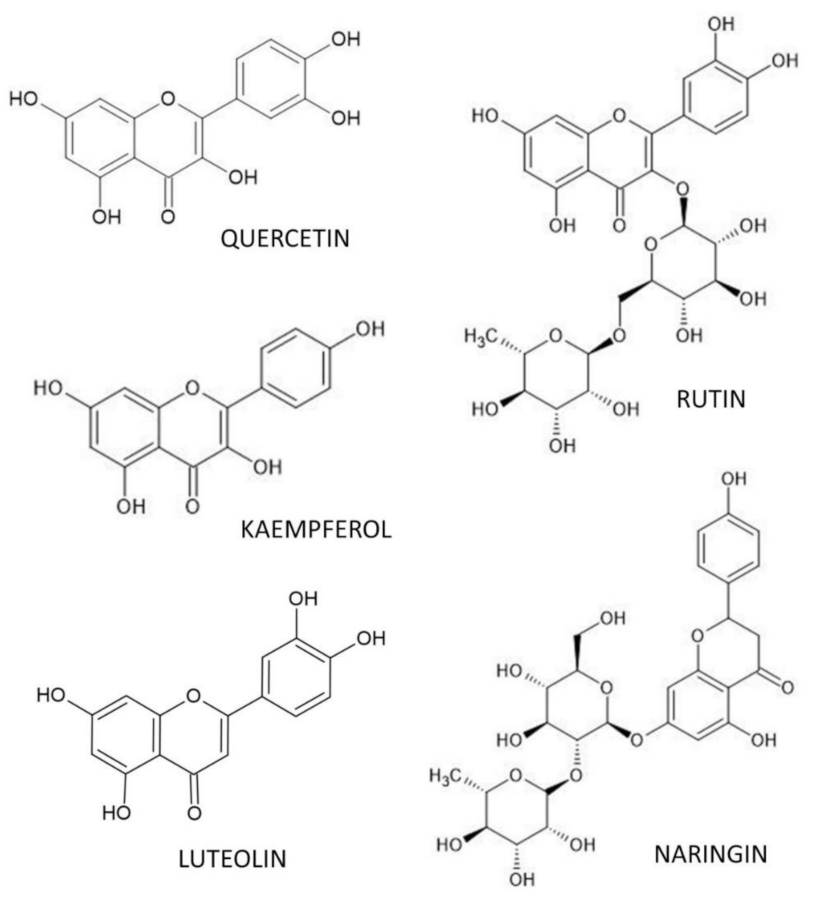
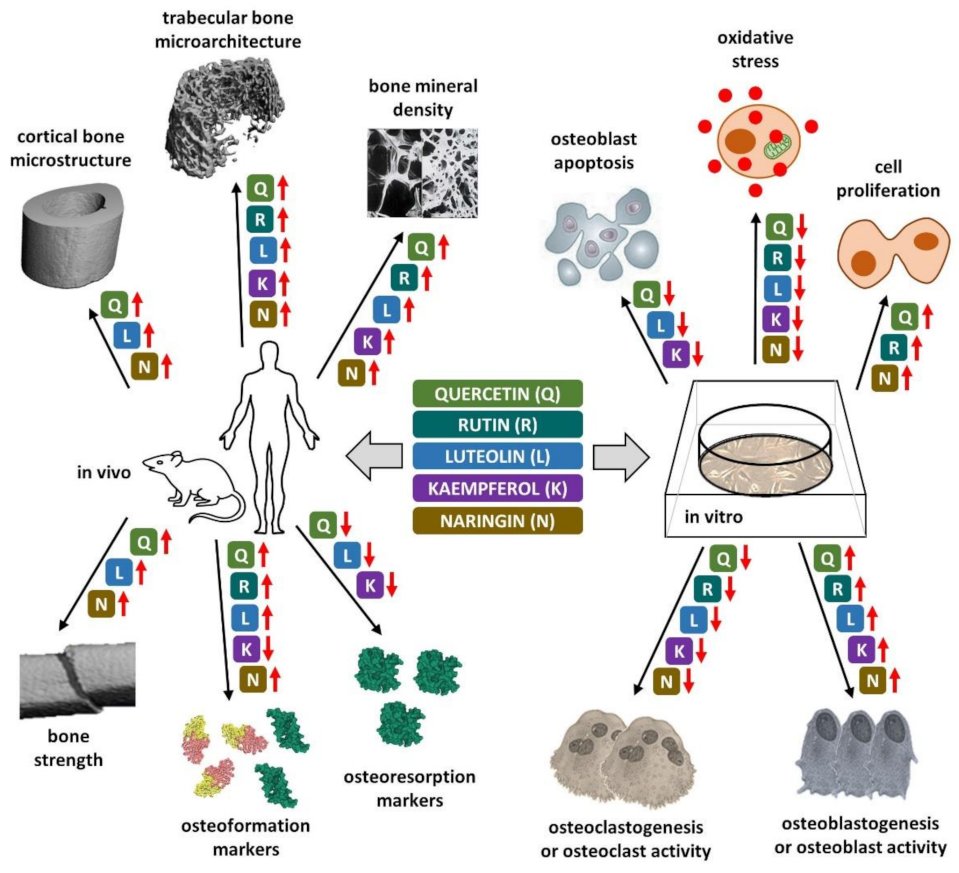
4. Flavonoid Polyphenols and Osteoporosis
4.1. Quercetin
4.2. Rutin
4.3. Luteolin
4.4. Kaempferol
4.5. Naringin
5. Conclusions
- -
- Foods with a high energy density, such as foods rich in PUFAs, fruits and vegetables, high in fibers and high-quality animal or plant-based proteins, should be selected as a matter of priority to ensure sufficient vitamins and minerals.
- -
- Supplements, such as calcium carbonate or calcium citrate, may be used to improve skeletal health if there are dietary deficiencies.
- -
- Vitamin D deficiency can be corrected by either extending time spent outdoors, taking supplements, or in combination.
- -
- Foods and beverages with a poor nutrient density, such as foods made from simple carbohydrates, carbonated and sugar-sweetened beverages or products high in Na or SFAs should be either reduced or excluded.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. New Horizons in Osteoporosis. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Ström, O.; Borgström, F.; Kanis, J.A.; Compston, J.; Cooper, C.; McCloskey, E.V.; Jönsson, B. Osteoporosis: Burden, Health Care Provision and Opportunities in the EU: A Report Prepared in Collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2011, 6, 59–155. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.-P.; Guéguen, L.; Palacios, C.; Shearer, M.J.; Weaver, C.M. Minerals and Vitamins in Bone Health: The Potential Value of Dietary Enhancement. Br. J. Nutr. 2009, 101, 1581–1596. [Google Scholar] [CrossRef]
- Warensjö Lemming, E.; Byberg, L. Is a Healthy Diet Also Suitable for the Prevention of Fragility Fractures? Nutrients 2020, 12, 2642. [Google Scholar] [CrossRef]
- Prentice, A. The Relative Contribution of Diet and Genotype to Bone Development. Proc. Nutr. Soc. 2001, 60, 45–52. [Google Scholar] [CrossRef]
- Ilesanmi-Oyelere, B.L.; Kruger, M.C. Nutrient and Dietary Patterns in Relation to the Pathogenesis of Postmenopausal Osteoporosis-A Literature Review. Life 2020, 10, 220. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Das, U.N. Catechins and Osteoporosis. Nutrition 2013, 29, 697–699. [Google Scholar] [CrossRef]
- Goltzman, D. The Aging Skeleton. In Human Cell Transformation: Advances in Cell Models for the Study of Cancer and Aging; Advances in Experimental Medicine and Biology; Rhim, J.S., Dritschilo, A., Kremer, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 153–160. ISBN 978-3-030-22254-3. [Google Scholar]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of Disease: Is Osteoporosis the Obesity of Bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Cici, D.; Rotondo, C.; Maruotti, N.; Cantatore, F.P. Molecular Basis of Bone Aging. Int. J. Mol. Sci. 2020, 21, 3679. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andía, J.B.; Carrillo-López, N.; Messina, O.D.; Hamdy, N.A.T.; Panizo, S.; Ferrari, S.L.; On behalf of the International Osteoporosis Foundation (IOF). Working Group on Bone and Cardiovascular Diseases Pathophysiology of Vascular Calcification and Bone Loss: Linked Disorders of Ageing? Nutrients 2021, 13, 3835. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor Activator of Nuclear Factor KappaB Ligand and Osteoprotegerin Regulation of Bone Remodeling in Health and Disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef]
- Eghbali-Fatourechi, G.; Khosla, S.; Sanyal, A.; Boyle, W.J.; Lacey, D.L.; Riggs, B.L. Role of RANK Ligand in Mediating Increased Bone Resorption in Early Postmenopausal Women. J. Clin. Investig. 2003, 111, 1221–1230. [Google Scholar] [CrossRef]
- Hejazi, J.; Davoodi, A.; Khosravi, M.; Sedaghat, M.; Abedi, V.; Hosseinverdi, S.; Ehrampoush, E.; Homayounfar, R.; Shojaie, L. Nutrition and Osteoporosis Prevention and Treatment. Biomed. Res. Ther. 2020, 7, 3709–3720. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological Agents and Natural Compounds: Available Treatments for Osteoporosis. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.J.; Lim, W. Osteoporosis Therapies and Their Mechanisms of Action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, e4218472. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Lorincz, C.; Manske, S.L.; Zernicke, R. Bone Health: Part 1, Nutrition. Sports Health 2009, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- EuroFIR—European Food Information Resource. Available online: https://www.eurofir.org/ (accessed on 21 December 2021).
- Food Composition Data|EFSA. Available online: https://www.efsa.europa.eu/en/microstrategy/food-composition-data (accessed on 21 December 2021).
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.; Buttriss, J.L. Vitamin D: An Overview of Vitamin D Status and Intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Favell, D.J. A Comparison of the Vitamin C Content of Fresh and Frozen Vegetables. Food Chem. 1998, 62, 59–64. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total Antioxidant and Ascorbic Acid Content of Fresh Fruits and Vegetables: Implications for Dietary Planning and Food Preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Effect of Alternative Preservation Steps and Storage on Vitamin C Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Heaney, R.P.; Layman, D.K. Amount and Type of Protein Influences Bone Health. Am. J. Clin. Nutr. 2008, 87, 1567S–1570S. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Protein and Calcium: Antagonists or Synergists? Am. J. Clin. Nutr. 2002, 75, 609–610. [Google Scholar] [CrossRef]
- Medicine, I. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P. Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Frankenfeld, C.L. Dietary Protein Intake above the Current RDA and Bone Health: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Promislow, J.H.E.; Goodman-Gruen, D.; Slymen, D.J.; Barrett-Connor, E. Protein Consumption and Bone Mineral Density in the Elderly: The Rancho Bernardo Study. Am. J. Epidemiol. 2002, 155, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Coin, A.; Perissinotto, E.; Enzi, G.; Zamboni, M.; Inelmen, E.M.; Frigo, A.C.; Manzato, E.; Busetto, L.; Buja, A.; Sergi, G. Predictors of Low Bone Mineral Density in the Elderly: The Role of Dietary Intake, Nutritional Status and Sarcopenia. Eur. J. Clin. Nutr. 2008, 62, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, J.E.; Looker, A.C.; Insogna, K.L. Low Dietary Protein and Low Bone Density. Calcif. Tissue Int. 2000, 66, 313. [Google Scholar] [CrossRef]
- Bonjour, J.-P. The Dietary Protein, IGF-I, Skeletal Health Axis. Horm. Mol. Biol. Clin. Investig. 2016, 28, 39–53. [Google Scholar] [CrossRef]
- Darling, A.L.; Millward, D.J.; Lanham-New, S.A. Dietary Protein and Bone Health: Towards a Synthesised View. Proc. Nutr. Soc. 2021, 80, 165–172. [Google Scholar] [CrossRef]
- Deane, C.S.; Bass, J.J.; Crossland, H.; Phillips, B.E.; Atherton, P.J. Animal, Plant, Collagen and Blended Dietary Proteins: Effects on Musculoskeletal Outcomes. Nutrients 2020, 12, 2670. [Google Scholar] [CrossRef]
- Conigrave, A.D.; Brown, E.M.; Rizzoli, R. Dietary Protein and Bone Health: Roles of Amino Acid-Sensing Receptors in the Control of Calcium Metabolism and Bone Homeostasis. Annu. Rev. Nutr. 2008, 28, 131–155. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Päivärinta, E.; Pellinen, T.; Viitakangas, H.; Risteli, J.; Erkkola, M.; Lamberg-Allardt, C.; Pajari, A.-M. Partial Replacement of Animal Proteins with Plant Proteins for 12 Weeks Accelerates Bone Turnover Among Healthy Adults: A Randomized Clinical Trial. J. Nutr. 2021, 151, 11–19. [Google Scholar] [CrossRef]
- Liu, Z.; Jeppesen, P.B.; Gregersen, S.; Chen, X.; Hermansen, K. Dose- and Glucose-Dependent Effects of Amino Acids on Insulin Secretion from Isolated Mouse Islets and Clonal INS-1E Beta-Cells. Rev. Diabet. Stud. 2008, 5, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Wang, W.; Liu, J. Insulin Stimulates Osteoblast Proliferation and Differentiation through ERK and PI3K in MG-63 Cells. Cell Biochem. Funct. 2010, 28, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated With Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence From Discordant Monozygotic Twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Torricelli, P.; Giavaresi, G.; Carpi, A.; Nicolini, A.; Giardino, R. Effect of L-Lysine and L-Arginine on Primary Osteoblast Cultures from Normal and Osteopenic Rats. Biomed. Pharmacother. 2001, 55, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, Vegetarianism, Bone Mineral Density, and Fracture Risk: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Chung, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Shi, J.; Wallace, T.C.; et al. Animal versus Plant Protein and Adult Bone Health: A Systematic Review and Meta-Analysis from the National Osteoporosis Foundation. PLoS ONE 2018, 13, e0192459. [Google Scholar] [CrossRef]
- Sahni, S.; Cupples, L.A.; Mclean, R.R.; Tucker, K.L.; Broe, K.E.; Kiel, D.P.; Hannan, M.T. Protective Effect of High Protein and Calcium Intake on the Risk of Hip Fracture in the Framingham Offspring Cohort. J. Bone Miner. Res. 2010, 25, 2770–2776. [Google Scholar] [CrossRef]
- Castaneda, C. Muscle Wasting and Protein Metabolism1. J. Anim. Sci. 2002, 80, E98–E105. [Google Scholar] [CrossRef]
- Bettis, T.; Kim, B.-J.; Hamrick, M.W. Impact of Muscle Atrophy on Bone Metabolism and Bone Strength: Implications for Muscle-Bone Crosstalk with Aging and Disuse. Osteoporos. Int. 2018, 29, 1713–1720. [Google Scholar] [CrossRef]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and Its Relationship with Bone Mineral Density in Middle-Aged and Elderly European Men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Won, C.W.; Kim, B.S.; Choi, H.R.; Moon, M.Y. The Association between the Low Muscle Mass and Osteoporosis in Elderly Korean People. J. Korean Med. Sci. 2014, 29, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Chen, J.; Yu, H.; Zheng, Y.; Zhao, J.; Zhu, J. Recent Advances in Seafood Bioactive Peptides and Their Potential for Managing Osteoporosis. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Peptides from Marine Processing Waste and Shellfish: A Review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive Compounds from Marine Processing Byproducts—A Review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; König, D. Specific Bioactive Collagen Peptides in Osteopenia and Osteoporosis: Long-Term Observation in Postmenopausal Women. J. Bone Metab. 2021, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Reddi, S.; Mada, S.; Kumar, N.; Kumar, R.; Ahmad, N.; Karvande, A.; Kapila, S.; Kapila, R.; Trivedi, R. Antiosteopenic Effect of Buffalo Milk Casein-Derived Peptide (NAVPITPTL) in Ovariectomized Rats. Int. J. Pept. Res. Ther. 2019, 25, 1147–1158. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential Role of Bioactive Peptides in Prevention and Treatment of Chronic Diseases: A Narrative Review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Arab, L. Biomarkers of Fat and Fatty Acid Intake. J. Nutr. 2003, 133 (Suppl. 3), 925S–932S. [Google Scholar] [CrossRef]
- Bajželj, B.; Laguzzi, F.; Röös, E. The Role of Fats in the Transition to Sustainable Diets. Lancet Planet. Health 2021, 5, e644–e653. [Google Scholar] [CrossRef]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty Acids and Osteoarthritis: Different Types, Different Effects. Jt. Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Cholesterol: Factors Determining Blood Levels. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 335–340. ISBN 978-0-12-384885-7. [Google Scholar]
- Degirolamo, C.; Rudel, L.L. Dietary Monounsaturated Fatty Acids Appear Not to Provide Cardioprotection. Curr. Atheroscler. Rep. 2010, 12, 391–396. [Google Scholar] [CrossRef]
- Kuna, A.; Achinna, P. Mono Unsaturated Fatty Acids for CVD and Diabetes: A Healthy Choice. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 236. [Google Scholar] [CrossRef]
- Abdullah, M.M.H.; Jew, S.; Jones, P.J.H. Health Benefits and Evaluation of Healthcare Cost Savings If Oils Rich in Monounsaturated Fatty Acids Were Substituted for Conventional Dietary Oils in the United States. Nutr. Rev. 2017, 75, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids, Olive Oil and Health Status: A Systematic Review and Meta-Analysis of Cohort Studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef]
- Kato, I.; Toniolo, P.; Zeleniuch-Jacquotte, A.; Shore, R.E.; Koenig, K.L.; Akhmedkhanov, A.; Riboli, E. Diet, Smoking and Anthropometric Indices and Postmenopausal Bone Fractures: A Prospective Study. Int. J. Epidemiol. 2000, 29, 85–92. [Google Scholar] [CrossRef]
- Romero Márquez, J.M.; Varela López, A.; Navarro Hortal, M.D.; Badillo Carrasco, A.; Quiles Morales, J.L. Molecular Interactions between Dietary Lipids and Bone Tissue during Aging. Int. J. Mol. Sci. 2021, 22, 6473. [Google Scholar] [CrossRef]
- Corwin, R.L.; Hartman, T.J.; Maczuga, S.A.; Graubard, B.I. Dietary Saturated Fat Intake Is Inversely Associated with Bone Density in Humans: Analysis of NHANES III. J. Nutr. 2006, 136, 159–165. [Google Scholar] [CrossRef]
- Orchard, T.S.; Cauley, J.A.; Frank, G.C.; Neuhouser, M.L.; Robinson, J.G.; Snetselaar, L.; Tylavsky, F.; Wactawski-Wende, J.; Young, A.M.; Lu, B.; et al. Fatty Acid Consumption and Risk of Fracture in the Women’s Health Initiative1234. Am. J. Clin. Nutr. 2010, 92, 1452–1460. [Google Scholar] [CrossRef]
- García-Martínez, O.; Rivas, A.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C. The Effect of Olive Oil on Osteoporosis Prevention. Int. J. Food Sci. Nutr. 2014, 65, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Martín, R.; Aliaga Vera, I.; Moreno-Corral, L.J.; Moran, J.M.; Lavado-Garcia, J.M.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M. Olive Oil Consumption and Bone Microarchitecture in Spanish Women. Nutrients 2018, 10, 968. [Google Scholar] [CrossRef]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective Effects of Fish Intake and Interactive Effects of Long-Chain Polyunsaturated Fatty Acid Intakes on Hip Bone Mineral Density in Older Adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Mangano, K.M.; McLean, R.R.; Hannan, M.T.; Kiel, D.P. Dietary Approaches for Bone Health: Lessons from the Framingham Osteoporosis Study. Curr. Osteoporos. Rep. 2015, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tartibian, B.; Hajizadeh Maleki, B.; Kanaley, J.; Sadeghi, K. Long-Term Aerobic Exercise and Omega-3 Supplementation Modulate Osteoporosis through Inflammatory Mechanisms in Post-Menopausal Women: A Randomized, Repeated Measures Study. Nutr. Metab. 2011, 8, 71. [Google Scholar] [CrossRef]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Dietary Intakes of Arachidonic Acid and α-Linolenic Acid Are Associated with Reduced Risk of Hip Fracture in Older Adults. J. Nutr. 2011, 141, 1146–1153. [Google Scholar] [CrossRef]
- Longo, A.B.; Ward, W.E. PUFAs, Bone Mineral Density, and Fragility Fracture: Findings from Human Studies. Adv. Nutr. 2016, 7, 299–312. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M.M. Bone Benefits of Fish Oil Supplementation Depend on Its EPA and DHA Content. Nutrients 2019, 11, 2701. [Google Scholar] [CrossRef]
- Kim, S.; Henneicke, H.; Caavanagh, L.L.; Macfarlane, E.; Thai, L.J.; Foong, D.; Gasparini, S.J.; Fong-Yee, C.; Swarbrick, M.M.; Seibel, M.J.; et al. Osteoblastic Glucocorticoid Signaling Exacerbates High-Fat-Diet- Induced Bone Loss and Obesity. Bone Res. 2021, 9, 40. [Google Scholar] [CrossRef]
- Macdonald, H.M.; New, S.A.; Golden, M.H.; Grubb, D.A.; Reid, D.M. Food Groups Affecting Perimenopausal and Early Postmenopausal Bone Loss in Scottish Women. Nutr. Asp. Osteoporos. 2001, 1, 399–408. [Google Scholar]
- Turcotte, A.-F.; O’Connor, S.; Morin, S.N.; Gibbs, J.C.; Willie, B.M.; Jean, S.; Gagnon, C. Association between Obesity and Risk of Fracture, Bone Mineral Density and Bone Quality in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0252487. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Vilaca, T. Obesity, Type 2 Diabetes and Bone in Adults. Calcif. Tissue Int. 2017, 100, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J. Effects of Obesity on Bone Metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Rinonapoli, G.; Pace, V.; Ruggiero, C.; Ceccarini, P.; Bisaccia, M.; Meccariello, L.; Caraffa, A. Obesity and Bone: A Complex Relationship. Int. J. Mol. Sci. 2021, 22, 13662. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Elazary, R.; Sherf-Dagan, S.; Goldenshluger, A.; Brodie, R.; Mintz, Y.; Weiss, R. Bone Health Following Bariatric Surgery: Implications for Management Strategies to Attenuate Bone Loss. Adv. Nutr. 2018, 9, 114–127. [Google Scholar] [CrossRef]
- Shapses, S.A.; Sukumar, D. Bone Metabolism in Obesity and Weight Loss. Annu. Rev. Nutr. 2012, 32, 287–309. [Google Scholar] [CrossRef]
- Kiely, L.J.; Hickey, R.M. Characterization and Analysis of Food-Sourced Carbohydrates. Methods Mol. Biol. 2022, 2370, 67–95. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Cohen, T.R.; Hazell, T.J.; Vanstone, C.A.; Plourde, H.; Rodd, C.J.; Weiler, H.A. A Family-Centered Lifestyle Intervention to Improve Body Composition and Bone Mass in Overweight and Obese Children 6 through 8 Years: A Randomized Controlled Trial Study Protocol. BMC Public Health 2013, 13, 383. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Larmas, M. A High Sucrose Diet Decreases the Mechanical Strength of Bones in Growing Rats. J. Nutr. 1998, 128, 1807–1810. [Google Scholar] [CrossRef]
- Terada, M.; Inaba, M.; Yano, Y.; Hasuma, T.; Nishizawa, Y.; Morii, H.; Otani, S. Growth-Inhibitory Effect of a High Glucose Concentration on Osteoblast-like Cells. Bone 1998, 22, 17–23. [Google Scholar] [CrossRef]
- Ericsson, Y.; Angmar-Månsson, B.; Flores, M. Urinary Mineral Ion Loss after Sugar Ingestion. Bone Miner. 1990, 9, 233–237. [Google Scholar] [CrossRef]
- Douard, V.; Sabbagh, Y.; Lee, J.; Patel, C.; Kemp, F.W.; Bogden, J.D.; Lin, S.; Ferraris, R.P. Excessive Fructose Intake Causes 1,25-(OH)(2)D(3)-Dependent Inhibition of Intestinal and Renal Calcium Transport in Growing Rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Tsanzi, E.; Light, H.R.; Tou, J.C. The Effect of Feeding Different Sugar-Sweetened Beverages to Growing Female Sprague-Dawley Rats on Bone Mass and Strength. Bone 2008, 42, 960–968. [Google Scholar] [CrossRef]
- Ogur, R.; Uysal, B.; Ogur, T.; Yaman, H.; Oztas, E.; Ozdemir, A.; Hasde, M. Evaluation of the Effect of Cola Drinks on Bone Mineral Density and Associated Factors. Basic Clin. Pharmacol. Toxicol. 2007, 100, 334–338. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of Soft Drink Consumption on Nutrition and Health: A Systematic Review and Meta-Analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Jakeman, S.A.; Henry, C.N.; Martin, B.R.; McCabe, G.P.; McCabe, L.D.; Jackson, G.S.; Peacock, M.; Weaver, C.M. Soluble Corn Fiber Increases Bone Calcium Retention in Postmenopausal Women in a Dose-Dependent Manner: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2016, 104, 837–843. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Jang, K.-H.; Lee, E.-Y.; Cho, Y.; Kang, S.-A.; Ha, W.-K.; Choue, R. The Effect of Chicory Fructan Fiber on Calcium Absorption and Bone Metabolism in Korean Postmenopausal Women. Nutr. Sci. 2004, 7, 151–157. [Google Scholar]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A Combination of Prebiotic Short- and Long-Chain Inulin-Type Fructans Enhances Calcium Absorption and Bone Mineralization in Young Adolescents. Am. J. Clin. Nutr. 2005, 82, 471–476. [Google Scholar] [CrossRef]
- McArdle, P.D.; Mellor, D.; Rilstone, S.; Taplin, J. The Role of Carbohydrate in Diabetes Management. Pract. Diabetes 2016, 33, 237–242. [Google Scholar] [CrossRef]
- Jackuliak, P.; Payer, J. Osteoporosis, Fractures, and Diabetes. Int. J. Endocrinol. 2014, 2014, e820615. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Omelka, R. Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats. Molecules 2021, 26, 2616. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, A.; Gurtu, S. Potential Benefits of Honey in Type 2 Diabetes Mellitus: A Review. Public Health 2013, 5, 18. [Google Scholar]
- Kraemer, K.; Badham, J.; Christian, P.; Hyun Rah, J. Sight and Life—Micronutrients, Macro Impact by Sight and Life. Available online: https://issuu.com/sight_and_life/docs/micronutriens_macro_impact (accessed on 21 December 2021).
- Cashman, K.D. Calcium Intake, Calcium Bioavailability and Bone Health. Br. J. Nutr. 2002, 87 (Suppl 2), S169–S177. [Google Scholar] [CrossRef]
- Nieves, J.W. Osteoporosis: The Role of Micronutrients. Am. J. Clin. Nutr. 2005, 81, 1232S–1239S. [Google Scholar] [CrossRef]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Effects of Calcium Supplementation on Bone Density in Healthy Children: Meta-Analysis of Randomised Controlled Trials. BMJ 2006, 333, 775. [Google Scholar] [CrossRef]
- Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; Mishra, G.; Mueck, A.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; Stute, P.; et al. Calcium in the Prevention of Postmenopausal Osteoporosis: EMAS Clinical Guide. Maturitas 2018, 107, 7–12. [Google Scholar] [CrossRef]
- Celotti, F.; Bignamini, A. Dietary Calcium and Mineral/Vitamin Supplementation: A Controversial Problem. J. Int. Med. Res. 1999, 27, 1–14. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, Vitamin D, Vitamin K2, and Magnesium Supplementation and Skeletal Health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium Intake and Bone Mineral Density: Systematic Review and Meta-Analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Langford, J.R.; Liporace, F.A. Essential Nutrients for Bone Health and a Review of Their Availability in the Average North American Diet. Open Orthop. J. 2012, 6, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sunyecz, J.A. The Use of Calcium and Vitamin D in the Management of Osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium Intake and Disease Prevention. Arq. Bras. Endocrinol. Metabol. 2006, 50, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Diet, Nutrition, and Bone Health. J. Nutr. 2007, 137, 2507S–2512S. [Google Scholar] [CrossRef]
- Pastore, S.M.; Gomes, P.C.; Rostagno, H.S.; Albino, L.F.T.; Calderano, A.A.; Vellasco, C.R.; da Viana, G.S.; de Almeida, R.L. Calcium Levels and Calcium: Available Phosphorus Ratios in Diets for White Egg Layers from 42 to 58 Weeks of Age. R. Bras. Zootec. 2012, 41, 2424–2432. [Google Scholar] [CrossRef]
- Block, G.D.; Wood, R.J.; Allen, L.H. A Comparison of the Effects of Feeding Sulfur Amino Acids and Protein on Urine Calcium in Man. Am. J. Clin. Nutr. 1980, 33, 2128–2136. [Google Scholar] [CrossRef]
- Zemel, M.B.; Schuette, S.A.; Hegsted, M.; Linkswiler, H.M. Role of the Sulfur-Containing Amino Acids in Protein-Induced Hypercalciuria in Men. J. Nutr. 1981, 111, 545–552. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research Progress on Applications of Calcium Derived from Marine Organisms. Sci. Rep. 2020, 10, 18425. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Puchała, M.; Józefiak, D. Effects on Performance and Eggshell Quality of Particle Size of Calcium Sources in Laying Hens’ Diets with Different Ca Concentrations. Arch. Anim. Breed. 2015, 58, 301–307. [Google Scholar] [CrossRef]
- Brennan, O.; Sweeney, J.; O’Meara, B.; Widaa, A.; Bonnier, F.J.; Byrne, H.J.; O’Gorman, D.M. A Natural, Calcium-Rich Marine Multi-Mineral Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Omelka, R.; Martiniakova, M.; Svik, K.; Slovak, L.; Payer, J.; Oppenbergerova, I.; Kovacova, V.; Babikova, M.; Soltesova-Prnova, M. The Effects of Eggshell Calcium (Biomin H®) and Its Combinations with Alfacalcidol (1α-Hydroxyvitamin D3) and Menaquinone-7 (Vitamin K2) on Ovariectomy-Induced Bone Loss in a Rat Model of Osteoporosis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Vorland, C.J.; Stremke, E.R.; Moorthi, R.N.; Hill Gallant, K.M. Effects of Excessive Dietary Phosphorus Intake on Bone Health. Curr. Osteoporos. Rep. 2017, 15, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Butusov, M.; Jernelov, A. Phosphorus. An Element That Could Have Been Called Lucifer; Springer: New York, NY, USA, 2013; Volume 9, ISBN 978-1-4614-6802-8. [Google Scholar]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Lamberg-Allardt, C.J.E. High Phosphorus Intakes Acutely and Negatively Affect Ca and Bone Metabolism in a Dose-Dependent Manner in Healthy Young Females. Br. J. Nutr. 2006, 96, 545–552. [Google Scholar]
- Kemi, V.E.; Rita, H.J.; Kärkkäinen, M.U.M.; Viljakainen, H.T.; Laaksonen, M.M.; Outila, T.A.; Lamberg-Allardt, C.J.E. Habitual High Phosphorus Intakes and Foods with Phosphate Additives Negatively Affect Serum Parathyroid Hormone Concentration: A Cross-Sectional Study on Healthy Premenopausal Women. Public Health Nutr. 2009, 12, 1885–1892. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Watson, P.; Lappe, J.M. Phosphate and Carbonate Salts of Calcium Support Robust Bone Building in Osteoporosis. Am. J. Clin. Nutr. 2010, 92, 101–105. [Google Scholar] [CrossRef]
- Rafferty, K.; Heaney, R.P. Nutrient Effects on the Calcium Economy: Emphasizing the Potassium Controversy. J. Nutr. 2008, 138, 166S–171S. [Google Scholar] [CrossRef]
- Lee, A.W.; Cho, S.S. Association between Phosphorus Intake and Bone Health in the NHANES Population. Nutr. J. 2015, 14, 28. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The Importance of Mineral Elements for Humans, Domestic Animals and Plants—A Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar] [CrossRef]
- Koyama, Y.; Rittling, S.R.; Tsuji, K.; Hino, K.; Salincarnboriboon, R.; Yano, T.; Taketani, Y.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin Deficiency Suppresses High Phosphate Load-Induced Bone Loss via Specific Modulation of Osteoclasts. Endocrinology 2006, 147, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Morita, K.; Qiao, N.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Colas, but Not Other Carbonated Beverages, Are Associated with Low Bone Mineral Density in Older Women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2006, 84, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Wyshak, G. Teenaged Girls, Carbonated Beverage Consumption, and Bone Fractures. Arch. Pediatr. Adolesc. Med. 2000, 154, 610–613. [Google Scholar] [CrossRef]
- Omelka, R.; Meliskova, V.; Conka, J.; Kovacova, V.; Sranko, P.; Celec, P.; Martiniakova, M. No Effect of Long-Term Cola Intake on Quantitative Characteristics of Femoral Bone in Mice. In Proceedings of the Osteoporosis International: WCO-IOF-ESCEO World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases, Florence, Italy, 23–26 March 2017; pp. S588–S589. [Google Scholar]
- Kristensen, M.; Jensen, M.; Kudsk, J.; Henriksen, M.; Mølgaard, C. Short-Term Effects on Bone Turnover of Replacing Milk with Cola Beverages: A 10-Day Interventional Study in Young Men. Osteoporos. Int. 2005, 16, 1803–1808. [Google Scholar] [CrossRef]
- Dennehy, C.; Tsourounis, C. A Review of Select Vitamins and Minerals Used by Postmenopausal Women. Maturitas 2010, 66, 370–380. [Google Scholar] [CrossRef]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An Update on Physiological, Clinical and Analytical Aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High Magnesium Inhibits Human Osteoblast Differentiation in Vitro. Magnes. Res. 2011, 24, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Rimnac, C.M.; Bansal, M.; Federman, M.; Lian, J.; Boyan, B.D. Effect of Short-Term Hypomagnesemia on the Chemical and Mechanical Properties of Rat Bone. J. Orthop. Res. 1992, 10, 774–783. [Google Scholar] [CrossRef]
- Belluci, M.M.; Giro, G.; Del Barrio, R.A.L.; Pereira, R.M.R.; Marcantonio, E.; Orrico, S.R.P. Effects of Magnesium Intake Deficiency on Bone Metabolism and Bone Tissue around Osseointegrated Implants. Clin. Oral Implants Res. 2011, 22, 716–721. [Google Scholar] [CrossRef]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; LaCroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium Intake, Bone Mineral Density, and Fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Nieves, J.W. Skeletal Effects of Nutrients and Nutraceuticals, beyond Calcium and Vitamin D. Osteoporos. Int. 2013, 24, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, L.B.; Ritenbaugh, C.; Aickin, M.; Lohman, T.G.; Going, S.B.; Weber, J.L.; Greaves, K.A.; Boyden, T.W.; Pamenter, R.W.; Hall, M.C. Nutrients, Body Composition and Exercise Are Related to Change in Bone Mineral Density in Premenopausal Women. J. Nutr. 1995, 125, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, Magnesium, and Fruit and Vegetable Intakes Are Associated with Greater Bone Mineral Density in Elderly Men and Women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Bancerz, B.; Duś-Żuchowska, M.; Cichy, W.; Matusiewicz, H. Effect of Magnesium on Human Health. Gastroenterol. Rev. 2013, 7, 359–366. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Zioła-Frankowska, A.; Kubaszewski, Ł.; Rogala, P.; Frankowski, M. Urban and Rural Area Differences in the Interaction between Oxidative Process Elements in Human Femoral Bone. Environ. Sci. Pollut. Res. 2018, 25, 30475–30487. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Wiechuła, D.; Loska, K. Original Article/Artykuł Oryginalny. J. Orthop. Trauma Surg. Rel. Res. 2008, 2, 17–24. [Google Scholar]
- Zioła-Frankowska, A.; Kubaszewski, Ł.; Dąbrowski, M.; Kowalski, A.; Rogala, P.; Strzyżewski, W.; Łabędź, W.; Uklejewski, R.; Novotny, K.; Kanicky, V.; et al. The Content of the 14 Metals in Cancellous and Cortical Bone of the Hip Joint Affected by Osteoarthritis. BioMed Res. Int. 2015, 2015, e815648. [Google Scholar] [CrossRef]
- Kuo, H.W.; Kuo, S.M.; Chou, C.H.; Lee, T.C. Determination of 14 Elements in Taiwanese Bones. Sci. Total Environ. 2000, 255, 45–54. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in IBD. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, E.; Wells, G.; Shea, B.; Gillespie, W.; Weaver, B.; Zytaruk, N.; Cranney, A.; Adachi, J.; Tugwell, P.; Josse, R.; et al. Meta-Analyses of Therapies for Postmenopausal Osteoporosis. VIII: Meta-Analysis of the Efficacy of Vitamin D Treatment in Preventing Osteoporosis in Postmenopausal Women. Endocr. Rev. 2002, 23, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on Falls: A Meta-Analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Warensjö, E.; Byberg, L.; Melhus, H.; Gedeborg, R.; Mallmin, H.; Wolk, A.; Michaëlsson, K. Dietary Calcium Intake and Risk of Fracture and Osteoporosis: Prospective Longitudinal Cohort Study. BMJ 2011, 342, d1473. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Lips, P.; Bouillon, R.; Bischoff-Ferrari, H.A.; Vanderschueren, D.; Haentjens, P. Need for Additional Calcium to Reduce the Risk of Hip Fracture with Vitamin d Supplementation: Evidence from a Comparative Metaanalysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2007, 92, 1415–1423. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Pamphile, R.; Paris, E.; Kempf, C.; Schlichting, M.; Arnaud, S.; Garnero, P.; Meunier, P.J. Combined Calcium and Vitamin D3 Supplementation in Elderly Women: Confirmation of Reversal of Secondary Hyperparathyroidism and Hip Fracture Risk: The Decalyos II Study. Osteoporos. Int. 2002, 13, 257–264. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of Vitamin D Supplementation on Musculoskeletal Health: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.; Rizkallah, M.; Maalouf, G.; Matta, J.; Frenn, F.; Berro, A.-J.; Barakat, A.; Bachour, F.; Sebaaly, A.; Howayek, M.; et al. Positive Correlations Between Free Vitamin D and Bone Variables in a Group of Young Lebanese Men. J. Clin. Densitom. 2018, 21, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.P.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of Calcium or Calcium in Combination with Vitamin D Supplementation to Prevent Fractures and Bone Loss in People Aged 50 Years and Older: A Meta-Analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Cranney, A.; Horsley, T.; O’Donnell, S.; Weiler, H.; Puil, L.; Ooi, D.; Atkinson, S.; Ward, L.; Moher, D.; Hanley, D.; et al. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Evid. Rep. Technol. Assess. (Full Rep.) 2007, 158, 1–235. [Google Scholar]
- Chung, M.; Balk, E.M.; Brendel, M.; Ip, S.; Lau, J.; Lee, J.; Lichtenstein, A.; Patel, K.; Raman, G.; Tatsioni, A.; et al. Vitamin D and Calcium: A Systematic Review of Health Outcomes. Evid. Rep. Technol. Assess. (Full Rep.) 2009, 183, 1–420. [Google Scholar]
- LeBoff, M.S.; Kohlmeier, L.; Hurwitz, S.; Franklin, J.; Wright, J.; Glowacki, J. Occult Vitamin D Deficiency in Postmenopausal US Women with Acute Hip Fracture. JAMA 1999, 281, 1505–1511. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Men’s Health 2021, 39, 208–221. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. Factors Influencing the Absorption of Vitamin D in GIT: An Overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Need, A.G.; Morris, H.A.; Horowitz, M.; Nordin, C. Effects of Skin Thickness, Age, Body Fat, and Sunlight on Serum 25-Hydroxyvitamin D. Am. J. Clin. Nutr. 1993, 58, 882–885. [Google Scholar] [CrossRef]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group Obesity and Hypovitaminosis D: Causality or Casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women: A Randomized Controlled Trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Devaki, S.J.; Raveendran, R.L. Vitamin C: Sources, Functions, Sensing and Analysis; IntechOpen: London, UK, 2017; ISBN 978-953-51-3422-0. [Google Scholar]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent; IntechOpen: London, UK, 2017; ISBN 978-953-51-3422-0. [Google Scholar]
- Finck, H.; Hart, A.R.; Jennings, A.; Welch, A.A. Is There a Role for Vitamin C in Preventing Osteoporosis and Fractures? A Review of the Potential Underlying Mechanisms and Current Epidemiological Evidence. Nutr. Res. Rev. 2014, 27, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, O.; Łukasik, Z.; Makowska, J.; Walczak, K. Role of Vitamin C in Osteoporosis Development and Treatment—A Literature Review. Nutrients 2020, 12, 2394. [Google Scholar] [CrossRef]
- Fain, O. Musculoskeletal Manifestations of Scurvy. Jt. Bone Spine 2005, 72, 124–128. [Google Scholar] [CrossRef]
- Simon, J.A.; Hudes, E.S. Relation of Ascorbic Acid to Bone Mineral Density and Self-Reported Fractures among US Adults. Am. J. Epidemiol. 2001, 154, 427–433. [Google Scholar] [CrossRef]
- Arslan, A.; Orkun, S.; Aydin, G.; Keles, I.; Tosun, A.; Arslan, M.; Caglayan, O. Effects of Ovariectomy and Ascorbic Acid Supplement on Oxidative Stress Parameters and Bone Mineral Density in Rats. Libyan J. Med. 2011, 6. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, K.M.; Lim, S.; Choi, S.H.; Moon, J.H.; Kim, J.H.; Kim, S.W.; Jang, H.C.; Shin, C.S. Favorable Effect of Dietary Vitamin C on Bone Mineral Density in Postmenopausal Women (KNHANES IV, 2009): Discrepancies Regarding Skeletal Sites, Age, and Vitamin D Status. Osteoporos. Int. 2015, 26, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Arabi, A. Vitamins and Bone Health: Beyond Calcium and Vitamin D. Nutr. Rev. 2011, 69, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Chuin, A.; Labonté, M.; Tessier, D.; Khalil, A.; Bobeuf, F.; Doyon, C.Y.; Rieth, N.; Dionne, I.J. Effect of Antioxidants Combined to Resistance Training on BMD in Elderly Women: A Pilot Study. Osteoporos. Int. 2009, 20, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. [Google Scholar]
- Carr, A.C.; Frei, B. Toward a New Recommended Dietary Allowance for Vitamin C Based on Antioxidant and Health Effects in Humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C Function and Status in Chronic Disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in Global Vitamin C Recommendations: A Review of RDA Criteria and Underlying Health Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary Factors and the Risk of Incident Kidney Stones in Men: New Insights after 14 Years of Follow-Up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef]
- Feskanich, D.; Weber, P.; Willett, W.C.; Rockett, H.; Booth, S.L.; Colditz, G.A. Vitamin K Intake and Hip Fractures in Women: A Prospective Study. Am. J. Clin. Nutr. 1999, 69, 74–79. [Google Scholar] [CrossRef]
- Lacombe, J.; Al Rifai, O.; Loter, L.; Moran, T.; Turcotte, A.-F.; Grenier-Larouche, T.; Tchernof, A.; Biertho, L.; Carpentier, A.C.; Prud’homme, D.; et al. Measurement of Bioactive Osteocalcin in Humans Using a Novel Immunoassay Reveals Association with Glucose Metabolism and β-Cell Function. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E381–E391. [Google Scholar] [CrossRef]
- Wei, J.; Karsenty, G. An Overview of the Metabolic Functions of Osteocalcin. Rev. Endocr. Metab. Disord. 2015, 16, 93–98. [Google Scholar] [CrossRef]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Bots, M.L.; Atsma, F.; Bartelink, M.-L.E.L.; Prokop, M.; Geleijnse, J.M.; Witteman, J.C.M.; Grobbee, D.E.; van der Schouw, Y.T. High Dietary Menaquinone Intake Is Associated with Reduced Coronary Calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Inoue, S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. Int. J. Mol. Sci. 2019, 20, 2844. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-J.; Kim, M.S.; Ahn, B.-Y. The Inhibitory Effect of Vitamin K on RANKL-Induced Osteoclast Differentiation and Bone Resorption. Food Funct. 2015, 6, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ouchi, Y.; Inoue, S. Vitamin K: Novel Molecular Mechanisms of Action and Its Roles in Osteoporosis. Geriatr. Gerontol. Int. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D.J. Vitamin K and the Prevention of Fractures: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Intern. Med. 2006, 166, 1256–1261. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Wan, S.-L.; Lu, Y.-J.; Ning, L.; Liu, C.; Fan, S.-W. Does Vitamin K2 Play a Role in the Prevention and Treatment of Osteoporosis for Postmenopausal Women: A Meta-Analysis of Randomized Controlled Trials. Osteoporos. Int. 2015, 26, 1175–1186. [Google Scholar] [CrossRef]
- Su, S.; He, N.; Men, P.; Song, C.; Zhai, S. The Efficacy and Safety of Menatetrenone in the Management of Osteoporosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Osteoporos. Int. 2019, 30, 1175–1186. [Google Scholar] [CrossRef]
- Kanellakis, S.; Moschonis, G.; Tenta, R.; Schaafsma, A.; van den Heuvel, E.G.H.M.; Papaioannou, N.; Lyritis, G.; Manios, Y. Changes in Parameters of Bone Metabolism in Postmenopausal Women Following a 12-Month Intervention Period Using Dairy Products Enriched with Calcium, Vitamin D, and Phylloquinone (Vitamin K(1)) or Menaquinone-7 (Vitamin K (2)): The Postmenopausal Health Study II. Calcif. Tissue Int. 2012, 90, 251–262. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Marković, Z.; Stupić, D.; Maletić, E.; Kontić, J.K.; Preiner, D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Lima, C.-Y.O.C. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; IntechOpen: London, UK, 2017; ISBN 978-953-51-2960-8. [Google Scholar]
- Taleb-Contini, S.H.; Salvador, M.J.; Balanco, J.M.F.; Albuquerque, S.; de Oliveira, D.C.R. Antiprotozoal Effect of Crude Extracts and Flavonoids Isolated from Chromolaena Hirsuta (Asteraceae). Phytother. Res. 2004, 18, 250–254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Osteoprotective Effects Of Kaempferol: The Evidence From In Vivo And In Vitro Studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef]
- Sharma, A.R.; Nam, J.-S. Kaempferol Stimulates WNT/β-Catenin Signaling Pathway to Induce Differentiation of Osteoblasts. J. Nutr. Biochem. 2019, 74, 108228. [Google Scholar] [CrossRef]
- Pang, J.L.; Ricupero, D.A.; Huang, S.; Fatma, N.; Singh, D.P.; Romero, J.R.; Chattopadhyay, N. Differential Activity of Kaempferol and Quercetin in Attenuating Tumor Necrosis Factor Receptor Family Signaling in Bone Cells. Biochem. Pharmacol. 2006, 71, 818–826. [Google Scholar] [CrossRef]
- Hirata, M.; Matsumoto, C.; Takita, M.; Miyaura, C.; Inada, M. Naringin Suppresses Osteoclast Formation and Enhances Bone Mass in Mice. J. Health Sci. 2009, 55, 463–467. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.-L.; Huo, X.-C.; Wang, J.-H.; Wang, D.-P.; Zhu, Q.-L.; Liu, B.; Xu, L.-L. Rutin Prevents the Ovariectomy-Induced Osteoporosis in Rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1911–1917. [Google Scholar]
- Gera, S.; Pooladanda, V.; Godugu, C.; Swamy Challa, V.; Wankar, J.; Dodoala, S.; Sampathi, S. Rutin Nanosuspension for Potential Management of Osteoporosis: Effect of Particle Size Reduction on Oral Bioavailability, in Vitro and in Vivo Activity. Pharm. Dev. Technol. 2020, 25, 971–988. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jung, J.W.; Ha, B.B.G.; Hong, J.M.; Park, E.K.; Kim, H.-J.; Kim, S.-Y. The Effects of Luteolin on Osteoclast Differentiation, Function in Vitro and Ovariectomy-Induced Bone Loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wang, C.; Yang, Q.; Wei, X.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Ma, X.; Liu, K.; et al. Luteolin Attenuates Glucocorticoid-Induced Osteoporosis by Regulating ERK/Lrp-5/GSK-3β Signaling Pathway in Vivo and in Vitro. J. Cell. Physiol. 2019, 234, 4472–4490. [Google Scholar] [CrossRef] [PubMed]
- Vakili, S.; Zal, F.; Mostafavi-pour, Z.; Savardashtaki, A.; Koohpeyma, F. Quercetin and Vitamin E Alleviate Ovariectomy-Induced Osteoporosis by Modulating Autophagy and Apoptosis in Rat Bone Cells. J. Cell. Physiol. 2021, 236, 3495–3509. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, Z.; Cai, G. Comparison of Neoeriocitrin and Naringin on Proliferation and Osteogenic Differentiation in MC3T3-E1. Phytomedicine 2011, 18, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yang, Y.; Xiao, X.; Sun, Y.; Zhou, Y.; Zhang, Y.; Dong, D.; Li, C.; Wu, X.; Li, Y.; et al. Quercetin Prevents Bone Loss in Hindlimb Suspension Mice via Stanniocalcin 1-Mediated Inhibition of Osteoclastogenesis. Acta Pharmacol. Sin. 2020, 41, 1476–1486. [Google Scholar] [CrossRef]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated Daily Intake and Seasonal Food Sources of Quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef]
- Tsuji, M.; Yamamoto, H.; Sato, T.; Mizuha, Y.; Kawai, Y.; Taketani, Y.; Kato, S.; Terao, J.; Inakuma, T.; Takeda, E. Dietary Quercetin Inhibits Bone Loss without Effect on the Uterus in Ovariectomized Mice. J. Bone Miner. Metab. 2009, 27, 673–681. [Google Scholar] [CrossRef]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin Rescued TNF-Alpha-Induced Impairments in Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and Improved Osteoporosis in Rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar]
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.-R.A.; Mohamed, E.K. Enhanced Therapeutic Benefit of Quercetin-Loaded Phytosome Nanoparticles in Ovariectomized Rats. Chem. Biol. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef]
- Ge, Y.; Feng, K.; Liu, X.; Zhu, Z.; Chen, H.; Chang, Y.; Sun, Z.; Wang, H.; Zhang, J.; Yu, D.; et al. Quercetin Inhibits Macrophage Polarization through the P-38α/β Signalling Pathway and Regulates OPG/RANKL Balance in a Mouse Skull Model. J. Cell. Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Quercetin, a Potent Suppressor of NF-ΚB and Smad Activation in Osteoblasts. Int. J. Mol. Med. 2011, 28, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.-T.; Nakagawa, H.; Notoya, M.; Yonezawa, T.; Udagawa, N.; Lee, I.-S.; Ohnishi, M.; Hagiwara, H.; Nagai, K. Quercetin Suppresses Bone Resorption by Inhibiting the Differentiation and Activation of Osteoclasts. Biol. Pharm. Bull. 2004, 27, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Raja, N.; Yun, H.S. Effect of Direct Loading of Phytoestrogens into the Calcium Phosphate Scaffold on Osteoporotic Bone Tissue Regeneration. J. Mater. Chem. B 2015, 3, 8694–8703. [Google Scholar] [CrossRef]
- Kim, D.-S.; Takai, H.; Arai, M.; Araki, S.; Mezawa, M.; Kawai, Y.; Murota, K.; Terao, J.; Ogata, Y. Effects of Quercetin and Quercetin 3-Glucuronide on the Expression of Bone Sialoprotein Gene. J. Cell. Biochem. 2007, 101, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Swarnkar, G.; Sharan, K.; Chakravarti, B.; Gautam, A.K.; Rawat, P.; Kumar, M.; Gupta, V.; Manickavasagam, L.; Dwivedi, A.K.; et al. A Naturally Occurring Rare Analog of Quercetin Promotes Peak Bone Mass Achievement and Exerts Anabolic Effect on Osteoporotic Bone. Osteoporos. Int. 2011, 22, 3013–3027. [Google Scholar] [CrossRef]
- Prouillet, C.; Mazière, J.-C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory Effect of Naturally Occurring Flavonols Quercetin and Kaempferol on Alkaline Phosphatase Activity in MG-63 Human Osteoblasts through ERK and Estrogen Receptor Pathway. Biochem. Pharmacol. 2004, 67, 1307–1313. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Tjhioe, W.; Zhao, J.; Lu, A.; Zhang, G.; Tan, R.X.; Zhou, M.; Xu, J.; Feng, H.T. Therapeutic Potential and Outlook of Alternative Medicine for Osteoporosis. Curr. Drug Targets 2017, 18, 1051–1068. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Yang, J.; Wang, Z.; Cheng, L. Quercetin Promotes Osteogenic Differentiation and Antioxidant Responses of Mouse Bone Mesenchymal Stem Cells through Activation of the AMPK/SIRT1 Signaling Pathway. Phytother. Res. 2021, 35, 2639–2650. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic Potential and Recent Advances in Drug Delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Lee, H.-H.; Jang, J.-W.; Lee, J.-K.; Park, C.-K. Rutin Improves Bone Histomorphometric Values by Reduction of Osteoclastic Activity in Osteoporosis Mouse Model Induced by Bilateral Ovariectomy. J. Korean Neurosurg. Soc. 2020, 63, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Beck, T.J.; Reeve, J.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Cummings, S.R. Prediction of Incident Hip Fracture Risk by Femur Geometry Variables Measured by Hip Structural Analysis in the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2008, 23, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Kyung, T.-W.; Lee, J.-E.; Shin, H.-H.; Choi, H.-S. Rutin Inhibits Osteoclast Formation by Decreasing Reactive Oxygen Species and TNF-α by Inhibiting Activation of NF-ΚB. Exp. Mol. Med. 2008, 40, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.T.; Steel, S.A.; Aye, M.; Doherty, S.M. The Effect of Prior Bisphosphonate Therapy on the Subsequent BMD and Bone Turnover Response to Strontium Ranelate. J. Bone Miner. Res. 2010, 25, 455–462. [Google Scholar] [CrossRef]
- Horcajada-Molteni, M.N.; Crespy, V.; Coxam, V.; Davicco, M.J.; Rémésy, C.; Barlet, J.P. Rutin Inhibits Ovariectomy-Induced Osteopenia in Rats. J. Bone Miner. Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef]
- Xiao, Y.; Wei, R.; Yuan, Z.; Lan, X.; Kuang, J.; Hu, D.; Song, Y.; Luo, J. Rutin Suppresses FNDC1 Expression in Bone Marrow Mesenchymal Stem Cells to Inhibit Postmenopausal Osteoporosis. Am. J. Transl. Res. 2019, 11, 6680–6690. [Google Scholar] [PubMed]
- Kotanidou, A.; Xagorari, A.; Bagli, E.; Kitsanta, P.; Fotsis, T.; Papapetropoulos, A.; Roussos, C. Luteolin Reduces Lipopolysaccharide-Induced Lethal Toxicity and Expression of Proinflammatory Molecules in Mice. Am. J. Respir. Crit. Care Med. 2002, 165, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Fatokun, A.A.; Tome, M.; Smith, R.A.; Darlington, L.G.; Stone, T.W. Protection by the Flavonoids Quercetin and Luteolin against Peroxide- or Menadione-Induced Oxidative Stress in MC3T3-E1 Osteoblast Cells. Nat. Prod. Res. 2015, 29, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin Inhibits an Endotoxin-Stimulated Phosphorylation Cascade and Proinflammatory Cytokine Production in Macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar]
- Choi, E.-M. Modulatory Effects of Luteolin on Osteoblastic Function and Inflammatory Mediators in Osteoblastic MC3T3-E1 Cells. Cell Biol. Int. 2007, 31, 870–877. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Jung, J.-W.; Kim, T.-H.; Hong, J.M.; Kim, H.-J.; Park, E.K. Effect of Luteolin on Bone Resorption, Bone Loss and Microarchitecture in Ovariectomized Mice. In Proceedings of the 55th Annual Meeting of the Orthopaedic Research Society, Las Vegas, NV, USA, 22-25 February 2009; Volume 1. [Google Scholar]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-Preventive and Therapeutic Effect of the Dietary Flavonoid Kaempferol: A Comprehensive Review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kumar, S.; Kumar, A.; Siddiqui, J.A.; Swarnkar, G.; Gupta, V.; Kendurker, A.; Dwivedi, A.K.; Romero, J.R.; Chattopadhyay, N. Kaempferol Has Osteogenic Effect in Ovariectomized Adult Sprague-Dawley Rats. Mol. Cell. Endocrinol. 2008, 289, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Matuszewska, A.; Nikodem, A.; Filipiak, J.; Landwójtowicz, M.; Sadanowicz, E.; Jędrzejuk, D.; Rzeszutko, M.; Zduniak, K.; Piasecki, T.; et al. Oral Administration of Kaempferol Inhibits Bone Loss in Rat Model of Ovariectomy-Induced Osteopenia. Pharmacol. Rep. 2017, 69, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yi, X.; Tu, S.; Cheng, C.; Luo, J. Kaempferol Promotes BMSC Osteogenic Differentiation and Improves Osteoporosis by Downregulating MiR-10a-3p and Upregulating CXCL12. Mol. Cell. Endocrinol. 2021, 520, 111074. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Shin, S.-H.; Kim, B.-J.; Kim, C.-H.; Kim, J.-H.; Kang, H.-M.; Park, B.-S.; Kim, I.-R. The Effects of Kaempferol-Inhibited Autophagy on Osteoclast Formation. Int. J. Mol. Sci. 2018, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Nie, Y.; Cao, D.-P.; Xue, Y.-Y.; Wang, J.-S.; Zhao, L.; Rahman, K.; Zhang, Q.-Y.; Qin, L.-P. Potential Antiosteoporotic Agents from Plants: A Comprehensive Review. Evid. Based Complement. Altern. Med. 2012, 2012, e364604. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, X.; Liu, S.; Nicholson, R.C.; Ni, X. Phytoestrogens Induce Differential Estrogen Receptor β-Mediated Responses in Transfected MG-63 Cells. Endocrine 2008, 34, 29–35. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic Compounds from Seeds of Cuscuta Chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Yu, K.E.; Alder, K.D.; Morris, M.T.; Munger, A.M.; Lee, I.; Cahill, S.V.; Kwon, H.-K.; Back, J.; Lee, F.Y. Re-Appraising the Potential of Naringin for Natural, Novel Orthopedic Biotherapies. Ther. Adv. Musculoskelet. 2020, 12, 1759720X20966135. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-M.; Yang, Y.-J.; Zhang, L.; Zhang, X.; Guan, F.-F.; Zhang, L.-F. Naringin Enhances CaMKII Activity and Improves Long-Term Memory in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2013, 14, 5576–5586. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.-Y.; Wang, X.-L.; Mok, S.-K.; Lai, W.-P.; Chow, H.-K.; Leung, P.-C.; Yao, X.-S.; Wong, M.-S. Naringin Improves Bone Properties in Ovariectomized Mice and Exerts Oestrogen-like Activities in Rat Osteoblast-like (UMR-106) Cells. Br. J. Pharmacol. 2010, 159, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Wang, F.; Dong, J.; Wang, D.; Sun, B.; Wang, B. Stimulation of Wnt/β-Catenin Signaling to Improve Bone Development by Naring.gin via Interacting with AMPK and Akt. Cell. Physiol. Biochem. 2015, 36, 1563–1576. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Wooley, P.H.; Xu, Z.; Yang, S.-Y. Naringin Promotes Osteoblast Differentiation and Effectively Reverses Ovariectomy-Associated Osteoporosis. J. Orthop. Sci. 2013, 18, 478–485. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, W.; Li, Y.; Zhu, Z.; Zhang, W. Effect of Naringin Treatment on Postmenopausal Osteoporosis in Ovariectomized Rats: A Meta-Analysis and Systematic Review. Evid. Based Complement. Altern. Med. 2021, 2021, e6016874. [Google Scholar] [CrossRef]
- Wu, J.-B.; Fong, Y.-C.; Tsai, H.-Y.; Chen, Y.-F.; Tsuzuki, M.; Tang, C.-H. Naringin-Induced Bone Morphogenetic Protein-2 Expression via PI3K, Akt, c-Fos/c-Jun and AP-1 Pathway in Osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef]
- Wong, R.W.K.; Rabie, A.B.M. Effect of Naringin on Bone Cells. J. Orthop. Res. 2006, 24, 2045–2050. [Google Scholar] [CrossRef]
| Type of Food | Amount of Nutrients per 100 g |
|---|---|
| Proteins (g) [24] | |
| meat (beef, pork, chicken) | 15.43–20.04 |
| sea fish | 18.88 |
| pike, trout | 11.00 |
| oatmeal | 13.10 |
| eggs | 12.40 |
| dairy products (yogurt, cheese, cream cheese) | 4.40–26.00 |
| legumes (chickpeas, beans, peas, lentils) | 20–24.20 |
| tofu | 7.80 |
| soy | 35.40 |
| cocoa powder | 22.70 |
| nuts (almonds, peanuts, pistachios) | 19.70–25.80 |
| seeds (poppy, sesame, pumpkins) | 20.40–24.50 |
| Saturated fatty acids (g) [25,26] | |
| milk fat | 63.40 |
| coconut oil | 86.50 |
| palm kernel oil | 81.50 |
| palm oil | 49.30 |
| cocoa butter | 59.70 |
| Monounsaturated fatty acids (g) [25,26] | |
| milk fat | 25.90 |
| palm oil | 37.00 |
| cocoa butter | 32.90 |
| olive oil | 73.00 |
| soybean oil | 22.70 |
| high linoleic acid sunflower oil | 19.50 |
| Polyunsaturated fatty acids (g) [25,26] | |
| palm oil | 9.30 |
| olive oil | 10.50 |
| soybean oil | 57.30 |
| high linoleic acid sunflower oil | 65.70 |
| Carbohydrates (g) [24] | |
| oatmeal | 68.10 |
| wheat flour | 73.10 |
| wheat white bread | 50.80 |
| legumes (chickpeas, beans, peas, lentils) | 58.00–60.50 |
| fruits (blackcurrant, grapes, bananas) | 17.20–21.80 |
| vegetables (potatoes, sweet corn, garlic) | 18.80–25.00 |
| nuts (peanuts, pistachios, chestnuts) | 18.20–53.00 |
| curry spice | 61.80 |
| black tea | 55.70 |
| bitter chocolate | 117.12 |
| Calcium (mg) [25,26] | |
| cow milk (natural) | 119.14 |
| hard cheese | 981.71–1218.00 |
| soft cheese | 732.86 |
| eggs | 57.06 |
| marjoram | 1388.00 |
| poppy seeds (natural) | 1513.71 |
| salmon (Atlantic) | 20.00 |
| almonds | 229.71 |
| green-leaf vegetables (head cabbage, curly kale) | 47.50–163.57 |
| legume-based dishes | 30.91 |
| tofu | 162.43 |
| seafood-based dishes | 38.05 |
| mineral water rich in calcium (250 mL) | 100.00 |
| Phosphorus (mg) [25,26] | |
| meat (chicken, duck, turkey, goat) | 188.57–234.86 |
| cow milk | 92.86 |
| hard cheese | 786.86 |
| soft cheese | 345.57 |
| cereals and cereal-like grains | 271.00 |
| seeds (linseed, pumpkin, sesame, sunflower, poppy) | 603.00–861.71 |
| nuts (cashew, peanuts, walnuts) | 519.57–369.71 |
| legume-based dishes | 132.37 |
| Magnesium (mg) [25,26] | |
| spinach | 61.99 |
| legume-based dishes | 41.20 |
| nuts (walnuts, hazelnuts, peanuts, almonds, cashew) | 150.71–262.14 |
| seeds (linseed, pumpkin, sesame, sunflower) | 272.00–358.83 |
| grain-based dishes | 100.00 |
| bitter chocolate | 164.29 |
| Vitamin D (μg) [27,28] | |
| D2/D3 in soybean oil | 700.00 |
| D2/D3 in sunflower oil | 11.20–14.50 |
| D2 in dry mushroom powder | 4420.00 |
| eggs | 3.20 |
| fishes (mackerel, salmon, sardines, tuna) | 3.20–8.00 |
| cod | trace |
| Vitamin C (mg) [29,30,31] | |
| citrus fruits (lemon, orange, grapefruit) products | 30.00–53.00 |
| broccoli | 34.80–93.10 |
| tomato products | 12.00 |
| peppers (red peppers, chili peppers) | 190.00–245.00 |
| green leafy vegetables (spinach, cabbage, kale, cauliflower) | 30.00–48.00 |
| potatoes | 25.00 |
| papaya | 61.00 |
| kiwifruit | 93.00 |
| red current | 80.00 |
| strawberry and its products | 54.00–60.00 |
| Vitamin K (μg) [25,26] | |
| dark green leafy vegetables (spinach, curly kale) | 362.50–817.00 |
| fruits (kiwifruit, blackcurrants, prunes, rose hip) | 25.00–92.00 |
| chickpea | 264.00 |
| liver (beef, chicken, veal) | 75.00–89.00 |
| parsley | 488.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. https://doi.org/10.3390/nu14030523
Martiniakova M, Babikova M, Mondockova V, Blahova J, Kovacova V, Omelka R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients. 2022; 14(3):523. https://doi.org/10.3390/nu14030523
Chicago/Turabian StyleMartiniakova, Monika, Martina Babikova, Vladimira Mondockova, Jana Blahova, Veronika Kovacova, and Radoslav Omelka. 2022. "The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis" Nutrients 14, no. 3: 523. https://doi.org/10.3390/nu14030523
APA StyleMartiniakova, M., Babikova, M., Mondockova, V., Blahova, J., Kovacova, V., & Omelka, R. (2022). The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients, 14(3), 523. https://doi.org/10.3390/nu14030523






