DXA-Derived Indices in the Characterisation of Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants, Study Description and Definition of Comorbidities
2.2. Anthropometric Measurements
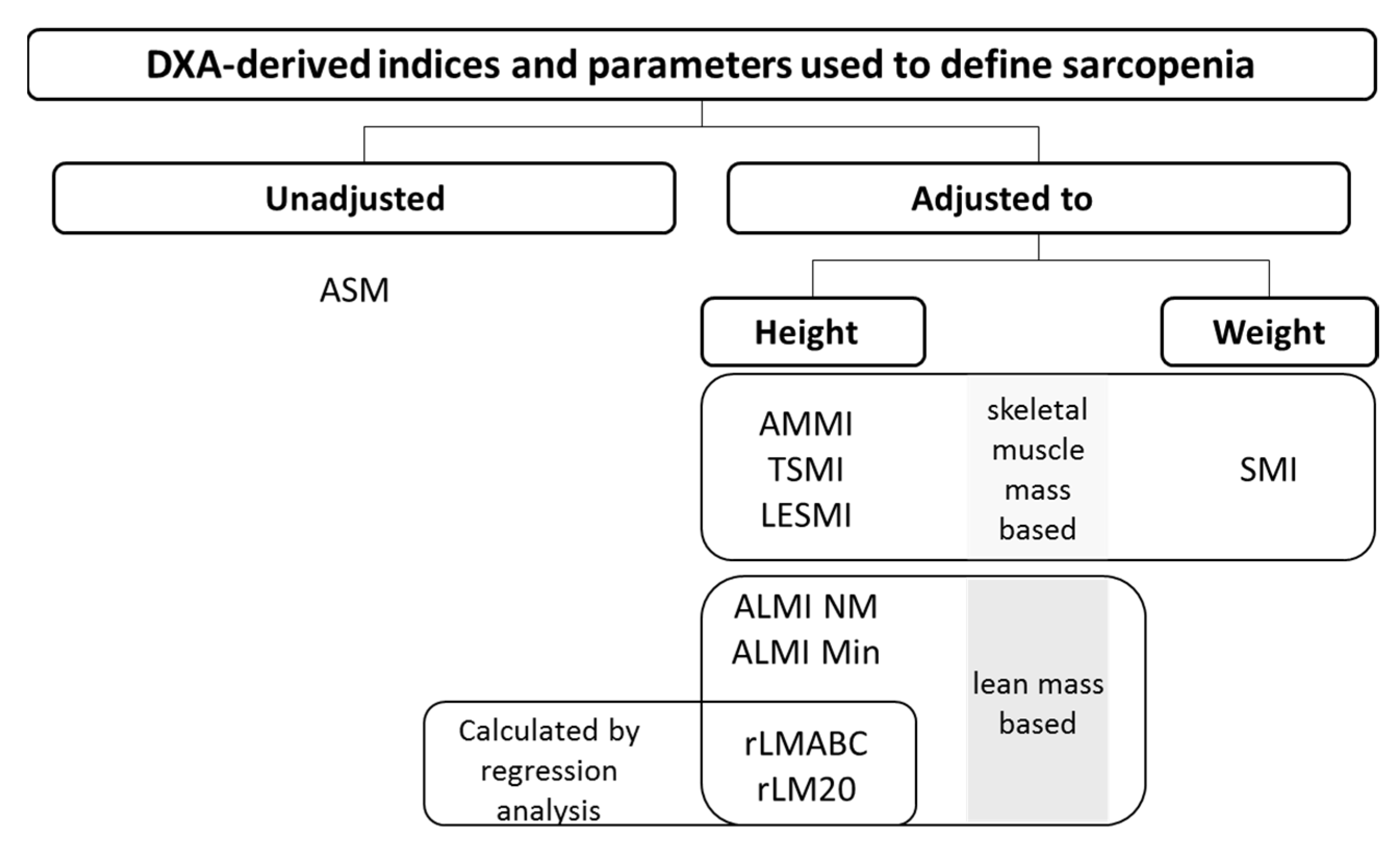
2.3. DXA Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Sarcopenia Prevalence According to Each DXA-Derived Index and ASM
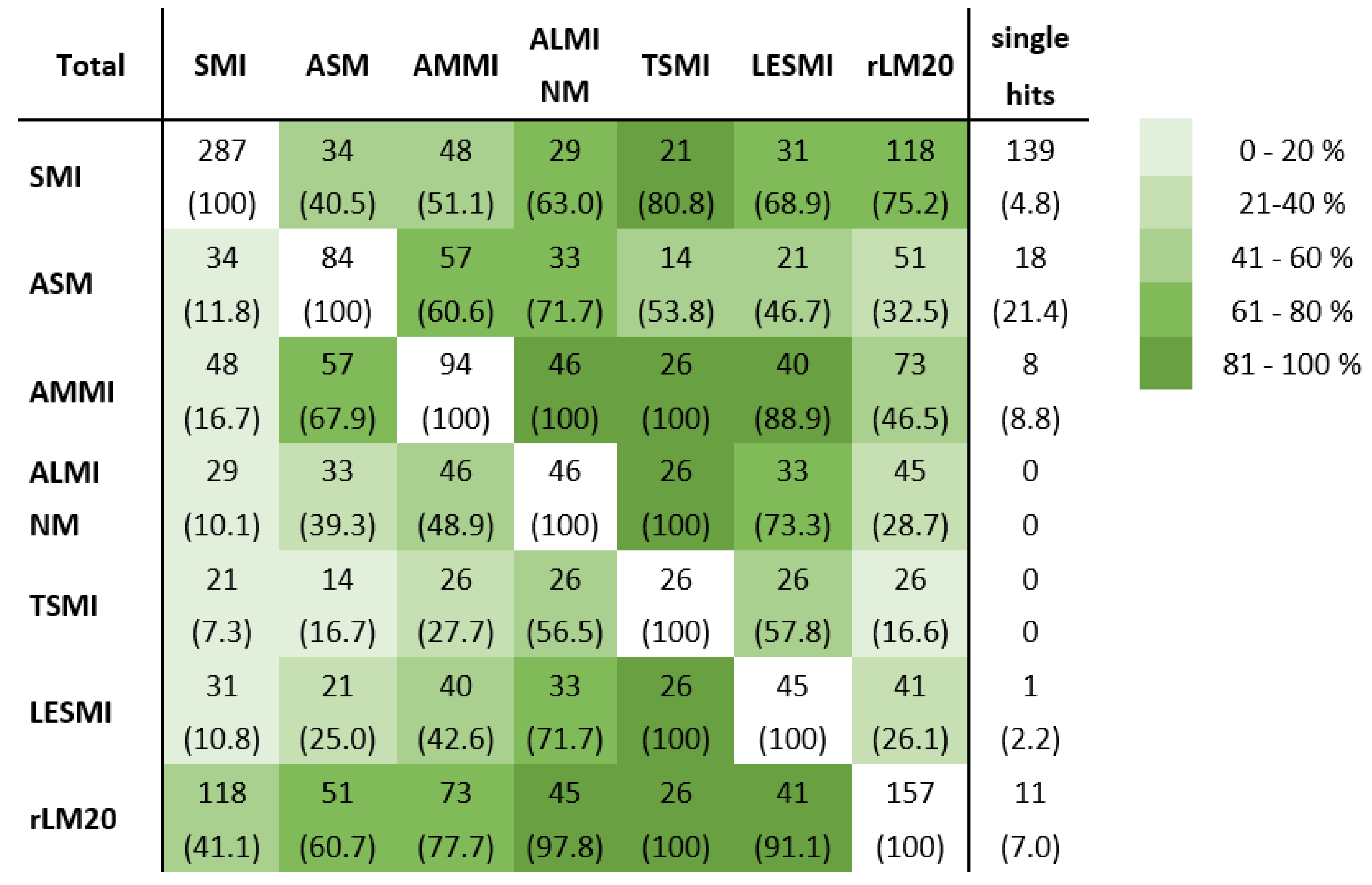
3.3. Sarcopenia Diagnosis Agreement
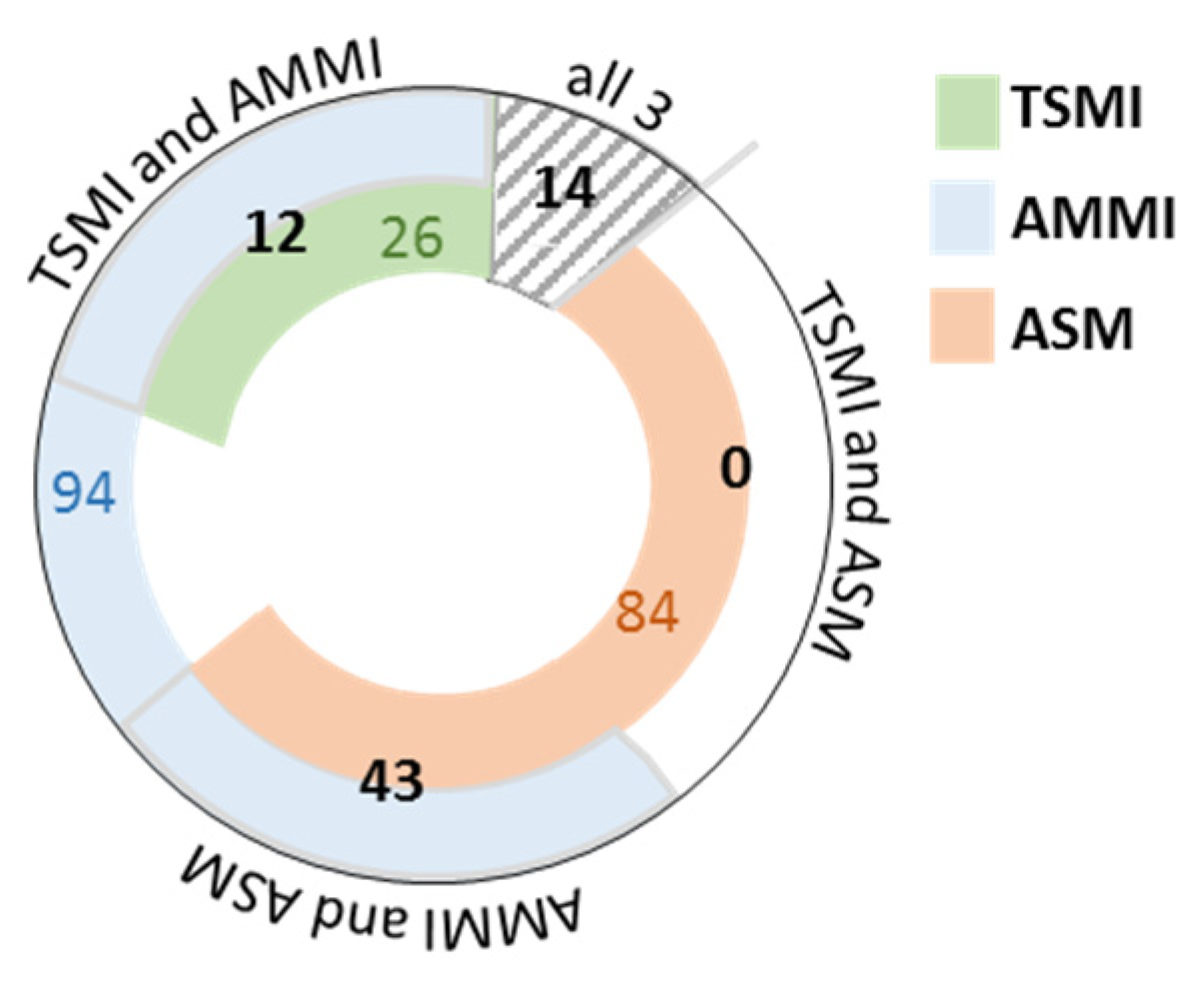
3.3.1. Comparison of Sarcopenia Diagnosis Agreement of All Investigated Indices
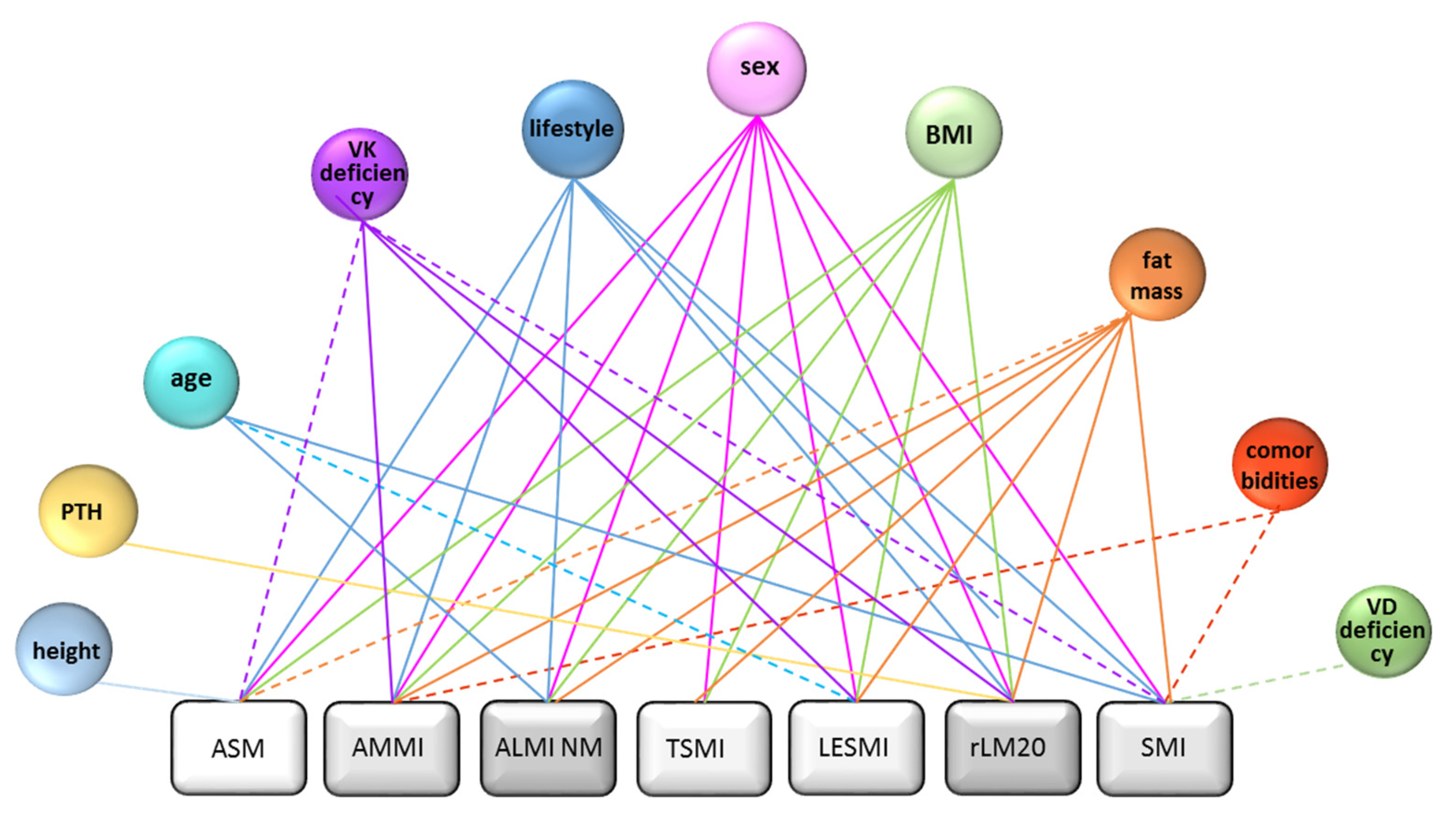
3.3.2. Analysis of Factors Affecting Sarcopenia Diagnosis Agreement
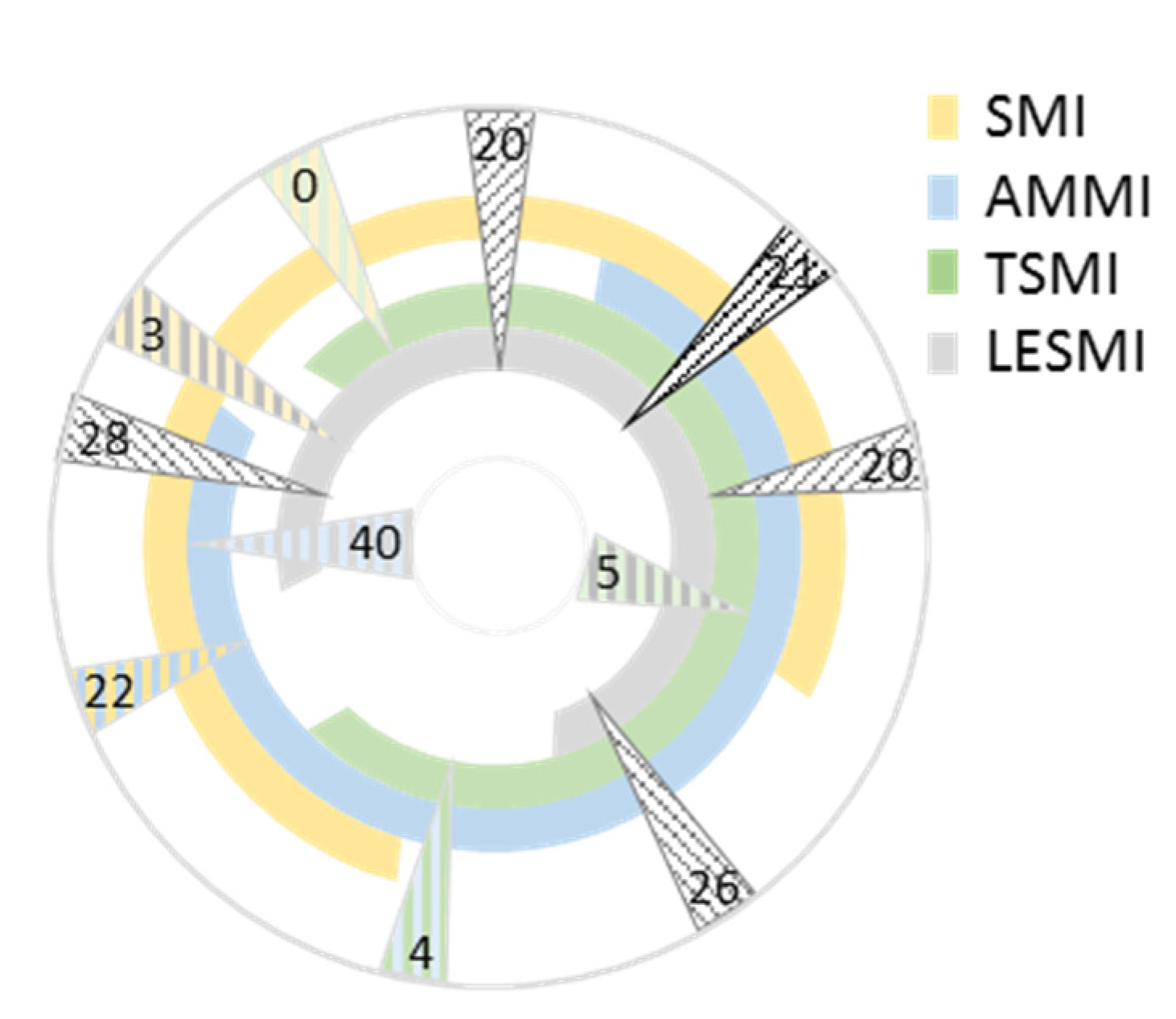
3.3.3. Underlying Muscle Mass Parameter and Sarcopenia Diagnosis Agreement
Skeletal Muscle Mass Based Indices
Lean Mass-Based Indices
Absence or Presence of an Adjustment and Sarcopenia Diagnosis Agreement
Type of Adjustment and Sarcopenia Diagnosis Agreement
Binary Logistic Regression Models to Determine Nutritional and Clinical Influencing Factors of DXA-Indices
3.4. Evaluation of the Reliability of Sarcopenia Diagnosis
3.4.1. Comparison of Diagnosis Reliability of Investigated Indices
3.4.2. Adjustment and Sarcopenia Diagnosis Reliability
Sarcopenia Diagnosis Reliability of Combined Height Adjusted Parameters
Sarcopenia Diagnosis Reliability of Adjustment Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALMI | appendicular lean mass index |
| ALMI Min | ALMI with reference values Minnesota |
| ALMI NM | ALMI with reference values New Mexico |
| AMMI | appendicular skeletal muscle mass index |
| ASM | appendicular skeletal muscle mass |
| LESMI | lower extremity skeletal muscle mass index |
| rLM20 | relative lean mass (20th percentile) |
| rLMABC | relative lean mass (20th percentile Health ABC study) |
| SMI | skeletal muscle mass index |
References
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.-F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, R.; Kuh, D.; Hardy, R. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- De Buyser, S.L.; Petrovic, M.; Taes, Y.E.; Toye, K.R.C.; Kaufman, J.-M.; Lapauw, B.; Goemaere, S. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing 2016, 45, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A. Sarcopenia and malnutrition: Commonly occurring conditions in the older population. Br. J. Nurs. 2021, 30, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Borba, V.Z.C.; Costa, T.L.; Moreira, C.A.; Boguszewski, C.L. Mechanisms of endocrine disease sarcopenia in endocrine and non-endocrine disorders. Eur. J. Endocrinol. 2019, 180, R185–R199. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Aspects Med. 2016, 50, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Grimby, G.; Saltin, B. The ageing muscle. Clin. Physiol. 1983, 3, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.A.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.-J.; Bae, G.-U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharm. Res. 2021, 44, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Rooks, D.; Roubenoff, R. Development of Pharmacotherapies for the Treatment of Sarcopenia. J. Frailty Aging 2019, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.F.; Berends, B.; Vedder, I.R.; Levolger, S.; Gupta, M.; Schuurmann, R.C.; de Vries, J.P.P.M.; Bokkers, R.P. Quantification of muscle mass in the legs of patients with peripheral arterial occlusive disease: Associations between volumetric and cross-sectional single-slice measurements for identification of atrophy and focal sarcopenia. J. Cardiovasc. Surg. (Torino) 2019, 60, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Park, S.-G.; Ryu, S.M.; Park, C.-H. New Skeletal Muscle Mass Index in Diagnosis of Sarcopenia. J. Bone Metab. 2018, 25, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, A.B.; Hannan, M.T.; Murabito, J.; Kiel, D.; McLean, R.R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The framingham study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Melton, J.L.; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, M.W.; Riggs, B.L. Epidemiology Sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Agein 2019, 48, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, D.N.; O’Brien, P.C.; Atkinson, E.J.; Nair, K.S. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am. J. Physiol.-Endocrinol. Metab. 1999, 277, 40–43. [Google Scholar] [CrossRef]
- Pagotto, V.; Silveira, E.A. Methods, diagnostic criteria, cutoff points, and prevalence of sarcopenia among older people. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemmler, W.; Teschler, M.; Weißenfels, A.; Sieber, C.C.; Freiberger, E.; Von Stengel, S. Prevalence of sarcopenia and sarcopenic obesity in older German men using recognized definitions: High accordance but low overlap! Osteoporos. Int. 2017, 28, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Stanley, A.; Pietrobelli, A.; Heo, M. Simple Skeletal Muscle Mass Estimation Formulas: What We Can Learn From Them. Front. Endocrinol. (Lausanne) 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Choi, K.M. Review Article Sarcopenia and Sarcopenic Obesity. Korean J. Intern. Med. 2016, 31, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Heymsfield, S.B.; Scherzer, R.; Pietrobelli, A.; Lewis, C.E.; Grunfeld, C. Body mass index as a phenotypic expression of adiposity: Quantitative contribution of muscularity in a population-based sample. Int. J. Obes. (Lond) 2009, 33, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.S.; Kim, T.H.; Kim, H.; Choi, E.H.; Kim, N.; Kong, I.D. Qualitative muscle mass index as a predictor of skeletal muscle function deficit in Asian older adults. Geriatr. Gerontol. Int. 2017, 17, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Kemmler, W.; von Stengel, S.; Kohl, M. Developing sarcopenia criteria and cutoffs for an older caucasian cohort–A strictly biometrical approach. Clin. Interv. Aging 2018, 13, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Patterns of Nutrient Intake in Relation to Sarcopenia and Its Components. Front. Nutr. 2021, 8, 645072. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Chen, K.-H.; Chen, C.; Chu, W.-C.; Kang, Y.-N. The optimal strategy of vitamin d for sarcopenia: A network meta-analysis of randomized controlled trials. Nutrients 2021, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
| Definition of Sarcopenia | ||||||
|---|---|---|---|---|---|---|
| Unit | Calculation | Cut-Off Values | References | Study/Organisation | ||
| males | females | |||||
| SMI | % | ASM/weight[kg] × 100 | <29.9 | <23.5 | Moon 2018 [19] | KNHANESV |
| LESMI | kg/m2 | LESM/height2 | <5.1 | <3.7 | Moon 2018 [19] | KNHANESV |
| rLM20 | n.a. | ALM adjusted for fat mass | 20th percentile | Newman 2003 [20] | Our study | |
| rLMABC | n.a. | and height | −2.29 | −1.73 | Newman 2003 [20] | ABC Health Study |
| TSMI | kg/m2 | TSM/height2 | 9 | 6 | Dufour 2013 [21] | Framingham Heart Study |
| ALMI | kg/m2 | ALM/height2 | <7.26 | <5.45 | Baumgartner 1998 [22] | New Mexico Elder Health Study |
| <6.77 | <4.51 | Melton 2000 [23] | Rochester Study | |||
| AMMI | kg/m2 | ASM/height2 | <7 | <5.5 | Gould 2014 [24] | EWGSOP2 |
| ASM | kg | lean mass arms and legs–bone mass arms and legs | <20 | <15 | Studenski 2014 [25] | EWGSOP2 |
| Male n = 340 | Female n = 448 | p-Value | |||
|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | |
| age [years] | 56.9 | 8.73 | 56.91 | 8.03 | 0.820 |
| BMI [kg/m2] | 27.5 | 3.87 | 25.64 | 4.74 | <0.001 |
| bone mass extremities [kg] | 1.69 | 0.22 | 1.14 | 0.17 | <0.001 |
| LMI [kg/m2] | 18.5 | 1.73 | 15.25 | 1.94 | <0.001 |
| ALMI-BMI | 1.01 | 0.12 | 0.73 | 0.10 | <0.001 |
| ALMI [kg/m2] | 8.71 | 0.99 | 6.82 | 1.04 | <0.001 |
| LESM [kg] | 18.9 | 2.75 | 13.23 | 2.22 | <0.001 |
| TSM [kg] | 34.4 | 4.90 | 23.16 | 3.77 | <0.001 |
| TSMI [kg/m2] | 10.9 | 1.29 | 8.51 | 1.33 | <0.001 |
| ASM [kg] | 25.8 | 3.68 | 17.41 | 2.83 | 0.002 |
| AMMI [kg/m2] | 8.18 | 0.97 | 6.40 | 1.00 | <0.001 |
| ALM [kg] | 27.5 | 3.83 | 18.56 | 2.94 | <0.001 |
| SMI [%] | 30.0 | 2.66 | 25.20 | 2.62 | <0.001 |
| LESMI [kg/m2] | 5.97 | 0.71 | 4.86 | 0.79 | <0.001 |
| rLM | −0.0033 | 0.9970 | −0.0038 | 0.9979 | 0.723 |
| Total | |||
|---|---|---|---|
| Total | Sarcopenia | SP | |
| SMI | 788 | 287 | 36.3 |
| LESMI | 788 | 45 | 5.7 |
| AMMI | 787 | 94 | 11.9 |
| ASM | 788 | 84 | 10.7 |
| rLM20 | 788 | 157 | 19.9 |
| rLMABC | 788 | 15 | 1.9 |
| ALMI Min | 788 | 5 | 0.6 |
| ALMI NM | 788 | 46 | 5.8 |
| TSMI | 788 | 26 | 3.3 |
| Number | Agreement [%] | ||||
|---|---|---|---|---|---|
| Total | Yes | No | Kappa * | p-Value * | |
| ASM | 64 | 76.6 | 23.4 | 0.745 | <0.001 |
| AMMI | 72 | 72.2 | 27.8 | 0.665 | <0.001 |
| ALMI NM | 36 | 61.1 | 36.1 | 0.752 | <0.001 |
| LESMI | 36 | 58.3 | 33.3 | 0.659 | <0.001 |
| SMI | 241 | 78.8 | 21.2 | 0.585 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweighofer, N.; Colantonio, C.; Haudum, C.W.; Hutz, B.; Kolesnik, E.; Mursic, I.; Pilz, S.; Schmidt, A.; Stadlbauer, V.; Zirlik, A.; et al. DXA-Derived Indices in the Characterisation of Sarcopenia. Nutrients 2022, 14, 186. https://doi.org/10.3390/nu14010186
Schweighofer N, Colantonio C, Haudum CW, Hutz B, Kolesnik E, Mursic I, Pilz S, Schmidt A, Stadlbauer V, Zirlik A, et al. DXA-Derived Indices in the Characterisation of Sarcopenia. Nutrients. 2022; 14(1):186. https://doi.org/10.3390/nu14010186
Chicago/Turabian StyleSchweighofer, Natascha, Caterina Colantonio, Christoph W. Haudum, Barbara Hutz, Ewald Kolesnik, Ines Mursic, Stefan Pilz, Albrecht Schmidt, Vanessa Stadlbauer, Andreas Zirlik, and et al. 2022. "DXA-Derived Indices in the Characterisation of Sarcopenia" Nutrients 14, no. 1: 186. https://doi.org/10.3390/nu14010186
APA StyleSchweighofer, N., Colantonio, C., Haudum, C. W., Hutz, B., Kolesnik, E., Mursic, I., Pilz, S., Schmidt, A., Stadlbauer, V., Zirlik, A., Pieber, T. R., Verheyen, N., & Obermayer-Pietsch, B. (2022). DXA-Derived Indices in the Characterisation of Sarcopenia. Nutrients, 14(1), 186. https://doi.org/10.3390/nu14010186









