Relative Validity and Reproducibility of a Semi-Quantitative Food Frequency Questionnaire for Determining Nutrient Intake in Older Adults in New Zealand: The REACH Study
Abstract
1. Introduction
2. Materials and Methods
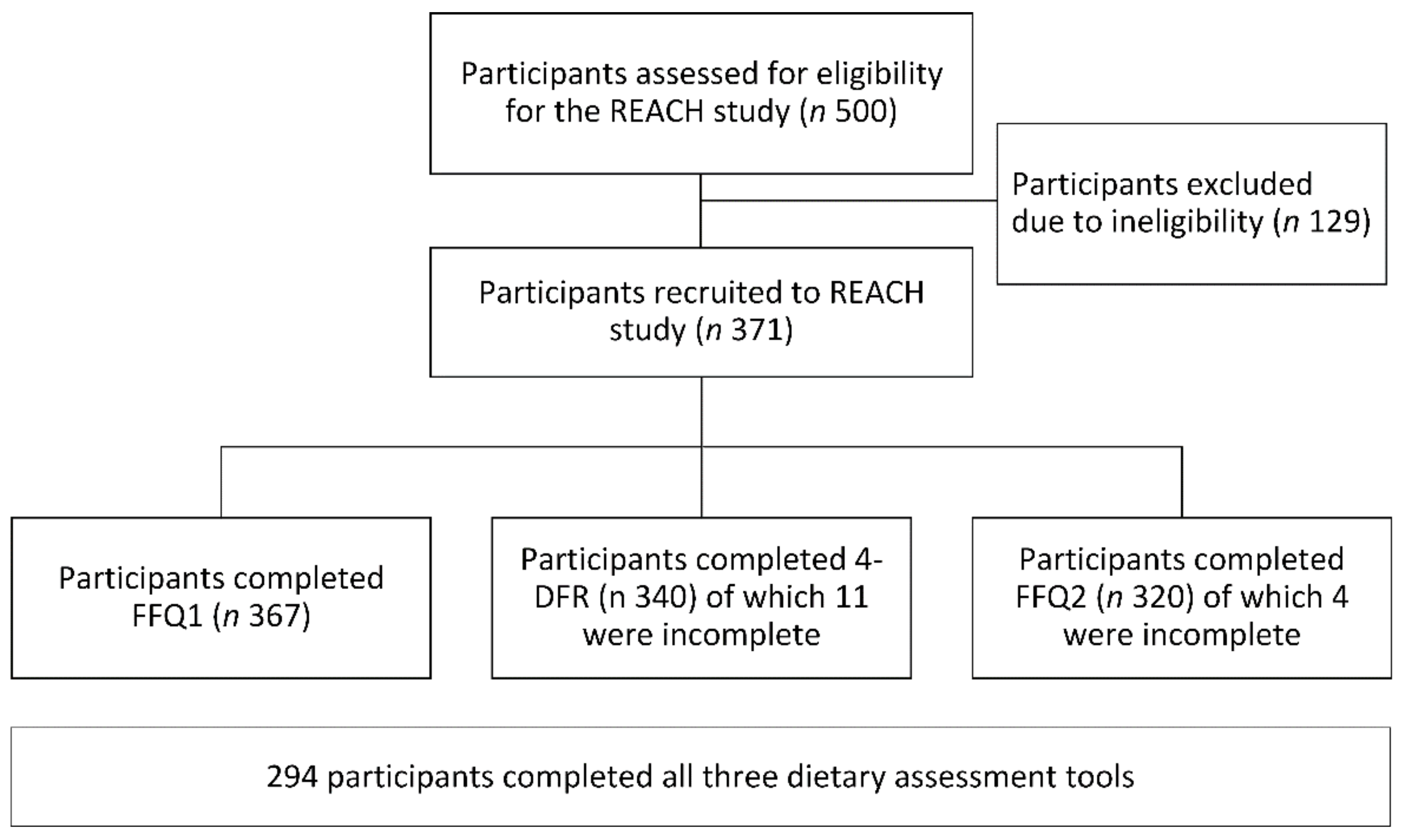
2.1. Participants and Recruitment
2.2. Development of the Semi-Quantitative FFQ
2.3. Data Collection
2.4. Data Entry and Management
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Relative Validity of Energy and Nutrient Intakes Derived from the REACH FFQ
3.3. Reproducibility of Energy and Nutrient Intakes Derived from the REACH FFQ
4. Discussion
4.1. Validity of the FFQ
4.2. Reproducibility of Nutrients from the FFQ
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, P.; Lynn, R. Health of Older People in New Zealand: A Statistical Reference; Ministry of Health: Wellington, New Zealand, 2002. Available online: https://www.health.govt.nz/publication/health-older-people-new-zealand-statistical-reference (accessed on 14 August 2021).
- Statistics New Zealand. National Population Projections: 2016(Base)–2068. Available online: https://www.stats.govt.nz/information-releases/national-population-projections-2016base2068 (accessed on 28 May 2021).
- Ministry of Health. Health Loss in New Zealand 1990–2013: A Report for the New Zealand Burden of Diseases, Injuries and Risk Factor Study; Ministry of Health: Wellington, New Zealand, 2016. Available online: https://www.health.govt.nz/publication/health-loss-new-zealand-1990-2013 (accessed on 14 August 2021).
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/186463 (accessed on 30 May 2021).
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Verlaan, S.; Mijnarends, D.M.; Schols, J.M.G.A.; de Groot, L.C.P.G.M.; Luiking, Y.C. Macronutrient intake and inadequacies of community-dwelling older adults, a systematic review. Ann. Nutr. Metab. 2015, 66, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone, density in men and women 65 years of age or older. N. Eng. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Lips, P.; Bouillon, R.; van Schoor, N.M.; Vanderschueren, D.; Verschueren, S.; Kuchuk, N.; Milisen, K.; Boonen, S. Reducing fracture risk with calcium and vitamin D. Clin. Endocrinol. 2010, 73, 277–285. [Google Scholar] [CrossRef]
- Yang, L.J.; Wu, G.H.; Yang, Y.L.; Wu, Y.H.; Zhang, L.; Wang, M.H.; Mo, L.Y.; Xue, G.; Wang, C.Z.; Weng, X.F. Nutrition, physical exercise, and the prevalence of sarcopenia in elderly residents in nursing homes in China. Med. Sci. Monit. 2019, 25, 4390–4399. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Hooson, J.; Hutchinson, J.; Warthon-Medina, M.; Hancock, N.; Greathead, K.; Knowles, B.; Vargas-Garcia, E.; Gibson, L.E.; Bush, L.A.; Margetts, B.; et al. A systematic review of reviews identifying UK validated dietary assessment tools for inclusion on an interactive guided website for researchers: www.nutritools.org. Crit. Rev. Food Sci. Nutr. 2019, 60, 1265–1289. [Google Scholar] [CrossRef]
- Watanabe, D.; Nanri, H.; Yoshida, T.; Yamaguchi, M.; Sugita, M.; Nozawa, Y.; Okabe, Y.; Itoi, A.; Goto, C.; Yamada, Y.; et al. Validation of energy and nutrition intake in Japanese elderly individuals estimated based on a short food frequency questionnaire compared against a 7-day dietary record: The Kyoto-Kameoka study. Nutrients 2019, 11, 688. [Google Scholar] [CrossRef]
- Cade, J.E.; Burley, V.J.; Warm, D.L.; Thompson, R.L.; Margetts, B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004, 17, 5–22. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Dietary assessment methodology. In Nutrition in the Prevention and Treatment of Disease, 4th ed.; Coulston, A.M., Boushey, C., Ferruzzi, M.G., Delahanty, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 5–48. [Google Scholar]
- Jia, X.; Craig, L.C.A.; Aucott, L.S.; Milne, A.C.; McNeill, G. Repeatability and validity of a food frequency questionnaire in free-living older people in relation to cognitive function. J. Nutr. Health Aging 2008, 12, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. 24-hour recall and diet record methods. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 50–70. [Google Scholar]
- Willett, W. Food frequency methods. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 71–96. [Google Scholar]
- Willett, W. Reproducibility and validity of food frequency questionnaires. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 97–142. [Google Scholar]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Heath Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R. Principles of Nutritional Assessment, 2nd ed.; Oxford Unversity Press: New York, NY, USA, 2005. [Google Scholar]
- Sam, C.H.Y.; Skidmore, P.; Skeaff, S.; Parackal, S.; Wall, C.; Bradbury, K.E. Relative validity and reproducibility of a short food frequency questionnaire to assess nutrient intakes of New Zealand adults. Nutrients 2020, 12, 619. [Google Scholar] [CrossRef]
- Beck, K.L.; Houston, Z.L.; McNaughton, S.A.; Kruger, R. Development and evaluation of a food frequency questionnaire to assess nutrient intakes of adult women in New Zealand. Nutr. Diet 2020, 77, 253–259. [Google Scholar] [CrossRef]
- Metcalf, P.; Swinburn, B.; Scragg, R.; Dryson, E. Reproducibility and validity of a food frequency questionnaire in European and Polynesian New Zealanders. Ethn. Health 1997, 2, 297–308. [Google Scholar] [CrossRef]
- Wilson, P.; Horwath, C. Validation of a short food frequency questionnaire for assessment of dietary calcium intake in women. Eur. J. Clin. Nutr. 1996, 50, 220–228. [Google Scholar]
- Horwath, C.C. Validity of a short food frequency questionnaire for estimating nutrient intake in elderly people. Br. J. Nutr. 1993, 70, 3–14. [Google Scholar] [CrossRef]
- Ingram, M.A.; Stonehouse, W.; Russell, K.G.; Meyer, B.J.; Kruger, R. The New Zealand PUFA Semiquantitative Food Frequency Questionnaire is a valid and reliable tool to assess PUFA intakes in healthy New Zealand adults. J. Nutr. 2012, 142, 1968–1974. [Google Scholar] [CrossRef]
- Sam, C.H.Y.; Skeaff, S.; Skidmore, P.M.L. A comprehensive FFQ developed for use in New Zealand adults: Reliability and validity for nutrient intakes. Public Health Nutr. 2014, 17, 287–296. [Google Scholar] [CrossRef]
- Mumme, K.; von Hurst, P.R.; Conlon, C.A.; Jones, B.; Haskell-Ramsay, C.F.; Stonehouse, W.; Heath, A.-L.M.; Coad, J.; Beck, K.L. Study protocol: Associations between dietary patterns, cognitive function and metabolic syndrome in older adults—A cross-sectional study. BMC Public Health 2019, 19, 535. [Google Scholar] [CrossRef]
- Mumme, K.; Conlon, C.; von Hurst, P.R.; Jones, B.; de Seymour, J.; Heath, A.-L.; Stonehouse, W.; Coad, J.; Haskell-Ramsay, C.; Beck, K.L. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary patterns and food group intake in older New Zealand adults: The REACH study. J. Acad. Nutr. Diet. 2021, 121, 2389–2400. [Google Scholar] [CrossRef]
- Beck, K.L.; Kruger, R.; Conlon, C.A.; Heath, A.-L.M.; Coad, J.; Matthys, C.; Jones, B.; Stonehouse, W. The relative validity and reproducibility of an iron food frequency questionnaire for identifying iron-related dietary patterns in young women. J. Acad. Nutr. Diet. 2012, 112, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- The New Zealand Institute for Plant & Food Research Limited; Ministry of Health. New Zealand Food Composition Database 2017: New Zealand FOODfiles(TM) 2016 Version 01. Available online: https://www.foodcomposition.co.nz/foodfiles (accessed on 2 May 2021).
- Momentive Inc. SurveyMonkey. Available online: https://www.momentive.ai (accessed on 2 November 2021).
- Marfell-Jones, M.; Stewart, A.; De Ridder, J. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012. [Google Scholar]
- Nelson, M.; Atkinson, M.; Meyer, J. A Photographic Atlas of Food Portion Sizes; MAFF Publications: London, UK, 1997. [Google Scholar]
- Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007, 16, 219–242. [Google Scholar] [CrossRef] [PubMed]
- FoodWorks 10 Premium, Version 10.0; Xyris Pty Ltd.: Brisbane, Australia, 2019.
- Food Standards Australia New Zealand. Australian Food Composition Database—Release 1. FSANZ. Available online: www.foodstandards.gov.au (accessed on 2 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019; R Version 3.6.1.; Available online: https://www.R-project.org (accessed on 21 January 2021).
- Willett, W. Issues in analysis and presentation of dietary data. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 306–333. [Google Scholar]
- Willett, W. Implications of total energy intake for epidemiologic analyses. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 261–287. [Google Scholar]
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Masson, L.F.; McNeill, G.; Tomany, J.O.; Simpson, J.A.; Peace, H.S.; Wei, L.; Grubb, D.A.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Smith, W.; Mitchell, P.; Reay, E.M.; Webb, K.; Harvey, P.W.J. Validity and reproducibility of a self-administered food frequency questionnaire in older people. Aust. N. Z. J. Public Health 1998, 22, 456–463. [Google Scholar] [CrossRef]
- Gilsing, A.; Mayhew, A.J.; Payette, H.; Shatenstein, B.; Kirkpatrick, S.I.; Amog, K.; Wolfson, C.; Kirkland, S.; Griffith, L.E.; Raina, P. Validity and reliability of a short diet questionnaire to estimate dietary intake in older adults in a subsample of the Canadian Longitudinal Study on Aging. Nutrients 2018, 10, 1522. [Google Scholar] [CrossRef]
- Malekahmadi, M.; Naeini, A.A.; Shab-Bidar, S.; Feizi, A.; Djazayery, A. Development, validity, and reliability of a food frequency questionnaire for antioxidants in elderly Iranian people. J. Res. Med. Sci. 2016, 21, 14. [Google Scholar] [CrossRef]
- Corrente, J.E.; Marchioni, D.M.L.; Fisberg, R.M. Validation of a FFQ (food frequency questionnaire) for older people. J. Life Sci. 2013, 7, 878. [Google Scholar] [CrossRef][Green Version]
- Zaragoza-Martí, A.; Ferrer-Cascales, R.; Hurtado-Sánchez, J.A.; Laguna-Pérez, A.; Cabañero-Martínez, M.J. Cross-cultural adaptation, validity, and reproducibility of the Mediterranean Islands Study food frequency questionnaire in the elderly population living in the Spanish Mediterranean. Nutrients 2018, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Lyons-Wall, P.; Martins, R.N.; Rainey-Smith, S.R. Validation and reliability of the Alzheimer’s Disease-Commonwealth Scientific and Industrial Research Organisation Food Frequency Questionnaire. Nutrients 2020, 12, 3605. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Bienias, J.L.; Evans, D.A.; Wilson, R.S. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am. J. Epidemiol. 2003, 158, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Hartman, A.M. Issues in reproducibility and validity of dietary studies. Am. J. Clin. Nutr. 1989, 50, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Correction for the effects of measurement error. In Nutritional Epidemiology, 3rd ed.; Oxford Scholarship Online: New York, NY, USA, 2012; pp. 288–305. [Google Scholar]
- Ministry of Health. Annual Data Explorerer 2019/20: New Zealand Health Survey [Data File]. Available online: https://minhealthnz.shinyapps.io/nz-health-survey-2019-20-annual-data-explorer/ (accessed on 30 August 2021).
- Stats NZ. Ethnicity, Culture, and Identity. Available online: https://www.stats.govt.nz/tools/2018-census-place-summaries/new-zealand#ethnicity-culture-and-identity (accessed on 30 August 2021).
| Characteristics | Mean (SD) | n (%) |
|---|---|---|
| Age (years) | 69.8 (2.6) | 294 (100%) |
| Female sex | 186 (63%) | |
| Ethnicity | ||
| European/other | 279 (95%) | |
| Māori/Pacific Islander | 9 (2%) | |
| Asian | 8 (3%) | |
| Education Status | ||
| No qualification/Secondary education only | 68 (23%) | |
| Post-Secondary | 118 (40%) | |
| University | 108 (37%) | |
| BMI (kg/m2) | 26.1 (4.4) | 294 (100%) |
| Underweight BMI: <18.5 kg/m2 | 2 (1%) | |
| Normal BMI 18.5–24.9 kg/m2 | 124 (42%) | |
| Overweight BMI: 25.0–29.9 kg/m2 | 129 (44%) | |
| Obese BMI: ≥30.0 kg/m2 | 39 (13%) |
| Nutrient | FFQ1 Daily Intake a Mean (SD) | 4-DFR Daily Intake a Mean (SD) | Mean Difference a,c (95% CI) | Percentage Difference a,d (%) | Mean Difference e p-Value | Effect Size f | Correlation Coefficients g | Correlation p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw a | Adj b | Raw a | Adj b | Raw a | Adj b | Raw a | Adj b | |||||
| Energy (MJ) | 7.5 (2.2) | 8.1 (1.9) | −0.6 (−0.9, −0.3) | −7.3 | <0.001 | 0.26 | 0.37 | - | <0.001 | - | ||
| Protein (g) | 80.5 (24.5) | 82.8 (19.9) | −2.3 (−5.1, 0.5) | −2.8 | 0.11 | <0.001 | 0.09 | 0.22 | 0.41 | 0.52 | <0.001 | <0.001 |
| Carbohydrate (g) | 178.7 (60.7) | 191.0 (59.3) | −12.3 (−19.2, −5.5) | −6.5 | <0.001 | 0.47 | 0.21 | 0.04 | 0.50 | 0.58 | <0.001 | <0.001 |
| Sugars (g) | 113.0 (42.6) | 88.8 (33.5) | 24.3 (19.6, 28.9) | 27.3 | <0.001 | <0.001 | 0.59 | 1.29 | 0.44 | 0.48 | <0.001 | <0.001 |
| Dietary fibre (g) | 26.2 (9.9) | 28.4 (10.0) | −2.1 (−3.3, −1.0) | −7.5 | <0.001 | 0.80 | 0.22 | 0.02 | 0.51 | 0.54 | <0.001 | <0.001 |
| Alcohol (g) e,g | 7.7 (9.1) | 10.0 (12.8) | −2.3 (−3.5, −1.1) | −23.2 | 0.001 c | 0.02 c | 0.22 | 0.14 | 0.78 e | 0.77 e | <0.001 | <0.001 |
| Total fat (g) | 73.3 (24.0) | 80.4 (25.4) | −7.1 (−10.5, −3.7) | −8.8 | <0.001 | 0.18 | 0.24 | 0.08 | 0.29 | 0.45 | <0.001 | <0.001 |
| SFA (g) | 31.6 (12.3) | 29.4 (11.2) | 2.1 (0.6, 3.73) | 7.3 | 0.008 | <0.001 | 0.16 | 0.55 | 0.31 | 0.42 | <0.001 | <0.001 |
| MUFA (g) | 23.3 (7.9) | 29.2 (10.6) | −6.0 (−7.3, −4.8) | −20.6 | <0.001 | <0.001 | 0.55 | 0.55 | 0.33 | 0.39 | <0.001 | <0.001 |
| PUFA (g) | 10.2 (4.0) | 13.2 (6.0) | −3.0 (−3.7, −2.4) | −23.0 | <0.001 | <0.001 | 0.51 | 0.47 | 0.35 | 0.48 | <0.001 | <0.001 |
| Cholesterol (mg) | 284.9 (134.1) | 292.7 (121.7) | −7.8 (−24.0, 8.4) | −2.7 | 0.34 | 0.25 | 0.06 | 0.07 | 0.40 | 0.55 | <0.001 | <0.001 |
| Thiamine (mg) | 1.0 (0.4) | 1.6 (0.9) | −0.5 (−0.6, −0.4) | −33.3 | <0.001 | <0.001 | 0.63 | 0.55 | 0.36 | 0.29 | <0.001 | <0.001 |
| Riboflavin (mg) | 3.0 (1.4) | 2.2 (0.8) | 0.8 (0.7, 1.0) | 39.1 | <0.001 | <0.001 | 0.63 | 0.97 | 0.36 | 0.31 | <0.001 | <0.001 |
| Niacin equiv. (mg) | 38.1 (11.5) | 37.7 (10.0) | 0.3 (−0.9, 1.6) | 0.9 | 0.60 | <0.001 | 0.03 | 0.36 | 0.48 | 0.48 | <0.001 | <0.001 |
| Vitamin B6 (mg) | 3.0 (1.0) | 2.5 (0.9) | 0.4 (0.3, 0.6) | 17.9 | <0.001 | <0.001 | 0.48 | 0.69 | 0.50 | 0.32 | <0.001 | <0.001 |
| Folate (μg) | 365.8 (134.2) | 376.6 (139.8) | −10.8 (−30.2, 8.3) | −2.9 | 0.28 | 0.03 | 0.06 | 0.13 | 0.24 | 0.19 | <0.001 | <0.001 |
| Vitamin B12 (μg) | 5.2 (4.3) | 4.3 (3.8) | 0.9 (0.4, 1.5) | 22.2 | 0.002 | <0.001 | 0.18 | 0.25 | 0.18 | 0.22 | 0.002 | <0.001 |
| β-carotene (mg) | 4.5 (2.2) | 3.7 (2.3) | 0.9 (0.6, 1.2) | 23.8 | <0.001 | <0.001 | 0.36 | 0.50 | 0.45 | 0.44 | <0.001 | <0.001 |
| Vitamin A (mg) | 1.5 (1.4) | 1.1 (1.0) | 0.4 (0.2, 0.6) | 32.9 | <0.001 | <0.001 | 0.23 | 0.30 | 0.12 | 0.17 | <0.05 | 0.004 |
| Vitamin C (mg) | 133.7 (70.9) | 125.2 (70.2) | 8.5 (−0,2, 17.2) | 6.8 | 0.06 | <0.001 | 0.11 | 0.25 | 0.42 | 0.39 | <0.001 | <0.001 |
| Vitamin E (mg) | 10.2 (3.9) | 11.1 (4.3) | −0.9 (−1.5, −0.4) | −8.3 | 0.001 | 0.76 | 0.20 | 0.02 | 0.34 | 0.49 | <0.001 | <0.001 |
| Calcium (mg) | 1193.2 (552.1) | 923.7 (341.1) | 269.5 (209.9, 329.2) | 29.2 | <0.001 | <0.001 | 0.52 | 0.85 | 0.40 | 0.36 | <0.001 | <0.001 |
| Iron (mg) | 10.0 (3.3) | 12.4 (3.9) | −2.3 (−2.8, −1.9) | −19.0 | <0.001 | <0.001 | 0.57 | 0.42 | 0.36 | 0.36 | <0.001 | <0.001 |
| Iodine (μg) | 87.0 (36.9) | 97.8 (67.6) | −10.8 (−18.8, −2.8) | −11.0 | 0.008 | 0.14 | 0.16 | 0.09 | 0.22 | 0.22 | <0.001 | <0.001 |
| Potassium (mg) | 3965.4 (1172.3) | 3644.0 (967.2) | 321.4 (190.5, 452.4) | 8.8 | <0.001 | <0.001 | 0.28 | 0.79 | 0.45 | 0.36 | <0.001 | <0.001 |
| Magnesium (mg) | 340.2 (100.4) | 381.9 (117.3) | −41.8 (−54.9, −28.7) | −10.9 | <0.001 | <0.001 | 0.37 | 0.21 | 0.46 | 0.49 | <0.001 | <0.001 |
| Phosphorus (mg) | 1476.4 (498.0) | 1516.5 (383.4) | −40.1 (−96.7, 16.5) | −2.6 | 0.16 | <0.001 | 0.08 | 0.21 | 0.40 | 0.38 | <0.001 | <0.001 |
| Selenium (μg) | 47.1 (18.6) | 75.1 (41.0) | −28.1 (−32.9, −23.2) | −37.4 | <0.001 | <0.001 | 0.67 | 0.59 | 0.17 | 0.14 | 0.005 | 0.02 |
| Zinc (mg) | 10.5 (3.4) | 10.2 (2.8) | 0.3 (−0.1, 0.7) | 3.0 | 0.14 | <0.001 | 0.09 | 0.40 | 0.40 | 0.26 | <0.001 | <0.001 |
| Nutrient | Correctly Classified—Same Tertiles (%) a | Grossly Misclassified—Opposite Tertiles (%) a | Weighted Kappa Statistics b | |||
|---|---|---|---|---|---|---|
| Raw c | Adjusted d | Raw c | Adjusted d | Raw c | Adjusted d | |
| Energy | 43.5 | 12.2 | 0.23 | |||
| Protein | 47.6 | 50.0 | 12.2 | 11.2 | 0.27 | 0.31 |
| Carbohydrate | 45.9 | 54.4 | 7.8 | 8.2 | 0.30 | 0.39 |
| Sugars | 44.9 | 46.9 | 10.9 | 12.2 | 0.26 | 0.26 |
| Dietary fibre | 44.2 | 50.7 | 12.2 | 9.2 | 0.23 | 0.34 |
| Alcohol | 68.0 | 68.7 | 2.7 | 2.0 | 0.61 | 0.62 |
| Total fat | 39.1 | 48.0 | 11.2 | 12.6 | 0.15 | 0.27 |
| SFA | 42.5 | 49.0 | 11.2 | 14.3 | 0.19 | 0.26 |
| MUFA | 39.8 | 45.9 | 14.6 | 13.3 | 0.16 | 0.24 |
| PUFA | 46.6 | 50.0 | 13.3 | 7.8 | 0.25 | 0.35 |
| Cholesterol | 42.5 | 47.3 | 15.3 | 9.2 | 0.18 | 0.30 |
| Thiamine | 45.2 | 43.5 | 11.2 | 12.9 | 0.26 | 0.22 |
| Riboflavin | 45.6 | 47.3 | 12.2 | 11.9 | 0.25 | 0.27 |
| Niacin equiv. | 54.1 | 52.4 | 6.5 | 10.9 | 0.41 | 0.34 |
| Vitamin B6 | 47.3 | 44.6 | 12.9 | 16.0 | 0.26 | 0.20 |
| Folate | 38.4 | 40.8 | 16.7 | 17.0 | 0.12 | 0.14 |
| Vitamin B12 | 44.2 | 50.3 | 11.2 | 10.9 | 0.25 | 0.32 |
| β-carotene | 43.9 | 44.2 | 13.3 | 13.6 | 0.22 | 0.22 |
| Vitamin A | 43.2 | 45.6 | 16.0 | 13.6 | 0.18 | 0.23 |
| Vitamin C | 44.2 | 45.2 | 11.6 | 11.2 | 0.24 | 0.26 |
| Vitamin E | 31.6 | 49.0 | 14.6 | 7.5 | 0.18 | 0.34 |
| Calcium | 48.3 | 42.5 | 11.6 | 11.9 | 0.29 | 0.22 |
| Iron | 44.2 | 40.8 | 12.2 | 16.3 | 0.23 | 0.15 |
| Iodine | 45.6 | 41.2 | 13.6 | 13.9 | 0.23 | 0.18 |
| Potassium | 42.9 | 40.5 | 10.2 | 14.6 | 0.24 | 0.16 |
| Magnesium | 46.9 | 51.7 | 8.8 | 10.9 | 0.30 | 0.33 |
| Phosphorus | 42.9 | 42.2 | 11.6 | 11.6 | 0.23 | 0.22 |
| Selenium | 42.9 | 44.6 | 18.4 | 17.3 | 0.15 | 0.18 |
| Zinc | 48.0 | 44.2 | 10.5 | 15.6 | 0.30 | 0.20 |
| Statistical Test | Validity b | Reproducibility c | ||
|---|---|---|---|---|
| Raw d | Energy-Adjusted e | Raw d | Energy-Adjusted e | |
| Correlation coefficient f, mean (SD) | 0.37 (0.11) Acceptable | 0.38 (0.13) Acceptable | 0.63 (0.10) Good | 0.65 (0.10) Good |
| Cross-classification g, mean (SD) | Poor | Poor | Good | Good |
| % in same tertiles | 45 (6) | 47 (6) | 61 (5) | 61 (5) |
| % in opposite tertiles | 12 (3) | 12 (3) | 4 (1) | 4 (2) |
| Weighted kappa value h, mean (SD) | 0.25 (0.09) Acceptable | 0.27 (0.10) Acceptable | 0.52 (0.06) Acceptable | 0.51 (0.08) Acceptable |
| Percentage difference k | Acceptable | Acceptable | Good | Good |
| % difference within ±10.9% | 52 | 43 | 93 | 96 |
| % difference between ±11 and 20% | 10 | 25 | 7 | 4 |
| % difference beyond ±20% | 38% | 32% | 0% | 0% |
| Difference between mean intakes m | 76% Poor | 78% Poor | 90% Poor | 21% Good |
| Bland–Altman n | Poor | Poor | Good | Good |
| Presence of bias % | 66 | 54 | 21 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.D.; Mumme, K.D.; Conlon, C.A.; von Hurst, P.R.; Gillies, N.; Heath, A.-L.; Coad, J.; Beck, K.L. Relative Validity and Reproducibility of a Semi-Quantitative Food Frequency Questionnaire for Determining Nutrient Intake in Older Adults in New Zealand: The REACH Study. Nutrients 2022, 14, 519. https://doi.org/10.3390/nu14030519
Yu AD, Mumme KD, Conlon CA, von Hurst PR, Gillies N, Heath A-L, Coad J, Beck KL. Relative Validity and Reproducibility of a Semi-Quantitative Food Frequency Questionnaire for Determining Nutrient Intake in Older Adults in New Zealand: The REACH Study. Nutrients. 2022; 14(3):519. https://doi.org/10.3390/nu14030519
Chicago/Turabian StyleYu, Angela D., Karen D. Mumme, Cathryn A. Conlon, Pamela R. von Hurst, Nicola Gillies, Anne-Louise Heath, Jane Coad, and Kathryn L. Beck. 2022. "Relative Validity and Reproducibility of a Semi-Quantitative Food Frequency Questionnaire for Determining Nutrient Intake in Older Adults in New Zealand: The REACH Study" Nutrients 14, no. 3: 519. https://doi.org/10.3390/nu14030519
APA StyleYu, A. D., Mumme, K. D., Conlon, C. A., von Hurst, P. R., Gillies, N., Heath, A.-L., Coad, J., & Beck, K. L. (2022). Relative Validity and Reproducibility of a Semi-Quantitative Food Frequency Questionnaire for Determining Nutrient Intake in Older Adults in New Zealand: The REACH Study. Nutrients, 14(3), 519. https://doi.org/10.3390/nu14030519







