Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples
2.3. Methods
2.3.1. Profile of Phenolic Compounds Composing TGS
2.3.2. Bioaccessibility of Bioactive Compounds
- Evaluation of the release of bioaccessible antioxidant compounds
- Inhibitors of carbohydrases
- Assessment of the effects of the bioaccessible compounds in cell models
2.3.3. Yogurt Shelf-Life
2.3.4. Sensory Analysis
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. TGS Polyphenolic Profile by UHPLC-MS/MS Analysis
3.2. Bioaccessibility of Bioactive Compounds from Tannat Grape Skin (TGS)
3.3. TGS Yogurt and Biscuit Bioaccessible Compounds
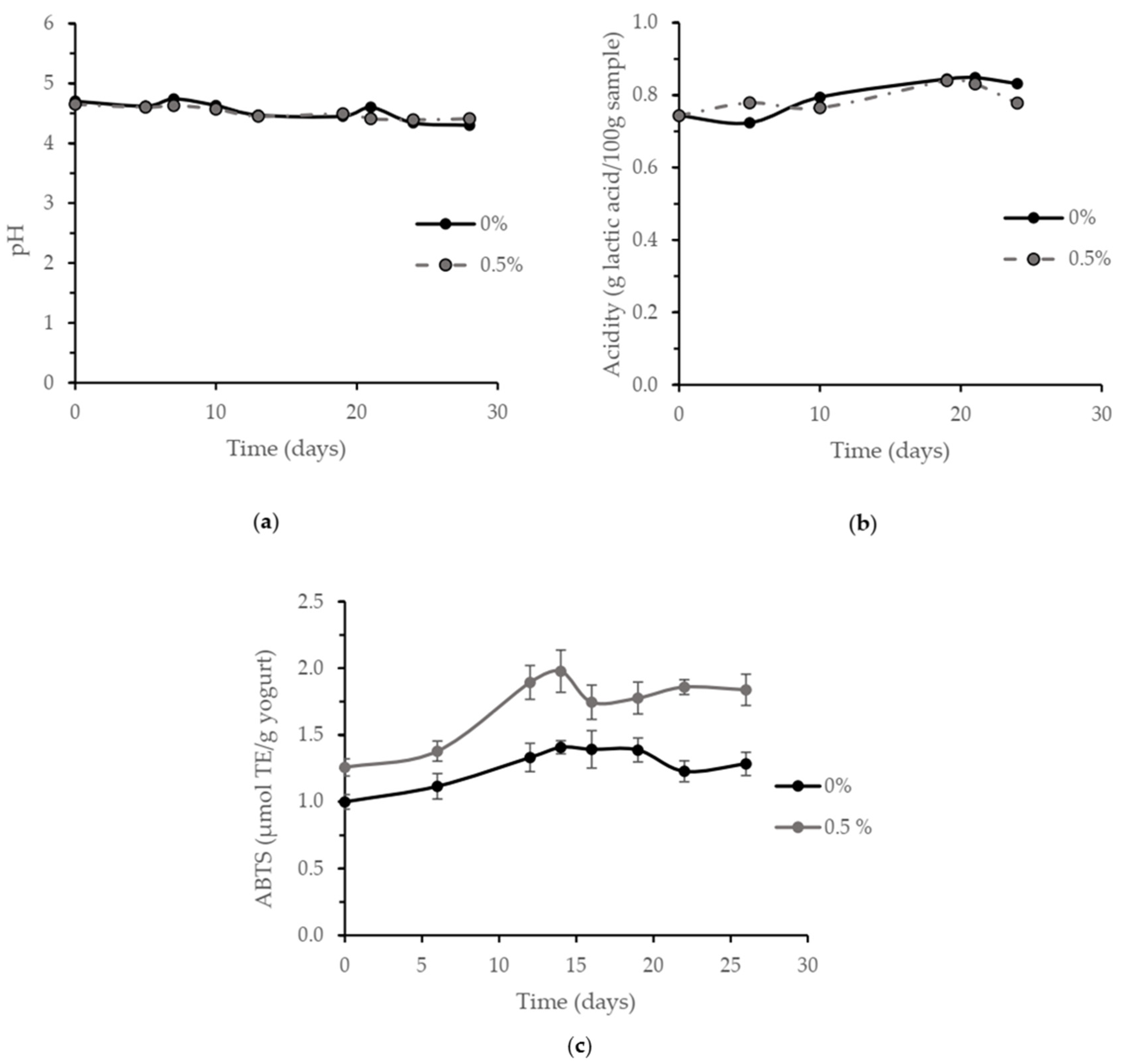
3.4. Yogurt Shelf-Life
3.5. Consumers’ Sensory Analysis of Healthy Sustainable Snacks
3.6. Intracellular Effects of TGS Yogurt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The effects of dietary fibre addition on the quality of common cereal products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Boido, E.; García-Marino, M.; Dellacassa, E.; Carrau, F.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterisation and evolution of grape polyphenol profiles of Vitis vinifera L. cv. Tannat during ripening and vinification. Aust. J. Grape Wine Res. 2011, 17, 383–393. [Google Scholar] [CrossRef]
- Da Silva, C.; Zamperin, G.; Ferrarini, A.; Minio, A.; Dal Molin, A.; Venturini, L.; Buson, G.; Tononi, P.; Avanzato, C.; Zago, E.; et al. The High Polyphenol Content of Grapevine Cultivar Tannat Berries Is Conferred Primarily by Genes That Are Not Shared with the Reference Genome. Plant Cell 2013, 25, 4777–4788. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of antioxidant, antidiabetic, antiobesity, and anti-inflammatory properties of a Tannat winemaking by-product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, I.M.; Cruz, F.A.; Alves, T.D.L.; De Gois, J.S.; Borges, D.L.G.; Cunha, H.P.; Da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: Phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α -glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Dubiard, C.G.; Cheynier, V.; Meudec, E.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Res. Int. 2016, 88, 284–292. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública MERCOSUR/GMC/RES. N° 01/12 Reglamento Tecnico Mercosur Sobre Informacion Nutricional Complementaria (Declaraciones de Propiedades Nutricionales). Available online: https://www.impo.com.uy/bases/decretos-internacional/402-2012/1 (accessed on 17 November 2021).
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; del Castillo, M.D. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules 2021, 26, 3480. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Dörnyei, Á.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Kilár, F.; Márk, L. Rapid MALDI-TOF-MS Detection of Anthocyanins in Wine and Grape Using Different Matrices. Food Anal. Methods 2011, 4, 108–115. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Gomes, T.M.; Toaldo, M.; Cristina, I.; Haas, S.; Burin, M.; Caliari, V.; Luna, A.S.; Santos De Gois, J.; Bordignon-Luiz, M.T. Differential contribution of grape peel, pulp, and seed to bioaccessibility of micronutrients and major polyphenolic compounds of red and white grapes through simulated human digestion. J. Funct. Foods 2019, 52, 699–708. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of simulated digestion on the phenolic components of red grapes and their corresponding wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Ostberg-Potthoff, J.J.; Berger, K.; Richling, E.; Winterhalter, P. Activity-Guided Fractionation of Red Fruit Extracts for the Identification of Compounds Influencing Glucose Metabolism. Nutrients 2019, 11, 1166. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Ohanna, K.; Inada, P.; Barcellos, T.; Silva, R.; Lobo, L.A.; Cavalcante, R.M.; Domingues, P.; Perrone, D.; Monteiro, M. Bioaccessibility of phenolic compounds of jaboticaba (Plinia jaboticaba) peel and seed after simulated gastrointestinal digestion and gut microbiota fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- El-Said, M.M.; Haggag, H.F.; Fakhr El-Din, H.M.; Gad, A.S.; Farahat, A.M. Antioxidant activities and physical properties of stirred yoghurt fortified with pomegranate peel extracts. Ann. Agric. Sci. 2014, 59, 207–212. [Google Scholar] [CrossRef]
- Oliveira, A.; Pintado, M. Stability of polyphenols and carotenoids in strawberry and peach yoghurt throughout in vitro gastrointestinal digestion. Food Funct. 2015, 6, 1611–1619. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional dairy products as a source of bioactive peptides and probiotics: Current trends and future prospectives. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Al-u’datt, D.G.F.; Alhamad, M.N.; Tranchant, C.C.; Rababah, T.; Gammoh, S.; Althnaibat, R.M.; Daradkeh, M.G.; Kubow, S. Characterization and biological properties of peptides isolated from dried fermented cow milk products by RP-HPLC: Amino acid composition, antioxidant, antihypertensive, and antidiabetic properties. J. Food Sci. 2021, 86, 3046–3060. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Kamal, H.; Kilari, B.P.; Gan, C.Y.; Maqsood, S. Identification and characterization of novel α-amylase and α-glucosidase inhibitory peptides from camel whey proteins. J. Dairy Sci. 2021, 104, 1364–1377. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağli, T.; Vančetović, J.; Gökmen, V. Effects of baking conditions and dough formulations on phenolic compound stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Takács, K.; Wiczkowski, W.; Cattaneo, S.; Szerdahelyi, E.; Stuknytė, M.; Casiraghi, M.C.; Nehir El, S.; De Noni, I. Occurrence of targeted nutrients and potentially bioactive compounds during in vitro digestion of wheat spaghetti. J. Funct. Foods 2018, 44, 118–126. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Blázquez-Duff, J.M.; del Castillo, M.D.; Miguel, E. Nutritional Quality, Sensory Analysis and Shelf Life Stability of Yogurts Containing Inulin-Type Fructans and Winery Byproducts for Sustainable Health. Foods 2020, 9, 1199. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública. Regalamento Bromatologico Nacional, 6th ed.; IMPO, Ed.; IMPO: Montevideo, Uruguay, 2012; ISBN 9781604138795. [Google Scholar]
- Şanlidere Aloĝlu, H.; Öner, Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Pereira Freitas, S.; Luiz de Oliveira Godoy, R.; Cristine Rodrigues de Oliveira, D.; Deliza, R.; Iacomini, M.; Mellinger-Silva, C.; Maria Correa Cabral, L. Antioxidant dietary fibre from grape pomace flour or extract: Does it make any difference on the nutritional and functional value? J. Funct. Foods 2019, 56, 276–285. [Google Scholar] [CrossRef]
- Górecka, D.; Pacholek, B.; Krzysztof, D.; Górecka, M. Rasperry pomace as a potential fiber source for cookies enrichment. Acta Sci. Pol. Technol. Aliment. 2010, 9, 451–462. [Google Scholar]
- Kuchtová, V.; Karovičová, J.; Kohajdová, Z.; Minarovičová, L.; Kimličková, V. Effects of white grape preparation on sensory quality of cookies. Acta Chim. Slovaca 2016, 9, 84–88. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lee, S.G.; Vance, T.M.; Wang, Y.; Kim, B.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract. Food Chem. 2016, 211, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; van Camp, J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells—A review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Nieto Fuentes, J.A. Comprehensive Study of the Grape (Vitis vinifera L.) as a Source of Bioavailable Phenolic Compounds with Biological Activity; Universidad Autónoma de Madrid: Madrid, Spain, 2015. [Google Scholar]
- Jin, Y.; Yu, Y.; Qi, Y.; Wang, F.; Yan, J.; Zou, H. Peptide profiling and the bioactivity character of yogurt in the simulated gastrointestinal digestion. J. Proteom. 2016, 141, 24–46. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Kozłowska, A.; Dzierżanowski, T. Targeting Inflammation by Anthocyanins as the Novel Therapeutic Potential for Chronic Diseases: An Update. Molecules 2021, 26, 4380. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Hernández-Mendoza, A.; Mata-Haro, V.; Vallejo-Córdoba, B.; Wall-Medrano, A.; Astiazarán-García, H.; Estrada-Montoya, M.D.C.; González-Córdova, A.F. Effect of Milk Fermented with Lactobacillus fermentum on the Inflammatory Response in Mice. Nutrients 2018, 10, 1039. [Google Scholar] [CrossRef]
- Salehi, F. Quality, physicochemical, and textural properties of dairy products containing fruits and vegetables: A review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.-L.; Flynn, A.; Heinonen, M.; Hulshof, K.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; Moseley, B.; et al. Danacol® and blood cholesterol Scientific substantiation of a health claim related to a low fat fermented milk product (Danacol®) enriched with plant sterols/stanols and lowering/reducing blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1177. [Google Scholar] [CrossRef]




| Yogurt Formulations (g) | Biscuits Formulations (g/100 g Dough Mix) | ||||
|---|---|---|---|---|---|
| Ingredients | Control | TGS | Ingredients | Control | TGS |
| UHT whole fluid milk (mL) | 800 | 800 | Butter | 10 | 10 |
| Skim milk powder | 16 | 16 | Sunflower oil | 4.25 | 4.25 |
| Modified cassava starch | 4 | 4 | Egg | 14 | 14 |
| Gelatin | 4 | 4 | Baking powder | 0.5 | 0.5 |
| CRL inulin (soluble fiber) | 10 | 10 | Salt | 0.08 | 0.08 |
| Stevia | 0.32 | 0.32 | Sweetener | 4 | 4 |
| Byproduct | 0 | 4 (0.5%) | Wheat flour | 67.17 | 47.17 |
| YO-MIX 495 LYO ferment | 250 DCU | Byproduct | 0 | 20 | |
| Negative ESI | ||||
|---|---|---|---|---|
| Compound 1 | TGS 2 | RT [min] | [M-H]− (m/z) | Fragments (m/z) |
| 3-Phenyllactic acid | 0.0268 | 10.6 | 165.0559 | 147.0455, 119.0504 |
| Salipurposid | 0.0019 | 11.8 | 433.1154 | 271.0607, 151.0041 |
| Astragalin isomer 1 | 0.0027 | 11.1 | 447.0944 | 284.0334, 227.0356 |
| Astragalin isomer 2 | 0.0071 | 11.2 | 447.0949 | 284.0334, 227.0356 |
| Caffeic acid | 0.0113 | 9.5 | 179.0354 | 135.0455 |
| cis-Aconitic acid | 0.0673 | 2.8 | 173.0094 | 129.0197, 85.0297 |
| Eriodictyol | 0.0021 | 13.6 | 287.0570 | 151.0041, 135.0456 |
| Gallic acid | 0.0990 | 3.7 | 169.0145 | 125.0247 |
| Isorhamnetin | 0.0311 | 14.9 | 315.0519 | 300.0283, 151.0037 |
| Myricetin | 0.0473 | 12.3 | 317.0310 | 178.9989, 151.0040 |
| Quercetin-3-galacturonide | 0.1190 | 10.7 | 477.0685 | 301.0361, 151.0039 |
| Quercetin | 0.1181 | 13.5 | 301.0361 | 151.0040, 107.0141 |
| Quercetin-3β-D-glucoside | 0.0578 | 10.7 | 463.0900 | 300.0282, 271.0254 |
| Syringic acid | 0.0003 | 11.2 | 197.0459 | 182.0225, 123.0091 |
| Vanillic acid | 0.0007 | 10.8 | 167.0352 | 152.0118, 123.0091 |
| Vanillyl alcohol | 0.0073 | 5.2 | 153.0561 | 138.0325, 123.0091 |
| Naringenin | 0.0036 | 14.8 | 271.0620 | 151.0041, 119.0505 |
| Positive ESI | ||||
| Compound 3 | TGS 2 | RT [min] | [M]+ (m/z) | Fragments (m/z) |
| Cyanidin 3-(6-O-acetylglucoside) | 0.00011 | 9.8 | 491.1184 | 287.0550 |
| Cyanidin-3-O-(6-p-coumaroyl) glucoside | 0.00032 | 10.9 | 595.1446 | 287.0550 |
| Cyanidin-3-pyranoside | 0.00021 | 8.5 | 449.1078 | 287.0550 |
| Delphinidin-3-(6-O-acetylglucoside) | 0.00014 | 9.2 | 507.1133 | 303.0500 |
| Delphinidin-3-O-(6-p-coumaroyl) glucoside | 0.00087 | 10.5 | 611.1395 | 303.0500 |
| Delphinidin-3-pyranoside | 0.00094 | 7.8 | 465.1027 | 303.0500 |
| Malvidin-3-(6-O-acetylglucoside) | 0.01076 | 10.4 | 535.1446 | 331.0800 |
| Malvidin-3-O-(6-p-coumaroyl) glucoside | 0.01766 | 11.5 | 639.1708 | 331.0800 |
| Malvidin-3-pyranoside | 0.02569 | 9.2 | 493.1340 | 331.0800 |
| Peonidin-3-(6-O-acetylglucoside) | 0.00105 | 10.4 | 505.1341 | 301.0700 |
| Peonidin-3-O-(6-p-coumaroyl) glucoside | 0.00194 | 11.5 | 609.1603 | 301.0700 |
| Peonidin-3-pyranoside | 0.00206 | 9.2 | 463.1235 | 301.0700 |
| Petunidin-3-(6-O-acetylglucoside) | 0.00134 | 9.9 | 521.1290 | 317.0700 |
| Petunidin-3-O-(6-p-coumaroyl) glucoside | 0.00294 | 11.0 | 625.1552 | 317.0700 |
| Petunidin-3-pyranoside | 0.00013 | 11.2 | 479.1184 | 317.0700 |
| Analysis | TGS | TGS Digest |
|---|---|---|
| TPC (mg GAE/g sample) | 29.85 ± 2.20 b | 7.41 ± 0.50 a |
| ABTS (µmol TE/g sample) | 28.28 ± 1.27 b | 18.13 ± 2.05 a |
| ORAC-FL (µmol TE/g sample) | 150.3 ± 11.1 b | 128.3 ± 13.3 a |
| α-glucosidase (IC50, mg/mL) | 11.67 ± 0.71 c | 8.23 ± 0.44 b |
| α-amylase (IC50, mg/mL) | 11.65 ± 0.11 b | 102.80 ± 8.93 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Ibañez, C.; Terán, D.; Gámbaro, A.; Medrano-Fernandez, A.; del Castillo, M.D. Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes. Nutrients 2022, 14, 419. https://doi.org/10.3390/nu14030419
Fernández-Fernández AM, Dellacassa E, Nardin T, Larcher R, Ibañez C, Terán D, Gámbaro A, Medrano-Fernandez A, del Castillo MD. Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes. Nutrients. 2022; 14(3):419. https://doi.org/10.3390/nu14030419
Chicago/Turabian StyleFernández-Fernández, Adriana Maite, Eduardo Dellacassa, Tiziana Nardin, Roberto Larcher, Cecilia Ibañez, Dahiana Terán, Adriana Gámbaro, Alejandra Medrano-Fernandez, and María Dolores del Castillo. 2022. "Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes" Nutrients 14, no. 3: 419. https://doi.org/10.3390/nu14030419
APA StyleFernández-Fernández, A. M., Dellacassa, E., Nardin, T., Larcher, R., Ibañez, C., Terán, D., Gámbaro, A., Medrano-Fernandez, A., & del Castillo, M. D. (2022). Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes. Nutrients, 14(3), 419. https://doi.org/10.3390/nu14030419










