Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection
Abstract
1. Saffron and Its Constituents
2. Neuro-Cognitive Protective Effects of Saffron in Humans
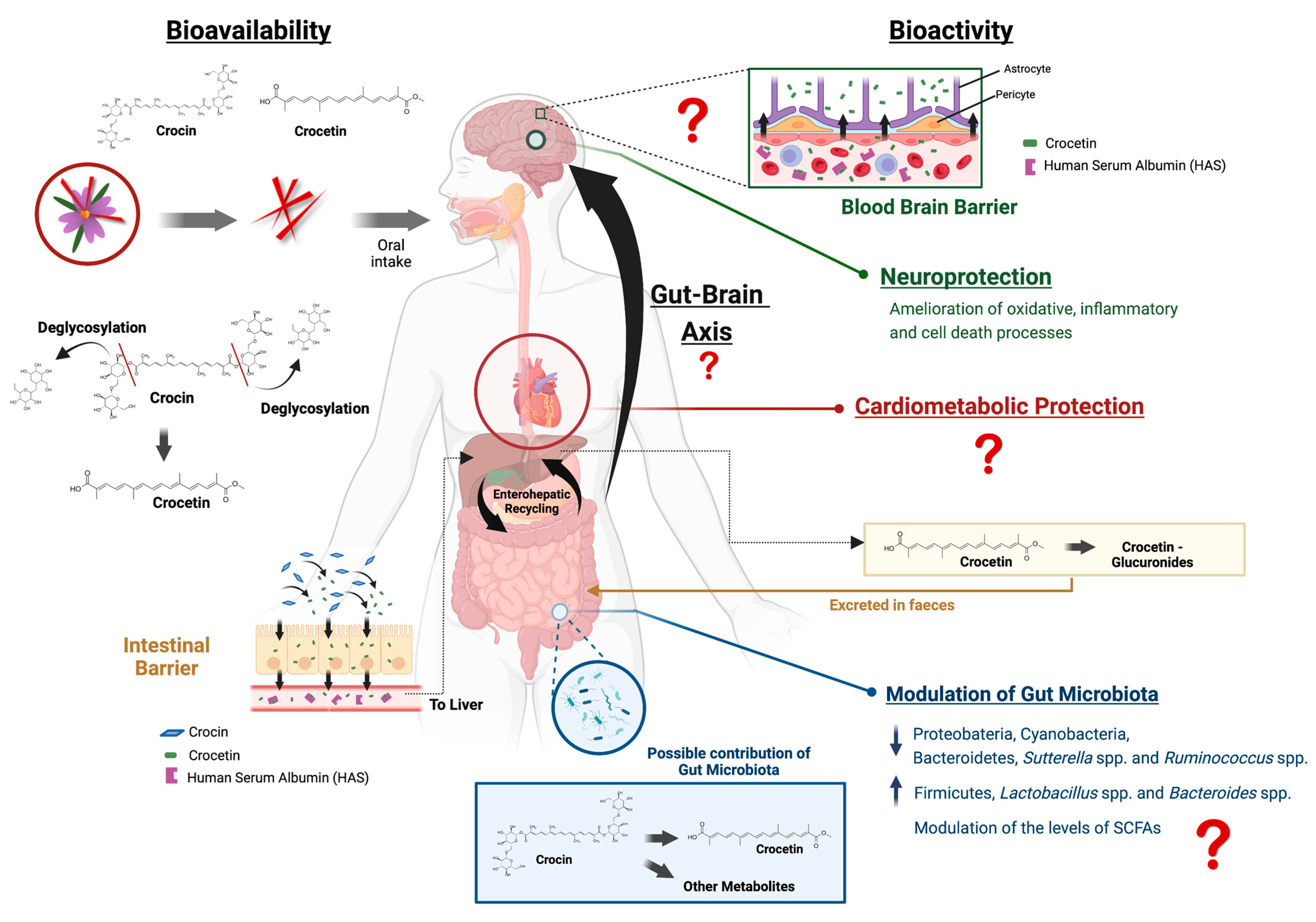
3. Bioavailability of the Main Saffron Bioactive Constituents
3.1. Host Metabolism
3.2. Microbial Metabolism of Saffron
4. Potential Mechanisms of Action Underlying the Neurological Responses of Saffron
4.1. In Vitro Cell Studies Supporting the Neurological Effects of the Main Saffron Compounds
4.2. The Effects of Saffron and Its Main Compounds in Animal Models of Neurological Diseases
4.3. The Role of the Interaction with the Microbiota in the Neurological Effects of Saffron
4.4. Cardiometabolic Benefits of Saffron and Potential Association with Brain Health
5. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Salinas, M.R.; Alonso, G.L. Bioactivity and bioavailability of the major metabolites of Crocus sativus L. flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Scuto, M.; Modafferi, S.; Rampulla, F.; Zimbone, V.; Tomasello, M.; Spano’, S.; Ontario, M.L.; Palmeri, A.; Trovato Salinaro, A.; Siracusa, R.; et al. Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: From molecular basis to therapy. Mech. Ageing Dev. 2022, 205, 111686. [Google Scholar]
- Roshanravan, N.; Ghaffari, S. The therapeutic potential of Crocus sativus Linn.: A comprehensive narrative review of clinical trials. Phytother. Res. 2022, 36, 98–111. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Loukidi, B.; Belyagoubi-Benhammou, N.; Gismondi, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Benmahieddine, A.; Rouigueb, K.; Ben Menni, D.; et al. Valorization of Algerian saffron: Stigmas and flowers as source of bioactive compounds. Waste Biomass Valorization 2021, 12, 6671–6683. [Google Scholar] [CrossRef]
- Rubio Moraga, A.; Ahrazem, O.; Rambla, J.L.; Granell, A.; Gómez Gómez, L. Crocins with high levels of sugar conjugation contribute to the yellow colours of early-spring flowering Crocus tepals. PLoS ONE 2013, 8, e71946. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- Khorasanchi, Z.; Shafiee, M.; Kermanshahi, F.; Khazaei, M.; Ryzhikov, M.; Parizadeh, M.R.; Kermanshahi, B.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine 2018, 43, 21–27. [Google Scholar]
- Farag, M.A.; Hegazi, N.; Dokhalahy, E.; Khattab, A.R. Chemometrics based GC-MS aroma profiling for revealing freshness, origin and roasting indices in saffron spice and its adulteration. Food Chem. 2020, 331, 127358. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Clemente-Villalba, J.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.-J. Novel insight into the volatile profile and antioxidant properties of Crocus sativus L. flowers. Antioxidants 2022, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Summary for CID 5281233, Crocin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Crocin (accessed on 31 October 2022).
- PubChem Compound Summary for CID 5281232, Crocetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Crocetin (accessed on 31 October 2022).
- PubChem Compound Summary for CID 61041, Safranal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Safranal (accessed on 31 October 2022).
- PubChem Compound Summary for CID 130796, Picrocrocin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Picrocrocin (accessed on 31 October 2022).
- PubChem Compound Summary for CID 5280863, Kaempferol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 31 October 2022).
- ubChem Compound Summary for CID 159287, Malvidin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Malvidin (accessed on 31 October 2022).
- PubChem Compound Summary for CID 370, Gallic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid (accessed on 31 October 2022).
- PubChem Compound Summary for CID 135, 4-Hydroxybenzoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Hydroxybenzoic-acid (accessed on 31 October 2022).
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrat, C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crops Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Cusano, E.; Consonni, R.; Petrakis, E.A.; Astraka, K.; Cagliani, L.R.; Polissiou, M.G. Integrated analytical methodology to investigate bioactive compounds in Crocus sativus L. flowers. Phytochem. Anal. 2018, 29, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, Y.; Yang, L.; Zou, Z.; Zhu, J.; Zhang, Y. Comparative metabolomics analysis of stigmas and petals in Chinese saffron (Crocus sativus) by widely targeted metabolomics. Plants 2022, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, S.F.; Hosseinzadeh, H. Saffron and its active ingredients against human disorders: A literature review on existing clinical evidence. Iran. J. Basic Med. Sci. 2022, 25, 913–933. [Google Scholar]
- Lopresti, A.L.; Smith, S.J. An examination into the mental and physical effects of a saffron extract (affron®) in recreationally-active adults: A randomized, double-blind, placebo-controlled study. J. Int. Soc. Sport. Nutr. 2022, 19, 219–238. [Google Scholar] [CrossRef]
- Salek, R.; Dehghani, M.; Mohajeri, S.A.; Talaei, A.; Fanipakdel, A.; Javadinia, S.A. Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: A double blind, randomized clinical trial. Phytother. Res. 2021, 35, 5143–5153. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Hadi, A.; Ostadrahimi, A. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int. J. Clin. Pract. 2021, 75, e14334. [Google Scholar] [CrossRef]
- Ahmadpanah, M.; Ramezanshams, F.; Ghaleiha, A.; Akhondzadeh, S.; Sadeghi Bahmani, D.; Brand, S. Crocus sativus L. (saffron) versus sertraline on symptoms of depression among older people with major depressive disorders–a double-blind, randomized intervention study. Psychiatry Res. 2019, 282, 112613. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Mostafavi, S.-A.; Keshavarz, S.A.; Mohammadi, M.R.; Hosseini, S.; Eshraghian, M.R. A placebo controlled randomized clinical trial of Crocus sativus L. (saffron) on depression and food craving among overweight women with mild to moderate depression. J. Clin. Pharm. Ther. 2020, 45, 134–143. [Google Scholar] [CrossRef]
- Kashani, L.; Esalatmanesh, S.; Eftekhari, F.; Salimi, S.; Foroughifar, T.; Etesam, F.; Safiaghdam, H.; Moazen-Zadeh, E.; Akhondzadeh, S. Efficacy of Crocus sativus (saffron) in treatment of major depressive disorder associated with post-menopausal hot flashes: A double-blind, randomized, placebo-controlled trial. Arch. Gynecol. Obstet. 2018, 297, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Moazen-Zadeh, E.; Abbasi, S.H.; Safi-Aghdam, H.; Shahmansouri, N.; Arjmandi-Beglar, A.; Hajhosseinn Talasaz, A.; Salehiomran, A.; Forghani, S.; Akhondzadeh, S. Effects of saffron on cognition, anxiety, and depression in patients undergoing coronary artery bypass grafting: A randomized double-blind placebo-controlled trial. J. Altern. Complement. Med. 2018, 24, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jalali, F.; Hashemi, S.F. The effect of saffron on depression among recovered consumers of methamphetamine living with HIV/AIDS. Subst. Use Misuse 2018, 53, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-García, A.M.; Prodanov, M. Affron®, a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kashani, L.; Eslatmanesh, S.; Saedi, N.; Niroomand, N.; Ebrahimi, M.; Hosseinian, M.; Foroughifar, T.; Salimi, S.; Akhondzadeh, S. Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: A double-blind, randomized clinical trial. Pharmacopsychiatry 2017, 50, 64–68. [Google Scholar] [CrossRef]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A.; et al. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: A double-blind, controlled clinical trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Sobhani, F.; Sadjadi, S.A.; Hosseinzadeh, H.; Mohajeri, S.A.; Rajabi, O.; Taherzadeh, Z.; Eslami, S. A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine 2017, 36, 145–152. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Jam, I.N.; Sahebkar, A.H.; Eslami, S.; Mokhber, N.; Nosrati, M.; Khademi, M.; Foroutan-Tanha, M.; Ghayour-Mobarhan, M.; Hadizadeh, F.; Ferns, G.; et al. The effects of crocin on the symptoms of depression in subjects with metabolic syndrome. Adv. Clin. Exp. Med. 2017, 26, 925–930. [Google Scholar]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016, 13, 195–199. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. An investigation into an evening intake of a saffron extract (affron®) on sleep quality, cortisol, and melatonin concentrations in adults with poor sleep: A randomised, double-blind, placebo-controlled, multi-dose study. Sleep Med. 2021, 86, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Pachikian, B.D.; Copine, S.; Suchareau, M.; Deldicque, L. Effects of saffron extract on sleep quality: A randomized double-blind controlled clinical trial. Nutrients 2021, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Takeda, R.; Mori, A. Effect of crocetin on quality of sleep: A randomized, double-blind, placebo-controlled, crossover study. Complement. Ther. Med. 2018, 41, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Baziar, S.; Aqamolaei, A.; Khadem, E.; Mortazavi, S.H.; Naderi, S.; Sahebolzamani, E.; Mortezaei, A.; Jalilevand, S.; Mohammadi, M.-R.; Shahmirzadi, M.; et al. Crocus sativus L. versus methylphenidate in treatment of children with attention-deficit/hyperactivity disorder: A randomized, double-blind pilot study. J. Child Adolesc. Psychopharmacol. 2019, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Karathanasi, E.; Lazarou, I.; Dovas, K.; Verykouki, E.; Karakostas, A.; Georgiadis, K.; Tsolaki, A.; Adam, K.; Kompatsiaris, I.; et al. Efficacy and Ssfety of Crocus sativus L. in patients with mild cognitive impairment: One year single-blind randomized, with parallel groups, clinical trial. J. Alzheimer’s Dis. 2016, 54, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Shafiee Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Shamabadi, A.; Hasanzadeh, A.; Akhondzadeh, S. The neuropsychotropic effects of Crocus sativus L. (saffron): An overview of systematic reviews and meta-analyses investigating its clinical efficacy in psychiatric and neurological disorders. Avicenna J. Phytomed. 2022, 12, 475–488. [Google Scholar]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are sbsorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Du, P.; Fu, J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 2007, 14, 633–636. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, F.; Zhen, L.; Zhu, X.; Wang, J.; Li, S.; Geng, J.; Sun, R.; Yu, X.; Chen, T.; et al. Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. J. Chromatogr. B 2017, 1044–1045, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Nepal, M.R.; Kang, M.J.; Jeong, T.C. Effects of intestinal microbiota on pharmacokinetics of crocin and crocetin in male sprague-dawley rats. Metabolites 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, M.; Sendker, J.; Hüwel, S.; Galla, H.J.; Brandt, S.; Düfer, M.; Riehemann, K.; Hensel, A. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine 2015, 22, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef] [PubMed]
- Chryssanthi, D.G.; Lamari, F.N.; Georgakopoulos, C.D.; Cordopatis, P. A new validated SPE-HPLC method for monitoring crocetin in human plasma—Application after saffron tea consumption. J. Pharm. Biomed. Anal. 2011, 55, 563–568. [Google Scholar] [CrossRef]
- Almodóvar, P.; Briskey, D.; Rao, A.; Prodanov, M.; Inarejos-García, A.M. Bioaccessibility and pharmacokinetics of a commercial saffron (Crocus sativus L.) extract. Evid.-Based Complement. Altern. Med. 2020, 2020, 1575730. [Google Scholar] [CrossRef]
- Miller, T.L.; Willett, S.L.; Moss, M.E.; Miller, J.; Belinka, B.A. Binding of crocetin to plasma albumin. J. Pharm. Sci. 1982, 71, 173–177. [Google Scholar] [CrossRef]
- Salem, A.A.; Lotfy, M.; Amin, A.; Ghattas, M.A. Characterization of human serum albumin’s interactions with safranal and crocin using multi-spectroscopic and molecular docking techniques. Biochem. Biophys. Rep. 2019, 20, 100670. [Google Scholar] [CrossRef]
- Christodoulou, E.; Grafakou, M.-E.; Skaltsa, E.; Kadoglou, N.; Kostomitsopoulos, N.; Valsami, G. Preparation, chemical characterization and determination of crocetin’s pharmacokinetics after oral and intravenous administration of saffron (Crocus sativus L.) aqueous extract to C57/BL6J mice. J. Pharm. Pharmacol. 2018, 71, 753–764. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z.; O’Callaghan, Y.C.; Galvin, K.; O’Brien, N.M. Changes in total and individual crocetin esters upon in vitro gastrointestinal digestion of saffron aqueous extracts. J. Agric. Food Chem. 2013, 61, 5318–5327. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; O’Callaghan, Y.C.; Galvin, K.; Tsimidou, M.Z.; O’Brien, N.M. Cellular transport and bioactivity of a major saffron apocarotenoid, picrocrocin (4-(β-d-Glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde). J. Agric. Food Chem. 2015, 63, 8662–8668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, J.; Hong, Y.; Jiao, L.; Li, S.; Sun, R.; Xie, Y.; Yan, C.; Aa, J.; Wang, G. Orally amdministered crocin protects against cerebral ischemia/reperfusion injury through the metabolic transformation of crocetin by gut microbiota. Front. Pharmacol. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Lu, H.; Li, M.; Yan, Q.; Gu, J.; Liu, L. Neuroprotective mechanism of crocin via PI3K/Akt/mTOR signaling pathway after cerebral infarction: An in vitro study. Am. J. Transl. Res. 2022, 14, 3164–3171. [Google Scholar]
- Dong, N.; Dong, Z.; Chen, Y.; Gu, X. Crocetin alleviates inflammation in MPTP-induced parkinson’s sisease models through improving mitochondrial functions. Park. Dis. 2020, 2020, 9864370. [Google Scholar]
- Forouzanfar, F.; Asadpour, E.; Hosseinzadeh, H.; Boroushaki, M.T.; Adab, A.; Dastpeiman, S.H.; Sadeghnia, H.R. Safranal protects against ischemia-induced PC12 cell injury through inhibiting oxidative stress and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 707–716. [Google Scholar] [CrossRef]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap1/Nrf2 signaling pathway. Cell. Mol. Biol. 2016, 62, 11–17. [Google Scholar] [CrossRef]
- Soeda, S.; Aritake, K.; Urade, Y.; Sato, H.; Shoyama, Y. Neuroprotective activities of saffron and crocin. In The Benefits of Natural Products for Neurodegenerative Diseases; Essa, M.M., Akbar, M., Guillemin, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 275–292. [Google Scholar]
- Zhang, G.-F.; Zhang, Y.; Zhao, G. Crocin protects PC12 cells against MPP+-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem. Int. 2015, 89, 101–110. [Google Scholar] [CrossRef]
- Inoue, E.; Shimizu, Y.; Masui, R.; Hayakawa, T.; Tsubonoya, T.; Hori, S.; Sudoh, K. Effects of saffron and its constituents, crocin-1, crocin-2, and crocetin on α-synuclein fibrils. J. Nat. Med. 2018, 72, 274–279. [Google Scholar] [CrossRef]
- Ghasemi Tigan, M.; Ghahghaei, A.; Lagzian, M. In-vitro and in-silico investigation of protective mechanisms of crocin against E46K α-synuclein amyloid formation. Mol. Biol. Rep. 2019, 46, 4279–4292. [Google Scholar] [CrossRef]
- Inoue, E.; Suzuki, T.; Shimizu, Y.; Sudo, K.; Kawasaki, H.; Ishida, N. Saffron ameliorated motor symptoms, short life span and retinal degeneration in Parkinson’s disease fly models. Gene 2021, 799, 145811. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-synuclein aggregation in parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.R.; Li, B.; Sun, C.; Fan, W.; Zhou, K.; Hughes, M.P.; Sawaya, M.R.; Jiang, L.; Eisenberg, D.S. The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc. Natl. Acad. Sci. USA 2020, 117, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.F.; El Awdan, S.A.; Hegazy, R.R.; Mansour, D.F.; Ogaly, H.A.; Abdelbaset, M. Neuroprotective effect of Crocus sativus against cerebral ischemia in rats. Metab. Brain Dis. 2020, 35, 427–439. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.L.; Shen, L.J.; Jiang, T.T.; Xia, J.J.; You, D.L.; Hu, S.Y.; Wang, L.; Wu, X. Crocin alleviates intracerebral hemorrhage-induced neuronal ferroptosis by facilitating Nrf2 nuclear translocation. Neurotox. Res. 2022, 40, 596–604. [Google Scholar] [CrossRef]
- Huang, A.; Jia, L. Crocin enhances hypothermia therapy in hypoxic ischemia-induced brain injury in mice. Acta Neurol. Belg. 2021, 121, 429–436. [Google Scholar] [CrossRef]
- Hadipour, M.; Meftahi, G.H.; Afarinesh, M.R.; Jahromi, G.P.; Hatef, B. Crocin attenuates the granular cells damages on the dentate gyrus and pyramidal neurons in the CA3 regions of the hippocampus and frontal cortex in the rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2021, 113, 101837. [Google Scholar] [CrossRef]
- Sun, X.-j.; Zhao, X.; Xie, J.-N.; Wan, H. Crocin alleviates schizophrenia-like symptoms in rats by upregulating silent information regulator-1 and brain derived neurotrophic factor. Compr. Psychiatry 2020, 103, 152209. [Google Scholar] [CrossRef]
- Rao, S.V.; Hemalatha, P.; Yetish, S.; Muralidhara, M.; Rajini, P.S. Prophylactic neuroprotective propensity of crocin, a carotenoid against rotenone induced neurotoxicity in mice: Behavioural and biochemical evidence. Metab. Brain Dis. 2019, 34, 1341–1353. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Shaterzadeh, H.; Forouzanfar, F.; Hosseinzadeh, H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 55, 206–213. [Google Scholar] [CrossRef]
- Karkoula, E.; Dagla, I.-V.; Baira, E.; Kokras, N.; Dalla, C.; Skaltsounis, A.-L.; Gikas, E.; Tsarbopoulos, A. A novel UHPLC-HRMS-based metabolomics strategy enables the discovery of potential neuroactive metabolites in mice plasma, following i.p. administration of the main Crocus sativus L. bioactive component. J. Pharm. Biomed. Anal. 2020, 177, 112878. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive review on alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.J.F.; Valero-Cases, E.; Rincon-Frutos, L. Food components with the potential to be used in the therapeutic approach of mental diseases. Curr. Pharm. Biotechnol. 2019, 20, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- Gates, E.J.; Bernath, A.K.; Klegeris, A. Modifying the diet and gut microbiota to prevent and manage neurodegenerative diseases. Rev. Neurosci. 2022, 33, 767–787. [Google Scholar] [CrossRef]
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect. Disord. 2020, 276, 476–486. [Google Scholar] [CrossRef]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-I ameliorates the disruption of lipid metabolism and dysbiosis of the gut microbiota induced by chronic corticosterone in mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef]
- Lin, S.; Li, Q.; Jiang, S.; Xu, Z.; Jiang, Y.; Liu, L.; Jiang, J.; Tong, Y.; Wang, P. Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J. Ethnopharmacol. 2021, 268, 113608. [Google Scholar] [CrossRef]
- Li, M.; Ding, L.; Hu, Y.-L.; Qin, L.-L.; Wu, Y.; Liu, W.; Wu, L.-L.; Liu, T.-H. Herbal formula LLKL ameliorates hyperglycaemia, modulates the gut microbiota and regulates the gut-liver axis in Zucker diabetic fatty rats. J. Cell. Mol. Med. 2021, 25, 367–382. [Google Scholar] [CrossRef]
- Banskota, S.; Brim, H.; Kwon, Y.H.; Singh, G.; Sinha, S.R.; Wang, H.; Khan, W.I.; Ashktorab, H. Saffron pre-treatment promotes reduction in tissue inflammatory profiles and alters microbiome composition in experimental colitis mice. Molecules 2021, 26, 3351. [Google Scholar] [CrossRef]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin prolongs recovery period of DSS-induced colitis via altering intestinal microbiome and increasing intestinal permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef] [PubMed]
- Vergoossen, L.W.M.; Jansen, J.F.A.; Backes, W.H.; Schram, M.T. Cardiometabolic determinants of early and advanced brain alterations: Insights from conventional and novel MRI techniques. Neurosci. Biobehav. Rev. 2020, 115, 308–320. [Google Scholar] [CrossRef] [PubMed]
- van Bussel, F.C.G.; Backes, W.H.; Hofman, P.A.M.; van Oostenbrugge, R.J.; van Boxtel, M.P.J.; Verhey, F.R.J.; Steinbusch, H.W.M.; Schram, M.T.; Stehouwer, C.D.A.; Wildberger, J.E.; et al. Cerebral pathology and cognition in diabetes: The merits of multiparametric neuroimaging. Front. Neurosci. 2017, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, M.; Ostadrahimi, A.; Tajaddini, A.; Asghari, S.; Barati, M.; Akbarzadeh, M.; Nikpayam, O.; Houshyar, J.; Roshanravan, N.; Alamdari, N.M. Effects of saffron supplementation on glycemia and inflammation in patients with type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical trial study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 527–534. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Sahebkar, A.; Aryaeian, N.; Pahlavani, N.; Fallah, S.; Moradi, N.; Abbasi, D.; Hosseini, A.F. Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. Syndr. Obes. 2019, 12, 2107–2115. [Google Scholar] [CrossRef]
- Moravej Aleali, A.; Amani, R.; Shahbazian, H.; Namjooyan, F.; Latifi, S.M.; Cheraghian, B. The effect of hydroalcoholic Saffron (Crocus sativus L.) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Phytother. Res. 2019, 33, 1648–1657. [Google Scholar] [CrossRef]
- Lu, C.; Ke, L.; Li, J.; Zhao, H.; Lu, T.; Mentis, A.F.A.; Wang, Y.; Wang, Z.; Polissiou, M.G.; Tang, L.; et al. Saffron (Crocus sativus L.) and health outcomes: A meta-research review of meta-analyses and an evidence mapping study. Phytomedicine 2021, 91, 153699. [Google Scholar] [CrossRef]
- Menezes, R.; Matafome, P.; Freitas, M.; García-Conesa, M.-T. Updated information of the effects of (poly)phenols against type-2 diabetes mellitus in humans: Reinforcing the recommendations for future research. Nutrients 2022, 14, 3563. [Google Scholar] [CrossRef]
- de Roos, B.; Aura, A.-M.; Bronze, M.; Cassidy, A.; Conesa, M.-T.G.; Gibney, E.R.; Greyling, A.; Kaput, J.; Kerem, Z.; Knežević, N.; et al. Targeting the delivery of dietary plant bioactives to those who would benefit most: From science to practical applications. Eur. J. Nutr. 2019, 58, 65–73. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Clayton, J.A. Sex influences in neurological disorders: Case studies and perspectives. Dialogues Clin. Neurosci. 2016, 18, 357–360. [Google Scholar] [CrossRef] [PubMed]
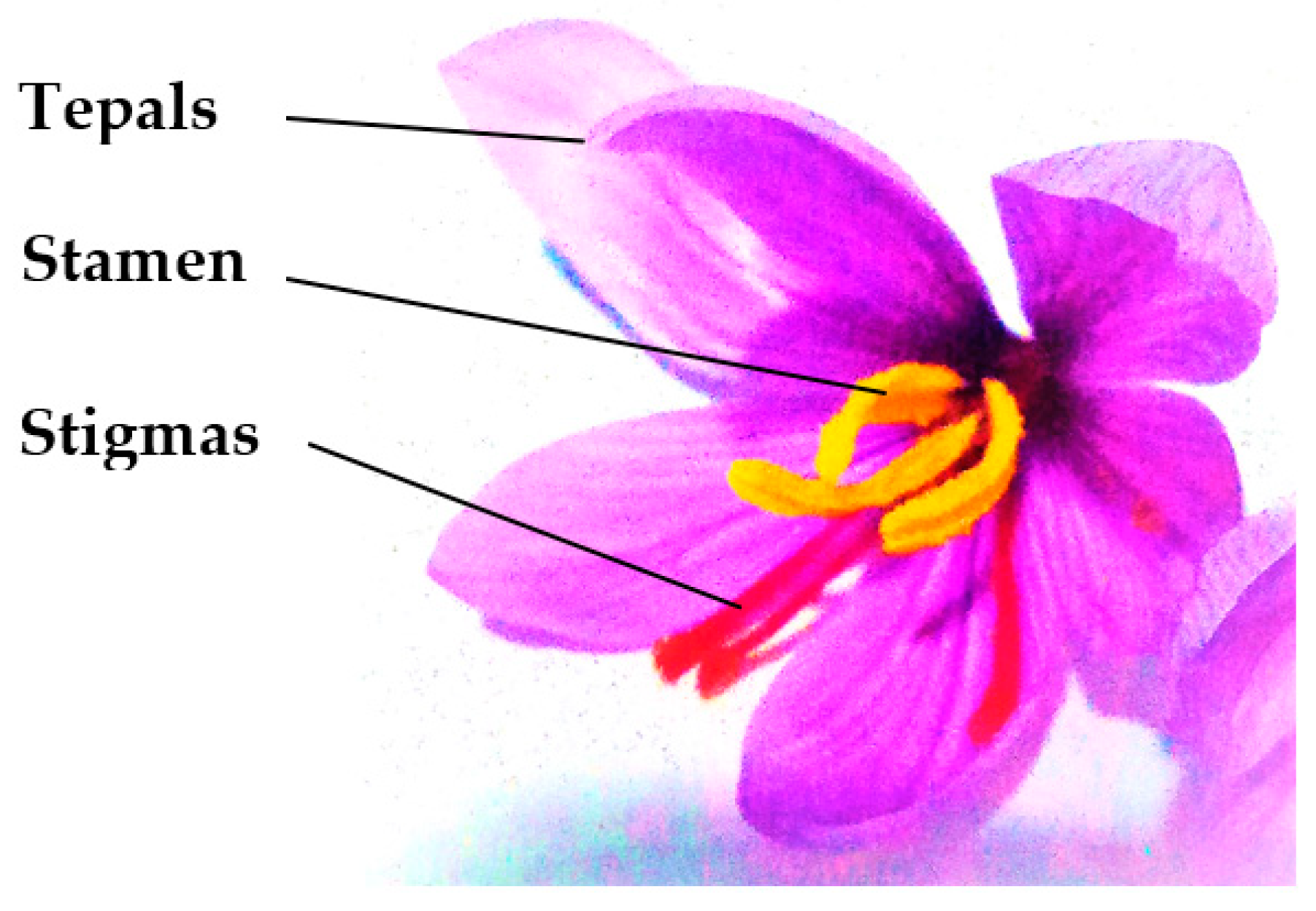
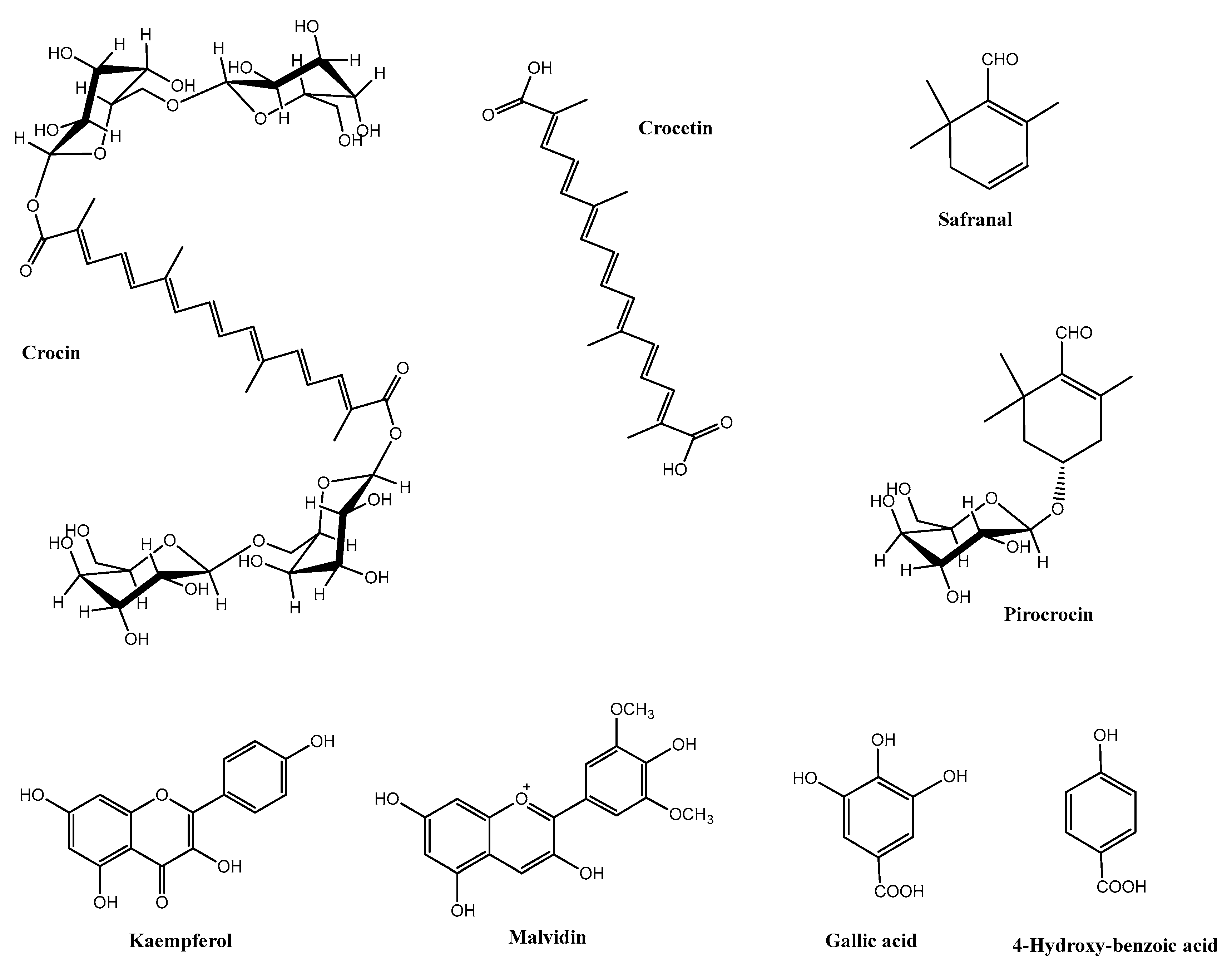
| Component | Saffron Spice (Stigmas) | Saffron Flower Waste (Mostly Tepals) |
|---|---|---|
| Primary constituents | ||
| Major nutrients | Carbohydrates, proteins, fats, fiber, reducing sugars | Carbohydrates, proteins, fats, fiber, reducing sugars |
| Vitamins | Thiamine, riboflavin | Vitamin C |
| Minerals | K, P, Mg, Ca, Fe, Na | K, P, Mg, Ca, Fe, Na |
| Other | Amino acids, organic acids | - |
| Secondary metabolites and bioactive compounds | ||
| Carotenoids | Crocin (trans-4-GG, trans-3-Gg, crocetin (trans- and cis- isomers), | trans-crocetin, trans-crocin, Esters of lutein |
| Terpenoids | Safranal, pirocrocin | - |
| Other volatile compounds | Ketones, aldehydes, esters, c13-norisoprenoids, saturated hydrocarbons, acetic acid | Acetoin, butanoic acid, acetic acid, butanal, carbolic acid, phenylethyl alcohol |
| Flavonoids | Kaempferol, quercetin, epicatechin, anthocyanins | Kaempferol, quercetin, anthocyanins, |
| Phenolic acids | 4-hydroxy benzoic acid, salycilic acid, gallic acid, vanillic acid, rosmarinic acid, chlorogenic acid | Gallic acid, 4-hydroxy-benzoic acid, salicylic acid, chlorogenic acid, hydroxy cinnamic acids, rosmarinic acid |
| Tannins | Hydrolysable and condensed tannins | Hydrolysable and condensed tannins |
| Ref. | Animal Model (N) | Administration Route | Administered Compound | Dose (mg/Kg) | Crocin | Crocetin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (µg/mL) | Tmax (min.) | AUC0-tmax (μg·h/mL) | Cmax (µg/mL) | Tmax (min.) | AUC0-tmax (μg·h/mL) | |||||
| [47] | ICR mice (8 male) | Intragastric gavage | Crocin (prepared from gardenia fruits) | 6.1 | - | - | - | 0.033 | 60 | - |
| Crocetin (prepared by saponification of the Amberlite resin-eluted pigments) | 2.1 | - | - | - | 0.115 | 60 | - | |||
| [48] | Sprague-Dawley rats (3 male + 3 female) | Intragastric gavage | Crocin (Nanjing Medical Company, Nanjing, China; 98%) | 40 | - | - | - | 0.830 ± 0.31 | 60 | - |
| [49] | Sprague-Dawley rats (32 male + 32 female) | Intragastric gavage | Crocin (Chengdu Biopurify Phytochemicals Ltd., Chengdu, China; 99.3%) | 29.3 | 0.050 ± 0.026 | 114 | 0.201 ± 0.097 | 0.688 ± 0.162 | 120 | 3.931 ± 0.930 |
| 58.6 | 0.056 ± 0.034 | 156 | 0.310 ± 0.212 | 1.278 ± 0.623 | 234 | 8.669 ± 2.744 | ||||
| 117.2 | 0.082 ± 0.039 | 114 | 0.394 ± 0.169 | 1.249 ± 0.788 | 246 | 10.61 ± 8.207 | ||||
| Crocetin (Chengdu Biopurify Phytochemicals Ltd.; 96.6%) | 9.8 | ND | ND | ND | 0.387 ± 0.228 | 162 | 3.062 ± 1.607 | |||
| 19.7 | ND | ND | ND | 0.572 ± 0.380 | 174 | 4.587 ± 3.009 | ||||
| 39.3 | ND | ND | ND | 0.658 ± 0.316 | 150 | 5.258 ± 2.594 | ||||
| [50] | Sprague-Dawley rats (Control—5 male) | Intragastric gavage | Crocin (Sigma, St. Louis, MO, USA) | 600 | 3.04 × 10−4 ± 1.53 × 10−4 | 168 ± 66 | 1.25 × 10−3 ± 6.09 × 10−4 | 1.03 × 10−2 ± 2.00 × 10−3 | 204 ± 54 | 6.79 × 10−2 ± 7.99 × 10−3 |
| Sprague-Dawley rats (Antibiotic pre-treatment—5 male) | 7.49 × 10−5 ± 3.01 × 10−5 | 150 ± 84 | 4.71 × 10−4 ± 4.48 × 10−4 | 9.73 × 10−3 ± 2.37 × 10−3 | 132 ± 24 | 3.51 × 10−2 ± 4.14 × 10−3 | ||||
| Ref. | Population (N, Gender, Age) | Product | Dose | Crocetin | ||
|---|---|---|---|---|---|---|
| Cmax (µg/mL) | Tmax (min.) | AUC0-tmax (μg·h/mL) | ||||
| [53] | 4 healthy volunteers (1 male + 3 female; 25 to 35 years) | Saffron Infusion (trans-crocin-4: 59.5 ± 0.2%; trans-crocin-3: 21.0 ± 0.4%; cis-crocin-4: 5.0 ± 0.1%; trans-crocin-2: 1.3 ± 0.2%; cis-crocin-3: 8.2 ± 0.2%; cis-crocin-1: 1.1 ± 0.3%) | 200 mg stigmas/150 mL hot water | 0.41–1.21 | 120 min | NA |
| [54] | 13 healthy volunteers (5 male + 8 female; 18 to 30 years) | affron® (Saffron Extract) (safranal: 0.04 ± 0.01%; picrocrocin: 3.21 ± 0.07%; kaempferol diglucoside: 0.13 ± 0.01%; crocins: 3.63 ± 0.05%; crocetin: 0.03 ± 0.01%) | 4 tablets—56 mg | 0.26 ± 0.12 | 60 min | 21.07 |
| 6 tablets—84 mg | 0.39 ± 0.10 | 90 min | 26.15 | |||
| In Vitro Model | Saffron Treatment | Reported Effects | ||||
|---|---|---|---|---|---|---|
| Ref. | Description | Biological Role in the Brain or Nervous System | Test Compound Purity | Concentration, Time of Exposure | Reported Mechanisms | Potential Neurological Benefit |
| Cell culture model | ||||||
| [62] | Mouse hippocampal neuronal cell line HT-22 treated with OGD to induce ischemia | Hippocampal neurons play a major role in the functioning of the human brain and in the memory | Crocin (Nanjing Jingzhu Biotechnology Co. Ltd., Nanjing, China) 98% | 1 µg/mL, 2 µg/mL and 5 µg/mL, 14 h | Improves cell viability, decreases apoptosis, decreases ROS, increases the expression of p-PI3K, p-Akt, and p-mTOR (activation of the PI3K/mTOR pathway) while decreases the expression of LC-3 II/I and BECN1 | Neuroprotective effects against cerebral infarction by inhibiting autophagy and reducing oxidative stress |
| [63] | Microglia BV2 cells isolated from a 10-days old female mouse treated with neurotoxin MPTP to induce PD like symptoms | Brain macrophages. Regulate brain development, maintenance of neuronal networks, and injury repair. Protect against infection and inflammation | Crocetin dialdehyde (Sigma, St.
Louis, MI, USA) >95% | 2.5 μM, 5 μM, 10 μM, 24 h | Decreases the expression of inflammatory associated genes (p-p65 and pro-/Cleaved Casp1) and cytokines (IL-1β, IL-6, IL-10, TNF-α, inducible nitric oxide synthase, COX2). Improves mitochondrial function by blocking intracellular reactive oxygen species (ROS) levels, increasing mitochondrial membrane potential and reducing the content of calcium in the cytosol | Inhibition of inflammatory conditions in the brain and amelioration of PD associated symptoms |
| [64] | PC-12 cells isolated from a rat male exposed to OGD to induce ischemia | PC-12 cells, isolated from adrenal medulla pheochromocytoma, mixture of neuroblastic cells and eosinophilic cells. Used to study biological functions of neuronal cells and brain diseases | Safranal (Sigma) ≥90% | 10–160 μM 2 h | Attenuation of oxidative brain injury via reducing intracellular ROS levels, oxidative DNA damage and cell apoptosis at 40 μM and 160 μM of safranal | Neuroprotection due to the modulation of oxidative stress and apoptotic responses in the brain |
| [66] | PC-12 cells isolated from a rat male (brain ischemia) | PC-12 cells, isolated from adrenal medulla pheochromocytoma of rats, mixture of neuroblastic cells and eosinophilic cells. Used to study biological functions of neuronal cells and brain diseases | Crocin NI | 1 µM, 10 μM, NI | Prevention of neuronal cell apoptosis, reduction of ROS generation, increased cellular levels of GSH by the activation of GR and restored SOD activity, especially at 10 μM of crocetin | Potential neuroprotection due to the antioxidant activity: reducing oxidative stress and neuronal apoptosis |
| [67] | PC-12 cell culture model isolated from a rat male treated with neurotoxin MPP+ to induce PD like symptoms | PC-12 cells, isolated from adrenal medulla pheochromocytoma of rats, mixture of neuroblastic cells and eosinophilic cells. Used to study biological functions of neuronal cells and brain diseases | Crocin Crocetin (Sigma) ≥95% ≥95% | 0.1 μM, 1 μM, 10 μM or 100 μM, 0, 3, 6 or 9 h after MPP+ exposure | Improvement of mitochondrial function and protection against cell apoptosis at 10 and 100 μM of crocin | Protection of cytotoxicity by inhibiting the activation of pro-apoptotic factor Casp12 by its anti-apoptotic activity |
| [65] | Primary dopaminergic cells isolated from rat embryos treated with rotenone to induce PD like symptoms | Dopaminergic cells are collections of neurons in the central nervous system that synthesize the neurotransmitter dopamine. The loss of these neurons is associated with neurological disorders such as PD | Safranal (Sigma) ≥ 90% ≥90% ≥90% | 10 μM, 15 μM, 20 μM, and 50 μM, 4 h | Suppression of ROS generation and cell apoptosis by the inhibition of Keap1 protein expression and promotion of nuclear translocation of Nrf2, especially at 50 μM of safranal | Neuroprotection against oxidative stress via the modulation of the Nrf2 signalling pathway that is involved in the protection of PD |
| [70] | Human neuronal cell line SH-SY5Y from a female neuroblastome subjected to α-Syn toxicity | Representative of human nervous cells | Saffron (Matsuura Yakugyo; Aichi, Japan) in DMSO | 6 µg/mL and 20 µg/mL | Saffron restores cell viability at the highest concentration | Cytoprotective effects in human cells |
| α-Syn PFF model | ||||||
| [68] | Solution of α-Syn fibrils | α-Syn is a 140-amino acid protein abundant in the human brain. Its abnormal aggregation and accumulation produces neurodegenerative diseases such as PD | Saffron powder (Matsuura Yakugyo, Japan); Crocin (Carbosynth & Adooq Bioscience, Irvine, CA, USA); Crocetin, safranal (Toronto Research Chemicals, North York, ON, Canada) NI | αS aggregation: 0.5 μL of saffron compounds, 3 h at 37 °C αS dissociation: 0.5 μL of saffron compounds, 15 min at 37 °C | Inhibition of α-Syn aggregation and promotion of the dissociation of α-Syn fibrils. Crocetin was the most potent bioactive compound | Effective neuroprotection to prevent and treat neurological disorders generated by abnormal α-Syn aggregation |
| [69] | Solution of E46K mutant forms of α-Syn fibrils | The E46K mutation of the α-Syn gene is linked to cause PD | Crocin (Booali Research Center, Mashhad, Iran) NI | Different molar ratios α-synuclein:crocin (1:0, 1:0.25, 1:0.5), different times | Inhibition of amyloid fibril formation in E46K α-Syn, interacting with its hydrophobic surface area | Potential therapeutic neuroprotection on the fibrillation pathway, inhibiting the dimerization and polymerization of α-Syn fibrils |
| Saffron Treatment | Reported Effects | |||||
|---|---|---|---|---|---|---|
| Ref. | Animal Model Sex | Test Compound Purity | Dose Administration Route Duration of Treatment | HED (mg/60 Kg Person) | Reported Mechanisms | Potential Neuroprotection |
| [70] | Drosophila model of PD overexpressing several human mutants α-Syn in their neurons or eyes Male | Saffron (Matsuura Yakugyo, Japan) suspended in distilled water; Crocetin extracted from saffron NI | Saffron: 3, 10 and 30 µg/mL; crocetin: 0.3, 1 and 3 µg/mL, p.o. (added into the feed) ≈80 days | ND | Improvement of the climbing ability, life expand, and eye phenotype but the results were α-Syn mutation-dependent. The results were especially seen at the higher concentrations of saffron and crocetin | Potential protective effect against PD progression by interfering with α-Syn aggregation process |
| [73] | Wistar rats (3-weeks-old) induced brain I/R injury Male | Saffron stigmas (from a local market) 300 g/3 L of 80% ethanol during 3 days, filtered and concentrated | 100 and 200 mg/kg b.w. i.p. 21 days prior to brain I/R injury and 4 days during I/R injury | ≈975 mg and ≈1950 mg | Attenuation of lipid peroxidation, decrease of NO and BNP, reversal of the depletion of GSH, upregulation of the expression of VEGF and decrease of the expression of CASP3 and Bax in brain tissue at both concentrations of saffron | Potential neuroprotection through the reduction of oxidative stress and apoptosis |
| [74] | Intracerebral hemorrhage mouse model Male | Crocin (Sigma) ≥95% | 40 mg/Kg b.w. i.p. 2 h after intracerebral hemorrhage | ≈195.0 mg | Improvement of brain edema and neurological defficient score. Increased SOD and GSH-Px activity while reduction of MDA. Inhibition of ferroptosis of neurons and molecular regulation of the expression of GPX4, FTH1, SLC7A11. Translocation of Nrf2. | Alleviation of intracerbral hemorrhage |
| [75] | C57BL/6J mice hypoxia-ischemia-induced NI | Crocin (Sigma) ≥95% | 10 mg/Kg b.w. i.p. every 12 h during 48 h | ≈50.0 mg | Reduction of MDA, NO and ROS in the brain and inhibition the expression of pro-inflammatory mediators (combined effect with hypothermia) | Improvement of the neurological function and neuroprotection by the reduction of the oxidative stress and inflammation in brain |
| [76] | Adult Wistar Albino rats Aβ1–42 AD-induced Male | Crocin powder dissolved in saline solution NI | 30 mg/kg b.w. i.p. once a day during 12 days | ≈300 mg | Improvement of synaptic loss and reduction of neuronal cell apoptosis | Protection of neuronal cell death which is responsible for cognitive impairment and dementia in AD |
| [77] | Neonatal Sprague Dawley rats treated with MK-801 to induce schizophrenia-like symptoms NI | Crocin (Chengdu Puri Technology Limited, Chengdu, China) dissolved in sterile saline solution ≥97% | 25 and 50 mg/kg b.w. i.p. once a day, on postnatal day 7 to day 14 (during 7 days) | ≈250 mg and ≈500 mg | Amelioration of schizophrenia symptoms via regulation of SIRT1 and downstream BDNF proteins expression in the hippocampus. Relief of the oxidative stress by reducing MDA levels and increasing the activity of CAT, GPx, and SOD in the hippocampus | Neuroprotection effects since SIRT1 participate in the pathogenesis of neurodegenerative disorders, playing important roles in several biological processes |
| [78] | CFT-Swiss mice (8-week old) treated with rotenone to induce PD like symptoms Male | Crocin (Sigma): 10 mg/mL prepared in double distilled water ≥95% | 25 mg/kg b.w. i.p. once a day during 7 days | ≈120 mg | Restoration of the levels of dopamine and α-Syn and enhancement of antioxidant enzymes and mitochondrial enzymes function in the striatal brain region | Neuroprotection by reducing the oxidative stress and improving mitochondrial function which is necessary for the high energy demand of the brain |
| [80] | Wild type C57BL/6J mice, aged 2–12 months Male and female | trans-crocin-4 isolated from Crocus sativus stigmas and dissolved in saline solution >90% | 50 mg/kg and 150 mg/kg b.w. i.p. duration NI | ≈250 mg and ≈750 mg | Changes in the metabolome, detection of some metabolites with important roles in neuroprotection (21-Hydroxypregnenolone and 17α-epiestriol participating in the steroid biosynthesis pathway; glutaryl carnitine and riboflavin related with the oxidative stress, and betaine involved in inhibitory neurotransmitter production pattways) | Potential prevention of neurological disorders trough changes in the metabolomic profile |
| [63] | C57BL/6J mice treated with MPTP to induce PD like symptoms Male | Crocetin dialdehyde (Sigma) ≥95% | 50 and 100 mg/Kg (NI per b.w. or per kg of feed) p.o. once a day during 11 days | ≈250 mg and 500 mg | Attenuation of the expression of the TH marker in the striatal brain regions at both concentrations of crocetin | Protection of dopaminergic neurons against toxin-induced damage |
| [79] | Adult Wistar rats of transient focal cerebral ischemia induced by MCAO Male | Safranal (Sigma) ≥90% | 72.5 and 145 mg/kg b.w. i.p. 0, 3, and 6 h after ischemia/reperfusion injury | ≈700 mg and ≈1400 mg | Inhibition of the oxidative stress in the brain tissues, reducing MDA levels and increasing the total sulfhydryl content at both concentrations of safranal | Potential neuroprotective effects by modulating the oxidative stress |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerdá-Bernad, D.; Costa, L.; Serra, A.T.; Bronze, M.R.; Valero-Cases, E.; Pérez-Llamas, F.; Candela, M.E.; Arnao, M.B.; Barberán, F.T.; Villalba, R.G.; et al. Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection. Nutrients 2022, 14, 5368. https://doi.org/10.3390/nu14245368
Cerdá-Bernad D, Costa L, Serra AT, Bronze MR, Valero-Cases E, Pérez-Llamas F, Candela ME, Arnao MB, Barberán FT, Villalba RG, et al. Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection. Nutrients. 2022; 14(24):5368. https://doi.org/10.3390/nu14245368
Chicago/Turabian StyleCerdá-Bernad, Débora, Leonor Costa, Ana Teresa Serra, Maria Rosário Bronze, Estefanía Valero-Cases, Francisca Pérez-Llamas, María Emilia Candela, Marino B. Arnao, Francisco Tomás Barberán, Rocío García Villalba, and et al. 2022. "Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection" Nutrients 14, no. 24: 5368. https://doi.org/10.3390/nu14245368
APA StyleCerdá-Bernad, D., Costa, L., Serra, A. T., Bronze, M. R., Valero-Cases, E., Pérez-Llamas, F., Candela, M. E., Arnao, M. B., Barberán, F. T., Villalba, R. G., García-Conesa, M.-T., & Frutos, M.-J. (2022). Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection. Nutrients, 14(24), 5368. https://doi.org/10.3390/nu14245368








