Increased Thoracic Fluid as the Most Distinctive Cardiovascular Hemodynamic Alteration in Men with Prolactinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Examination
2.3. Impedance Cardiography
2.4. Statistical Methods
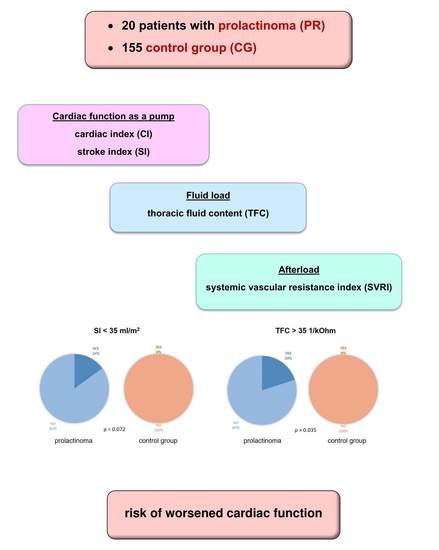
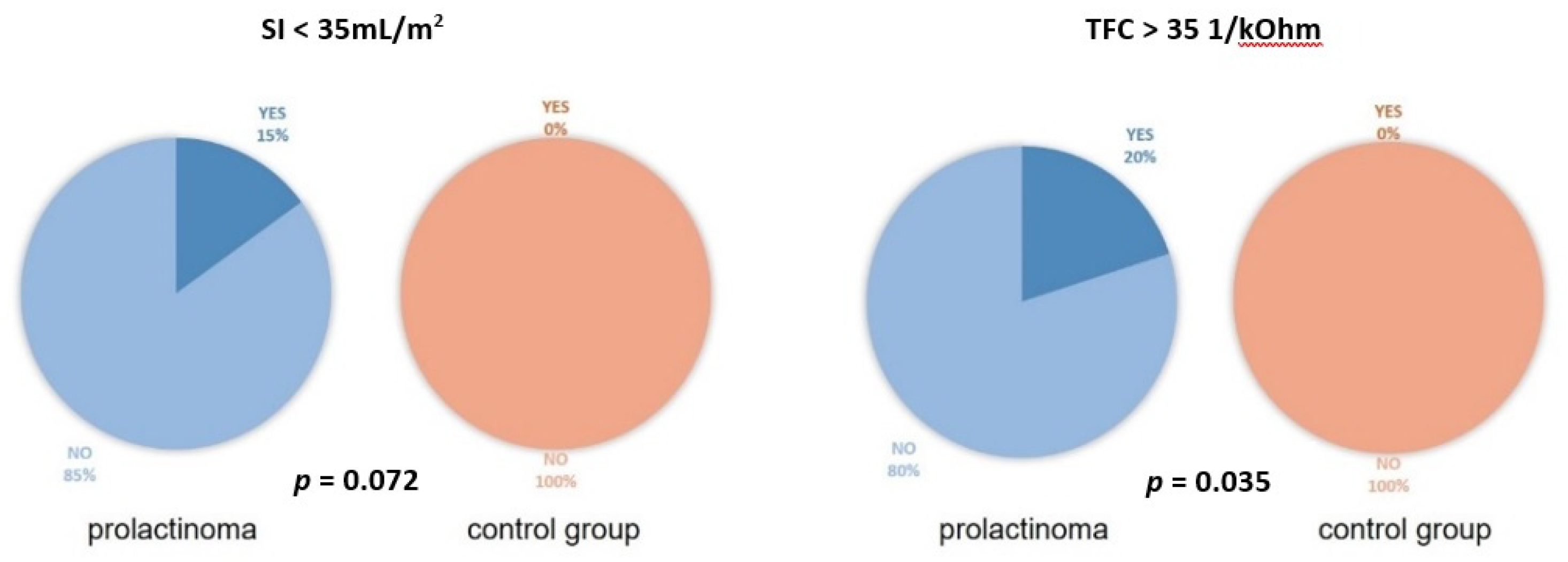
3. Results
3.1. Baseline Characteristics
3.2. ICG Variables
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casanueva, F.F.; Molitch, M.E.; Schlechte, J.A.; Abs, R.; Bonert, V.; Bronstein, M.D.; Brue, T.; Cappabianca, P.; Colao, A.; Fahlbusch, R.; et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin. Endocrinol. 2006, 65, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A. Endocrine Society. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Beckers, A. The Epidemiology of Pituitary Adenomas. Endocrinol. Metab. Clin. 2020, 49, 347–355. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Raappana, A.; Koivukangas, J.; Ebeling, T.; Pirilä, T. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J. Clin. Endocrinol. Metab. 2010, 95, 4268–4275. [Google Scholar] [CrossRef]
- Gruppetta, M.; Mercieca, C.; Vassallo, J. Prevalence and incidence of pituitary adenomas: A population based study in Malta. Pituitary 2013, 16, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Robbins, T.; Reddy, N.; Balachandran, K.; Gokhale, K.; Wijesinghe, H.; Cheng, K.K.; Karavitaki, N.; Wass, J.; Nirantharakumar, K. Males with prolactinoma are at increased risk of incident cardiovascular disease. Clin. Endocrinol. 2018, 88, 71–76. [Google Scholar] [CrossRef]
- Krogh, J.; Selmer, C.; Torp-Pedersen, C.; Gislason, G.H.; Kistorp, C. Hyperprolactinemia and the Association with All-Cause Mortality and Cardiovascular Mortality. Horm. Metab. Res. 2017, 49, 411–417. [Google Scholar] [CrossRef]
- Pala, N.A.; Laway, B.A.; Misgar, R.A.; Dar, R.A. Metabolic abnormalities in patients with prolactinoma: Response to treatment with cabergoline. Diabetol. Metab. Syndr. 2015, 7, 99. [Google Scholar] [CrossRef]
- dos Santos Silva, C.M.; Barbosa, F.R.; Lima, G.A.; Warszawski, L.; Fontes, R.; Domingues, R.C.; Gadelha, M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011, 19, 800–805. [Google Scholar] [CrossRef]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.S.; Topaloglu, O.; Sahin, M.; Tutal, E.; Gungunes, A.; Cakir, E.; Ozturk, I.U.; Karbek, B.; Ucan, B.; Ginis, Z.; et al. Preclinical atherosclerosis in patients with prolactinoma. Endocr. Pract. 2014, 20, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Erem, C.; Kocak, M.; Nuhoglu, I.; Yılmaz, M.; Ucuncu, O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin. Endocrinol. 2010, 73, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, A.; Daly, A.F.; Beckers, A. The epidemiology of prolactinomas. Pituitary 2005, 8, 3–6. [Google Scholar] [CrossRef]
- Reuwer, A.Q.; van Zaane, B.; van Wissen, M.; van Zanten, A.P.; Twickler, M.T.; Gerdes, V.E. Prolactin is involved in the systemic inflammatory response in myocardial infarction. Horm. Metab. Res. 2011, 43, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Shahar, E.; Redline, S.; Gottlieb, D.J.; Givelber, R.; Resnick, H.E. Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am. J. Epidemiol. 2004, 160, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Reuwer, A.Q.; Twickler, M.T.; Hutten, B.A.; Molema, F.W.; Wareham, N.J.; Dallinga-Thie, G.M.; Bogorad, R.L.; Goffin, V.; Smink-Bol, M.; Kastelein, J.J.; et al. Prolactin levels and the risk of future coronary artery disease in apparently healthy men and women. Circ. Cardiovasc. Genet. 2009, 2, 389–395. [Google Scholar] [CrossRef]
- Jiang, X.B.; Zhang, J.; Li, C.L.; Mao, Z.G.; Hu, B.; Zhu, Z.; Zhu, Y.H.; Wang, H.J. Subclinical impairment of left ventricular longitudinal function in patients with prolactinomas. Endocr. Pract. 2017, 23, 1379–1386. [Google Scholar] [CrossRef]
- Reuwer, A.Q.; Sondermeijer, B.M.; Battjes, S.; van Zijderveld, R.; Stuijver, D.J.; Bisschop, P.H.; Twickler, M.T.; Meijers, J.C.; Schlingemann, R.O.; Stroes, E.S. Microcirculation and atherothrombotic parameters in prolactinoma patients: A pilot study. Pituitary 2012, 15, 472–481. [Google Scholar] [CrossRef][Green Version]
- Krzesiński, P.; Gielerak, G.; Kowal, J. Impedance cardiography—A modern tool for monitoring therapy of cardiovascular diseases. Kardiol. Pol. 2009, 67, 65–71. [Google Scholar]
- Bhalla, V.; Isakson, S.; Bhalla, M.A.; Lin, J.P.; Clopton, P.; Gardetto, N.; Maisel, A.S. Diagnostic ability of B-type natriuretic peptide and impedance cardiography: Testing to identify left ventricular dysfunction in hypertensive patients. Am. J. Hypertens. 2005, 18, 73S–81S. [Google Scholar] [CrossRef] [PubMed]
- Krzesiński, P.; Gielerak, G.; Stańczyk, A.; Uziębło-Życzkowska, B.; Smurzyński, P.; Piotrowicz, K.; Skrobowski, A. What does impedance cardiography add more to the assessment of left ventricular diastolic function in essential hypertension? Pol Merkur Lek. 2015, 39, 352–358. [Google Scholar]
- Parrott, C.W.; Burnham, K.M.; Quale, C.; Lewis, D.L. Comparison of changes in ejection fraction to changes in impedance cardiography cardiac index and systolic time ratio. Congest. Heart Fail. 2004, 10, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Abdelhammed, A.I.; Smith, R.D.; Levy, P.; Smits, G.J.; Ferrario, C.M. Noninvasive hemodynamic profiles in hypertensive subjects. Am. J. Hypertens. 2005, 18, 51S–59S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Strobeck, J.E.; Silver, M.A. Beyond the four quadrants: The critical and emerging role of impedance cardiography in heart failure. Congest. Heart Fail. 2004, 10, 1–6. [Google Scholar] [CrossRef]
- Friedrich, N.; Schneider, H.J.; Spielhagen, C.; Markus, M.R.; Haring, R.; Grabe, H.J.; Buchfelder, M.; Wallaschofski, H.; Nauck, M. The association of serum prolactin concentration with inflammatory biomarkers—Cross-sectional findings from the population-based Study of Health in Pomerania. Clin. Endocrinol 2011, 75, 561–566. [Google Scholar] [CrossRef]
- Haring, R.; Friedrich, N.; Völzke, H.; Vasan, R.S.; Felix, S.B.; Dörr, M.; Meyer zu Schwabedissen, H.E.; Nauck, M.; Wallaschofski, H. Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. Eur. Heart J. 2014, 35, 1215–1221. [Google Scholar] [CrossRef]
- Melmed, S. Medical progress: Acromegaly. N. Engl. J. Med. 2006, 355, 2558–2573. [Google Scholar] [CrossRef]
- Pivonello, R.; Auriemma, R.S.; Grasso, L.F.; Pivonello, C.; Simeoli, C.; Patalano, R.; Galdiero, M.; Colao, A. Complications of acromegaly: Cardiovascular, respiratory and metabolic comorbidities. Pituitary 2017, 20, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Jurek, A.; Krzesiński, P.; Gielerak, G.; Witek, P.; Zieliński, G.; Kazimierczak, A.; Wierzbowski, R.; Banak, M.; Uziębło-Życzkowska, B. Cushing’s Disease: Assessment of Early Cardiovascular Hemodynamic Dysfunction With Impedance Cardiography. Front. Endocrinol. 2021, 12, 751743. [Google Scholar] [CrossRef] [PubMed]
- Jurek, A.; Krzesiński, P.; Gielerak, G.; Witek, P.; Zieliński, G.; Kazimierczak, A.; Wierzbowski, R.; Banak, M.; Uziębło-Życzkowska, B. Acromegaly: The Research and Practical Value of Noninvasive Hemodynamic Assessments via Impedance Cardiography. Front. Endocrinol. 2022, 12, 793280. [Google Scholar] [CrossRef] [PubMed]
| VARIABLE | Mean ± SD (Median; Interquartile Range) or n (%) |
|---|---|
| Age [years]. | 43 ± 13 (42; 31–52) |
| Male sex | 20 (100) |
| BMI [kg/m2] | 32 ± 7 (30; 28–35) |
| BMI 18.5–24.9 kg/m2 | 2 (10) |
| BMI 25–29.9 kg/m2 | 8 (40) |
| BMI ≥ 30 kg/m2 | 10 (50) |
| HR [bpm] | 68 ± 10 (69; 61–74) |
| SBP [mmHg] | 117 ± 17 (119; 102–130) |
| SBP ≥ 140 mmHg | 2 (10) |
| DBP [mmHg] | 76 ± 10 (77; 66–82) |
| DBP ≥ 90 mmHg | 2 (10) |
| AH | 8 (40) |
| DM | 3 (15) |
| Creatinine [mg/dL] | 0.80 |
| LVEF [%] | 64 ± 3 (64; 63–65) |
| VARIABLES | CG Mean ± SD (Median; IQR) or n (%) | PR Mean ± SD (Median; IQR) or n (%) | p-Value |
|---|---|---|---|
| BASELINE CHARACTERISTICS | |||
| Age [years] | 43 ± 9 (43; 37–50) | 43 ± 13 (42; 31–52) | 0.922 |
| BMI [kg/m2] | 30 ± 4 (29; 27–31) | 32 ± 7 (30; 28–35) | 0.525 |
| HR [bpm] | 66 ± 10 (67; 59–74) | 68 ± 10 (69; 61–74) | 0.692 |
| SBP [mmHg] | 119 ± 7 (117; 114–123) | 117 ± 17 (119; 102–130) | 0.567 |
| DBP [mmHg] | 75 ± 8 (74; 69–81) | 76 ± 10 (77; 66–82) | 0.684 |
| AH | 11/20 (55) | 8/20 (40) | 0.342 |
| Creatinine [mg/dL] | 0.96 ± 0.14 (0.94; 0.90–1.00) | 0.80 ± 0.13 (0.80; 0.70–0.85) | 0.001 |
| LVEF [%] | 66 ± 3 (66; 64–68) | 64 ± 3 (64; 63–65) | 0.051 |
| IMPEDANCE CARDIOGRAPHY | |||
| HR [bpm] | 67 ± 13 (68; 58–74) | 67 ± 10 (67; 61–72) | 0.989 |
| SBP [mmHg] | 117 ± 9 (117; 109–124) | 116 ± 16 (119; 102–131) | 0.682 |
| DBP [mmHg] | 75 ± 7 (75; 73–80) | 76 ± 10 (78; 66–82) | 0.762 |
| MBP [mmHg] | 85 ± 7 (85; 81–91) | 86 ± 11 (89; 76–92) | 0.873 |
| PP [mmHg] | 43 ± 8 (43; 38–48) | 40 ± 9 (39; 35–46) | 0.355 |
| SI [mL/m2] | 50 ± 11 (53; 41–58) | 47 ± 11 (45; 40–54) | 0.301 |
| CI [mL*m−2*min−1] | 3.3 ± 0.7 (3.4; 2.9–3.8) | 3.1 ± 0.6 (3.0; 2.7–3.4) | 0.211 |
| SVRI [dyn*s*cm−5*m2] | 1994 ± 498 (1871; 1721–2228) | 2127 ± 402 (2091; 1811–2378) | 0.359 |
| VI [1*1000−1*s−1] | 46 ± 13 (43; 35–60) | 43 ± 12 (43; 36–50) | 0.400 |
| ACI [1/100/s2] | 71 ± 25 (72; 49–95) | 67 ± 22 (70; 53–81) | 0.640 |
| HI [Ohm/s2] | 12 ± 3 (12; 10–15) | 10 ± 3 (10; 8–12) | 0.112 |
| TFC [1/kOhm] | 30 ± 4 (30; 28–32) | 30 ± 4 (31; 27–33) | 0.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, A.; Krzesiński, P.; Gielerak, G.; Witek, P.; Zieliński, G.; Kazimierczak, A.; Wierzbowski, R.; Banak, M.; Uziębło-Życzkowska, B. Increased Thoracic Fluid as the Most Distinctive Cardiovascular Hemodynamic Alteration in Men with Prolactinoma. Nutrients 2022, 14, 5369. https://doi.org/10.3390/nu14245369
Jurek A, Krzesiński P, Gielerak G, Witek P, Zieliński G, Kazimierczak A, Wierzbowski R, Banak M, Uziębło-Życzkowska B. Increased Thoracic Fluid as the Most Distinctive Cardiovascular Hemodynamic Alteration in Men with Prolactinoma. Nutrients. 2022; 14(24):5369. https://doi.org/10.3390/nu14245369
Chicago/Turabian StyleJurek, Agnieszka, Paweł Krzesiński, Grzegorz Gielerak, Przemysław Witek, Grzegorz Zieliński, Anna Kazimierczak, Robert Wierzbowski, Małgorzata Banak, and Beata Uziębło-Życzkowska. 2022. "Increased Thoracic Fluid as the Most Distinctive Cardiovascular Hemodynamic Alteration in Men with Prolactinoma" Nutrients 14, no. 24: 5369. https://doi.org/10.3390/nu14245369
APA StyleJurek, A., Krzesiński, P., Gielerak, G., Witek, P., Zieliński, G., Kazimierczak, A., Wierzbowski, R., Banak, M., & Uziębło-Życzkowska, B. (2022). Increased Thoracic Fluid as the Most Distinctive Cardiovascular Hemodynamic Alteration in Men with Prolactinoma. Nutrients, 14(24), 5369. https://doi.org/10.3390/nu14245369