Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cold-Brewed Jasmine Tea
2.2. Experimental Animal Design
2.3. Measurement of Serum Parameters and Hepatic Lipid Profiles
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Quantification of Gene Expression
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
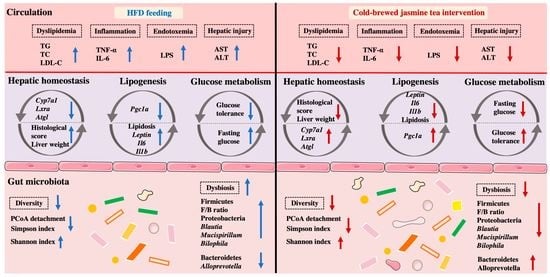
3. Results
3.1. CB-JT Suppressed HFD-Induced Abnormal Body Weight Gain, Organ Weight and Food Intake
3.2. CB-JT Improved the Serum Biochemical Parameters in HFD-Fed Mice
3.3. CB-JT Attenuated HFD-Induced Histological Injury
3.4. CB-JT Regulated HFD-Induced Abnormal Expression of Lipid Metabolism-Related Genes
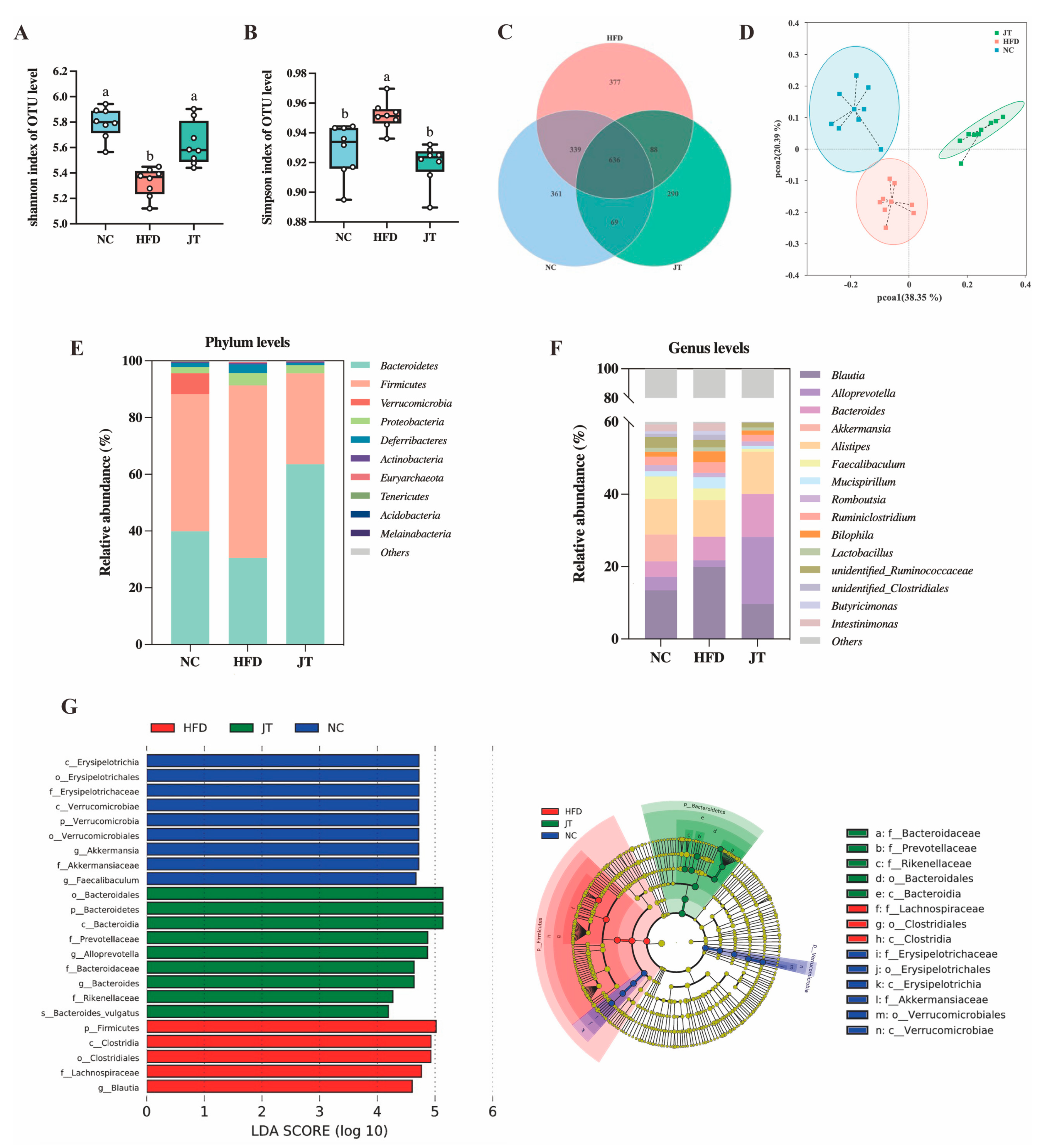
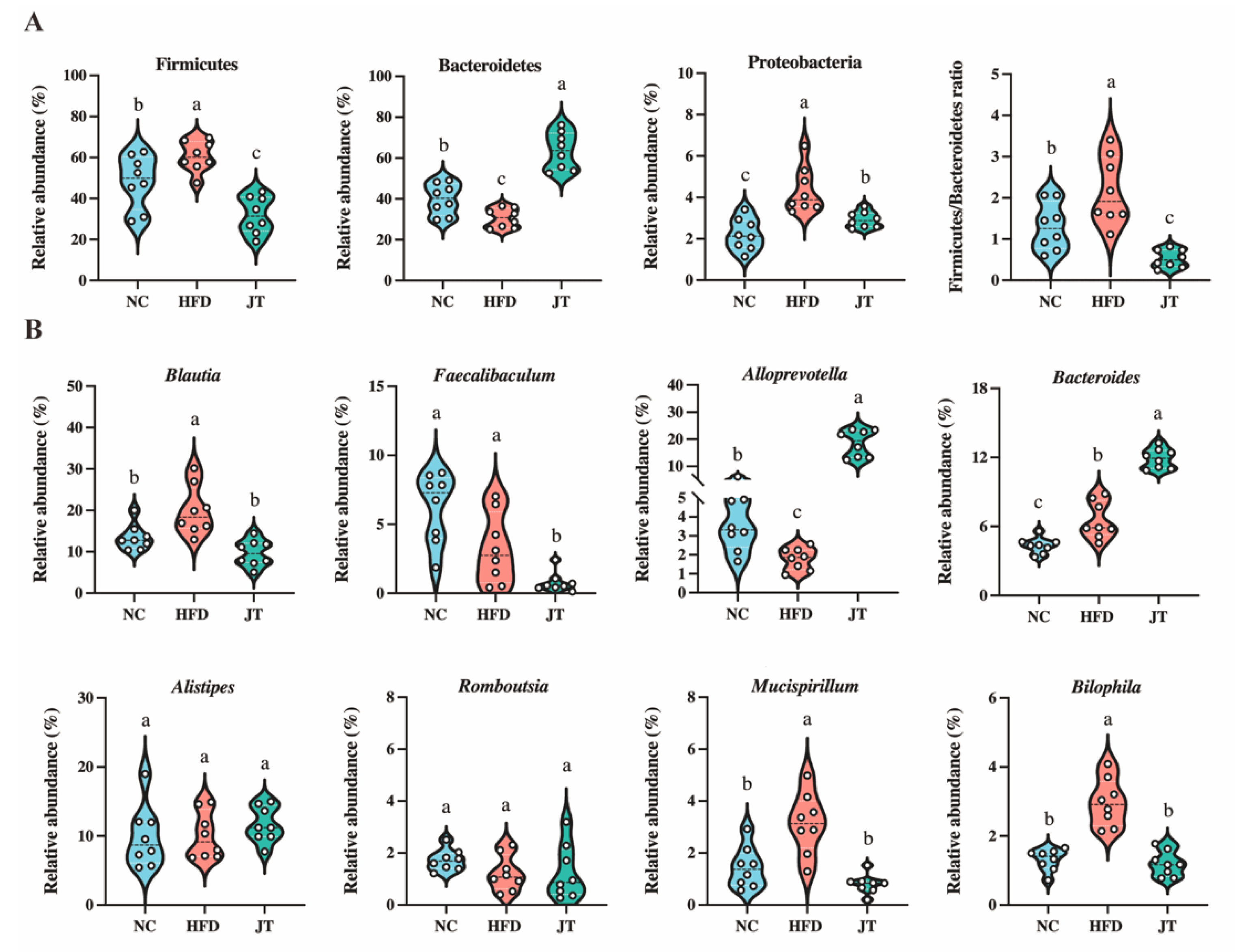
3.5. CB-JT Modulated HFD-Induced Gut Microbiota Disorder
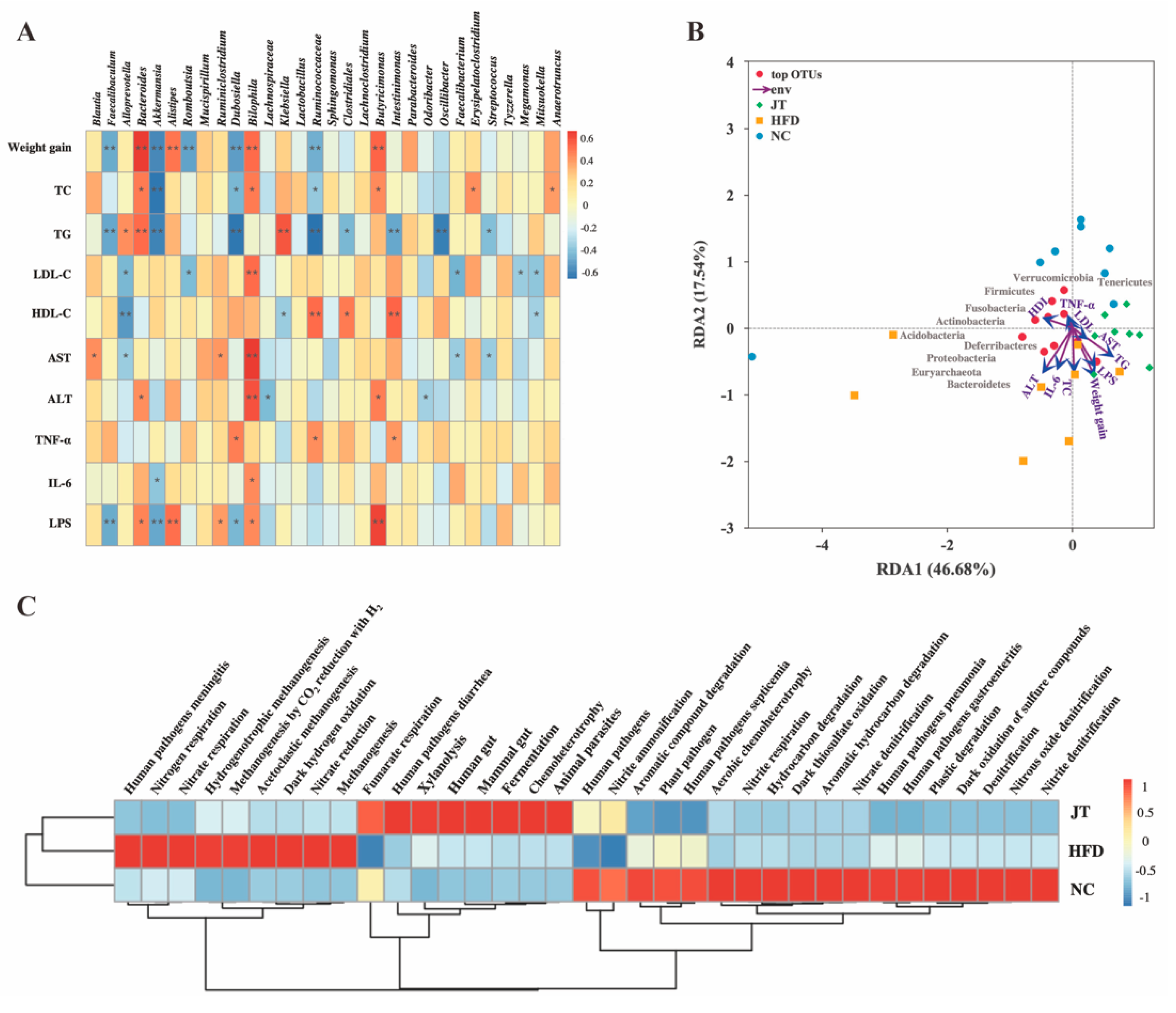
3.6. Correlation Analysis and Predictive Function Profiling of Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, P.; Li, D.; Hu, X.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2019, 59, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria Dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Sainsbury, E.; Magnusson, R.; Thow, A.-M.; Colagiuri, S. Explaining resistance to regulatory interventions to prevent obesity and improve nutrition: A case-study of a sugar-sweetened beverages tax in Australia. Food Policy 2020, 93, 101904. [Google Scholar] [CrossRef]
- Muller, M.; De Beer, D.; Truzzi, C.; Annibaldi, A.; Carloni, P.; Girolametti, F.; Damiani, E.; Joubert, E. Cold brewing of rooibos tea affects its sensory profile and physicochemical properties compared to regular hot, and boiled brewing. LWT 2020, 132, 109919. [Google Scholar] [CrossRef]
- Verbeke, W. Functional foods: Consumer willingness to compromise on taste for health? Food Qual. Prefer. 2006, 17, 126–131. [Google Scholar] [CrossRef]
- Lin, S.-D.; Yang, J.-H.; Hsieh, Y.-J.; Liu, E.-H.; Mau, J.-L. Effect of different brewing methods on quality of green tea. J. Food Process. Preserv. 2014, 38, 1234–1243. [Google Scholar] [CrossRef]
- Damiani, E.; Bacchetti, T.; Padella, L.; Tiano, L.; Carloni, P. Antioxidant activity of different white teas: Comparison of hot and cold tea infusions. J. Food Compos. Anal. 2014, 33, 59–66. [Google Scholar] [CrossRef]
- Jiang, J. The hypoglycemic effect of green tea brewed in cold water is more effective. Mol. Nutr. Food Res. 2019, 63, 1970042. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, A.; Kakuda, T.; Kubota, K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Ou, X.; Zhang, Y.; Li, S.; Xiong, Y.; Li, Q.; Huang, J.; Liu, Z. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sheng, J.; He, X.; Sun, J.; Wei, Z.; Liu, G.; Li, C.; Lin, B.; Li, L. Novel antioxidant and hypoglycemic water-soluble polysaccharides from jasmine tea. Foods 2021, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Inoue, N.; Ito, Y.; Kubota, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Sedative effects of the jasmine tea odor and (R)-(−)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur. J. Appl. Physiol. 2005, 95, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, R.; Tan, Y.; Wu, X.; Wen, Q.; Liu, Z.; Ouyang, S.-H.; Yin, Z.; Yang, H. Preventive consumption of green tea modifies the gut microbiota and provides persistent protection from high-fat diet-induced obesity. J. Funct. Foods 2020, 64, 103621. [Google Scholar] [CrossRef]
- Yoo, A.; Kim, M.J.; Ahn, J.; Jung, C.H.; Seo, H.D.; Ly, S.Y.; Ha, T.Y. Fuzhuan brick tea extract prevents diet-induced obesity via stimulation of fat browning in mice. Food Chem. 2022, 377, 132006. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.-T.; Gosslau, A. Multifunctional health-promoting effects of oolong tea and its products. Food Sci. Hum. Wellness 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Wang, C.; Lv, S.D.; Wang, J.X.; Qiu, X.L.; Wu, Y.S.; Meng, Q.X. Processing technologies affect the aroma but not the taste of teas: A study of Yunnan Biluochun, Jiangsu Biluochun, and other regular green teas. Int. J. Food Prop. 2017, 20, 1404–1421. [Google Scholar] [CrossRef]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef]
- Mondal, R.; Bhat, A. Temporal and environmental drivers of fish-community structure in tropical streams from two contrasting regions in India. PLoS ONE 2020, 15, e0227354. [Google Scholar] [CrossRef]
- Anari, R.; Amani, R.; Veissi, M. Sugar-sweetened beverages consumption is associated with abdominal obesity risk in diabetic patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S675–S678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, M.; Ho, C.-T.; Guo, X.; Wu, Z.; Weng, P.; Yan, M.; Cao, J. Metagenomics analysis of gut microbiota modulatory effect of green tea polyphenols by high fat diet-induced obesity mice model. J. Funct. Foods 2018, 46, 268–277. [Google Scholar] [CrossRef]
- Vu, D.C.; Alvarez, S. Phenolic, carotenoid and saccharide compositions of vietnamese camellia sinensis teas and herbal teas. Molecules 2021, 26, 6496. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Fong, W.P.; Cheung, Y.L.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J. Nutr. 1999, 129, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Ye, J.; Gaikwad, N.W. Comparative effect of black, green, oolong, and white tea intake on weight gain and bile acid metabolism. Nutrition 2019, 65, 208–215. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Chen, P.B.; Black, A.S.; Sobel, A.L.; Zhao, Y.; Mukherjee, P.; Molparia, B.; Moore, N.E.; Aleman Muench, G.R.; Wu, J.; Chen, W.; et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat. Biotechnol. 2020, 38, 1288–1297. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Wan, B.; Wang, Q.; Wan, X. Green tea polyphenols alter lipid metabolism in the livers of broiler chickens through increased phosphorylation of AMP-activated protein kinase. PLoS ONE 2017, 12, e0187061. [Google Scholar] [CrossRef]
- Suk, S.; Kwon, G.T.; Lee, E.; Jang, W.J.; Yang, H.; Kim, J.H.; Thimmegowda, N.R.; Chung, M.-Y.; Kwon, J.Y.; Yang, S.; et al. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. Mol. Nutr. Food Res. 2017, 61, 1700139. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, F.; Yuan, W.; Wei, Y.; Wei, M.; Zhou, Y.; Yang, Y.; Chang, Y.; Wu, X. Hepatic cholesterol accumulation ascribed to the activation of ileum Fxr-Fgf15 pathway inhibiting hepatic Cyp7a1 in high-fat diet-induced obesity rats. Life Sci. 2019, 232, 116638. [Google Scholar] [CrossRef] [PubMed]
- Attané, C.; Peyot, M.-L.; Lussier, R.; Poursharifi, P.; Zhao, S.; Zhang, D.; Morin, J.; Pineda, M.; Wang, S.; Dumortier, O.; et al. A beta cell ATGL-lipolysis/adipose tissue axis controls energy homeostasis and body weight via insulin secretion in mice. Diabetologia 2016, 59, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A.; Calabriso, N.; Wabitsch, M.; Storelli, C.; Wright, M.; De Caterina, R. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol. Res. 2016, 107, 125–136. [Google Scholar] [CrossRef]
- Santos, J.M.; Tewari, S.; Benite-Ribeiro, S.A. The effect of exercise on epigenetic modifications of PGC1: The impact on type 2 Diabetes. Med. Hypotheses 2014, 82, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional modulation of leptin expression and leptin action in obesity and obesity-associated complications. J. Nutr. Biochem. 2021, 89, 108561. [Google Scholar] [CrossRef]
- Matsusue, K.; Aibara, D.; Hayafuchi, R.; Matsuo, K.; Takiguchi, S.; Gonzalez, F.J.; Yamano, S. Hepatic PPARγ and LXRα independently regulate lipid accumulation in the livers of genetically obese mice. FEBS Lett. 2014, 588, 2277–2281. [Google Scholar] [CrossRef]
- Lee, G.; Kim, Y.Y.; Jang, H.; Han, J.S.; Nahmgoong, H.; Park, Y.J.; Han, S.M.; Cho, C.; Lim, S.; Noh, J.-R.; et al. SREBP1c-PARP1 axis tunes anti-senescence activity of adipocytes and ameliorates metabolic imbalance in obesity. Cell Metab. 2022, 34, 702.e705–718.e705. [Google Scholar] [CrossRef]
- Li, S.-Z.; Zeng, S.-L.; Liu, E.H. Anti-obesity natural products and gut microbiota. Food Res. Int. 2022, 151, 110819. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Vasques-Monteiro, I.M.L.; Silva-Veiga, F.M.; Miranda, C.S.; de Andrade Gonçalves, É.C.B.; Daleprane, J.B.; Souza-Mello, V. A rise in proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 2021, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Y.; Zhang, Y.; Yang, Y.; Su, W.; Yang, Y.; Sun, L.; Zhang, F.; Yu, J.; Wang, Y.; et al. Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR’s cholesterol-decreasing efficacy in patients. J. Adv. Res. 2022, 37, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Hu, R.; Guo, W.; Huang, Z.; Li, L.; Liu, B.; Lv, X. Extracts of ganoderma lucidum attenuate lipid metabolism and modulate gut microbiota in high-fat diet fed rats. J. Funct. Foods 2018, 46, 403–412. [Google Scholar] [CrossRef]
- Olson, C.A.; Iñiguez, A.J.; Yang, G.E.; Fang, P.; Pronovost, G.N.; Jameson, K.G.; Rendon, T.K.; Paramo, J.; Barlow, J.T.; Ismagilov, R.F.; et al. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host Microbe 2021, 29, 1378.e1376–1392.e1376. [Google Scholar] [CrossRef]
- Chen, S.; Xu, M.; Zhou, M.; He, Y.; Li, Y.; Lang, H.; Wei, X.; Yan, L.; Xu, H. Hibiscus manihot L. improves obesity in mice induced by a high-fat diet. J. Funct. Foods 2022, 89, 104953. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.N.; Tian, F.; Shen, H.Y.; Zhou, M.M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. eBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Wang, J.; Zhang, X.; Kou, R.; Chen, M.; Zhang, B.; Liu, J.; Peng, B.; Zhang, Y.; Wang, S. Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis. Nutrients 2022, 14, 5359. https://doi.org/10.3390/nu14245359
Li A, Wang J, Zhang X, Kou R, Chen M, Zhang B, Liu J, Peng B, Zhang Y, Wang S. Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis. Nutrients. 2022; 14(24):5359. https://doi.org/10.3390/nu14245359
Chicago/Turabian StyleLi, Ang, Jin Wang, Xuejiao Zhang, Ruixin Kou, Mengshan Chen, Bowei Zhang, Jingmin Liu, Bo Peng, Yan Zhang, and Shuo Wang. 2022. "Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis" Nutrients 14, no. 24: 5359. https://doi.org/10.3390/nu14245359
APA StyleLi, A., Wang, J., Zhang, X., Kou, R., Chen, M., Zhang, B., Liu, J., Peng, B., Zhang, Y., & Wang, S. (2022). Cold-Brewed Jasmine Tea Attenuates High-Fat Diet-Induced Obesity and Gut Microbial Dysbiosis. Nutrients, 14(24), 5359. https://doi.org/10.3390/nu14245359