Cognitive Decline Related to Diet Pattern and Nutritional Adequacy in Alzheimer’s Disease Using Surface-Based Morphometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Demographic Data
2.4. Dietary Assessment and Dementia Functional Survey
2.5. Cognitive Outcomes (Baseline and One Year Prior to Enrolment)
2.6. MRI Acquisition, Salient Regions of Interest and Composite Cortical Thickness
2.7. Statistical Analysis
3. Result
3.1. Factor Loading of 22 Food Frequencies
3.2. Gender Differences in BMI and DPs
3.3. Underweight-BMI Had Lower Cognitive Performance, Smaller Composite Cortical Thickness and Higher Lipid Profiles
3.4. Factors Related to RCD
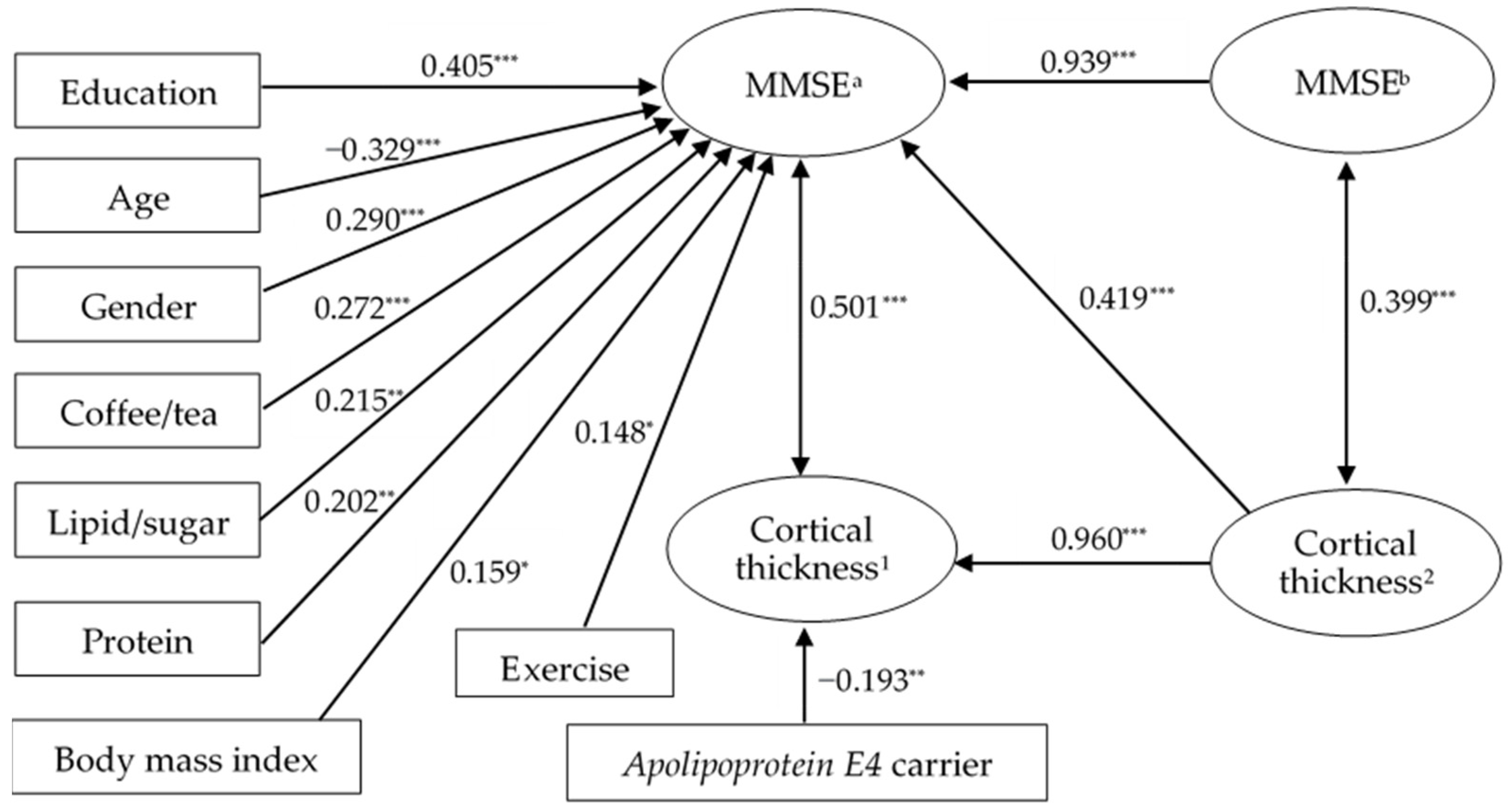
3.5. Modifiable and Non-Modifiable Factors Associated with Cognitive Performance
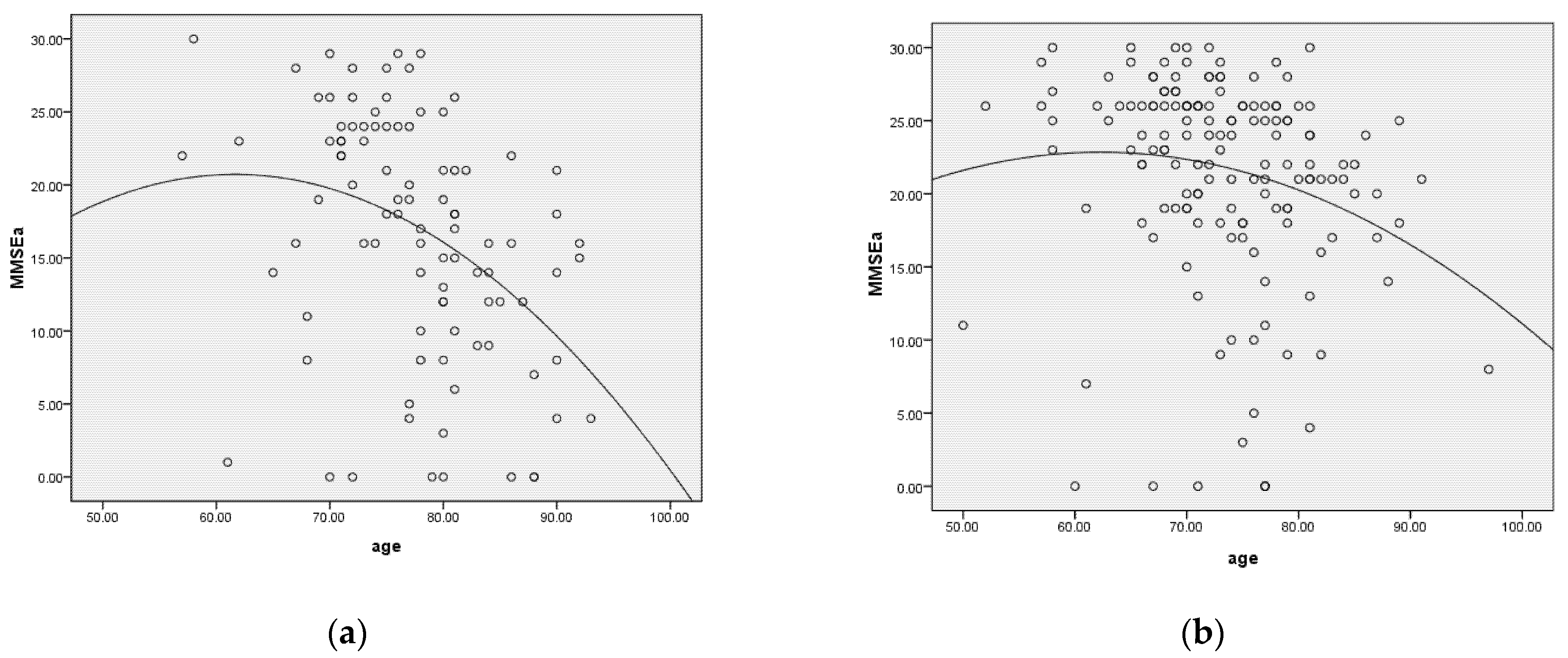
3.6. Spatiotemporal Cortical Degenerative Patterns
4. Discussion
4.1. Major Findings
4.2. DP-Related Factors and Cortical Atrophy
4.3. DP and Cognitive Functions in the AD Patients
4.4. Gender Effects in Cognitive Function, DP and Lifestyle in the AD Patients
4.5. Factors Associated with Rapid Decline
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. 3.6 Preprocessing of Baseline and Longitudinal Imaging Data
References
- Ramanan, V.K.; Saykin, A.J. Pathways to neurodegeneration: Mechanistic insights from GWAS in Alzheimer’s disease, Parkinson’s disease, and related disorders. Am. J. Neurodegener. Dis. 2013, 2, 145–175. [Google Scholar] [PubMed]
- Wiȩckowska-Gacek, A.; Mietelska-Porowska, A.; Chutorański, D.; Wydrych, M.; Długosz, J.; Wojda, U. Western diet induces impairment of liver-brain axis accelerating neuroinflammation and amyloid pathology in Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 654509. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Marko, D.M.; Fenech, R.K.; Yang, A.J.T.; MacPherson, R.E.K. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Aubertin-Leheudre, M. Nutrition to prevent or treat cognitive impairment in older adults: A GRADE recommendation. J. Prev. Alzheimer’s Dis. 2021, 8, 110–116. [Google Scholar] [CrossRef]
- McGurran, H.; Glenn, J.; Madero, E.; Bott, N. Risk reduction and prevention of Alzheimer’s disease: Biological mechanisms of diet. Curr. Alzheimer. Res. 2020, 17, 407–427. [Google Scholar] [CrossRef]
- Samuelsson, J.; Kern, S.; Zetterberg, H.; Blennow, K.; Rothenberg, E.; Wallengren, O.; Skoog, I.; Zettergren, A. A Western-style dietary pattern is associated with cerebrospinal fluid biomarker levels for preclinical Alzheimer’s disease-A population-based cross-sectional study among 70-year-olds. Alzheimer’s Dement. 2021, 7, e12183. [Google Scholar] [CrossRef]
- Van Asbroeck, S.; van Boxtel, M.P.J.; Steyaert, J.; Köhler, S.; Heger, I.; de Vugt, M.; Verhey, F.; Deckers, K. Increasing knowledge on dementia risk reduction in the general population: Results of a public awareness campaign. Prev. Med. 2021, 147, 106522. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Zhang, H.; Greenwood, D.C.; Risch, H.A.; Bunce, D.; Hardie, L.J.; Cade, J.E. Meat consumption and risk of incident dementia: Cohort study of 493,888 UK Biobank participants. Am. J. Clin. Nutr. 2021, 114, 175–184. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, W.; Ni, C.; Hu, S.; Shao, P.; Li, C.; Hua, Y.; Lang, H.; Wan, Y. Dietary patterns and risk of mild cognitive impairment among Chinese elderly: A cross-sectional study. PLoS ONE 2020, 15, e0235974. [Google Scholar] [CrossRef]
- Wesselman, L.M.P.; van Lent, D.M.; Schröder, A.; van de Rest, O.; Peters, O.; Menne, F.; Fuentes, M.; Priller, J.; Spruth, E.J.; Altenstein, S.; et al. Dietary patterns are related to cognitive functioning in elderly enriched with individuals at increased risk for Alzheimer’s disease. Eur. J. Nutr. 2021, 60, 849–860. [Google Scholar] [CrossRef] [PubMed]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Dearborn-Tomazos, J.L.; Wu, A.; Steffen, L.M.; Anderson, C.A.M.; Hu, E.A.; Knopman, D.; Mosley, T.H.; Gottesman, R.F. Association of Dietary Patterns in Midlife and Cognitive Function in Later Life in US Adults Without Dementia. JAMA Netw. Open 2019, 2, e1916641. [Google Scholar] [CrossRef]
- Mumme, K.D.; von Hurst, P.R.; Conlon, C.A.; Jones, B.; Haskell-Ramsay, C.F.; Stonehouse, W.; Heath, A.M.; Coad, J.; Beck, K.L. Study protocol: Associations between dietary patterns, cognitive function and metabolic syndrome in older adults—A cross-sectional study. BMC Public Health 2019, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, K.W.; Kim, M.-H.; Kim, H.J.; An, Y.S.; Chung, H.-K. Identifying Dietary Patterns Associated with Mild Cognitive Impairment in Older Korean Adults Using Reduced Rank Regression. Int. J. Environ. Res. Public Health 2018, 15, 100. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lo, Y.L.; Wu, S.Y.; Wang, P.N.; Pan, W.H. Dietary Patterns and Foods Associated With Cognitive Function in Taiwanese Older Adults: The Cross-sectional and Longitudinal Studies. J. Am. Med. Dir. Assoc. 2019, 20, 544–550.e4. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Dietary Patterns and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary Patterns and Risk of Dementia: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9874159. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimer’s Dis. JAD 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Prinelli, F.; Fratiglioni, L.; Kalpouzos, G.; Musicco, M.; Adorni, F.; Johansson, I.; Marseglia, A.; Xu, W. Specific nutrient patterns are associated with higher structural brain integrity in dementia-free older adults. NeuroImage 2019, 199, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Jung, C.C.; Chen, J.H.; Chiou, J.M.; Chen, T.F.; Chen, Y.F.; Tang, S.C.; Yeh, S.J.; Lee, M.S. Association of Dietary Patterns With Global and Domain-Specific Cognitive Decline in Chinese Elderly. J. Am. Geriatr. Soc. 2017, 65, 1159–1167. [Google Scholar] [CrossRef]
- Samadi, M.; Moradi, S.; Moradinazar, M.; Mostafai, R.; Pasdar, Y. Dietary pattern in relation to the risk of Alzheimer’s disease: A systematic review. Neurol. Sci. 2019, 40, 2031–2043. [Google Scholar] [CrossRef]
- Fieldhouse, J.L.P.; Doorduijn, A.S.; de Leeuw, F.A.; Verhaar, B.J.H.; Koene, T.; Wesselman, L.M.P.; Schueren, M.V.; Visser, M.; Rest, O.V.; Scheltens, P.; et al. A Suboptimal Diet is Associated with Poorer Cognition: The NUDAD Project. Nutrients 2020, 12, 703. [Google Scholar] [CrossRef]
- Cai, K.; Xu, H.; Guan, H.; Zhu, W.; Jiang, J.; Cui, Y.; Zhang, J.; Liu, T.; Wen, W. Identification of Early-Stage Alzheimer’s Disease Using Sulcal Morphology and Other Common Neuroimaging Indices. PLoS ONE 2017, 12, e0170875. [Google Scholar] [CrossRef]
- Guan, H.; Liu, T.; Jiang, J.; Tao, D.; Zhang, J.; Niu, H.; Zhu, W.; Wang, Y.; Cheng, J.; Kochan, N.A.; et al. Classifying MCI Subtypes in Community-Dwelling Elderly Using Cross-Sectional and Longitudinal MRI-Based Biomarkers. Front. Aging Neurosci. 2017, 9, 309. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, F.; Chinnici, G.; Bramerio, M.; Mai, R.; Sartori, I.; Cossu, M.; Lo Russo, G.; Castana, L.; Colombo, N.; Caborni, C.; et al. Validation of FreeSurfer-estimated brain cortical thickness: Comparison with histologic measurements. Neuroinformatics 2014, 12, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.; Penny, W.D.; Ridgway, G.R.; Ourselin, S.; Friston, K.J.; Alzheimer’s Disease Neuroimaging, I. Estimating anatomical trajectories with Bayesian mixed-effects modeling. Neuroimage 2015, 121, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, M.R.; Bernal-Rusiel, J.L.; Reuter, M.; Greve, D.N.; Fischl, B.; Alzheimer’s Disease Neuroimaging Initiative. Event time analysis of longitudinal neuroimage data. Neuroimage 2014, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Rusiel, J.L.; Reuter, M.; Greve, D.N.; Fischl, B.; Sabuncu, M.R.; Alzheimer’s Disease Neuroimaging Initiative. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage 2013, 81, 358–370. [Google Scholar] [CrossRef]
- Chen, G.; Saad, Z.S.; Britton, J.C.; Pine, D.S.; Cox, R.W. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 2013, 73, 176–190. [Google Scholar] [CrossRef]
- Bernal-Rusiel, J.L.; Greve, D.N.; Reuter, M.; Fischl, B.; Sabuncu, M.R.; Alzheimer’s Disease Neuroimaging Initiative. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage 2013, 66, 249–260. [Google Scholar] [CrossRef]
- Doorduijn, A.S.; Visser, M.; van de Rest, O.; Kester, M.I.; de Leeuw, F.A.; Boesveldt, S.; Fieldhouse, J.L.P.; van den Heuvel, E.; Teunissen, C.E.; Scheltens, P.; et al. Associations of AD Biomarkers and Cognitive Performance with Nutritional Status: The NUDAD Project. Nutrients 2019, 11, 1161. [Google Scholar] [CrossRef]
- Doorduijn, A.S.; de van der Schueren, M.A.E.; van de Rest, O.; de Leeuw, F.A.; Hendriksen, H.M.A.; Teunissen, C.E.; Scheltens, P.; van der Flier, W.M.; Visser, M. Nutritional Status Is Associated With Clinical Progression in Alzheimer’s Disease: The NUDAD Project. J. Am. Med. Dir. Assoc. 2020, 1–7. [Google Scholar] [CrossRef]
- Moran, C.; Beare, R.; Wang, W.; Callisaya, M.; Srikanth, V. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 2019, 92, e823–e830. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.K.; Rajagopalan, P.; Joshi, S.H.; Toga, A.W.; Thompson, P.M. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s Disease Neuroimaging Initiative. Neurobiol. Aging 2015, 36 (Suppl. S1), S203–S210. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Arbogast, S.; Dhana, K.; Aggarwal, N.T.; Zhang, S.; Agarwal, P.; Liu, X.; Laranjo, N.; Carey, V.; Sacks, F.; Barnes, L.L.; et al. Vitamin D Intake and Brain Cortical Thickness in Community-Dwelling Overweight Older Adults: A Cross-Sectional Study. J. Nutr. 2021, 151, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.; Liu, Y.; Ngandu, T.; Antikainen, R.; Hulkkonen, J.; Koikkalainen, J.; Kemppainen, N.; Lötjönen, J.; Levälahti, E.; Parkkola, R.; et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimer’s Res. Ther. 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Chang, Y.T.; Huang, C.W.; Huang, K.L.; Hsu, J.L.; Hsu, S.W.; Tsai, S.J.; Chang, W.N.; Lee, C.C.; Huang, S.H.; et al. Structural Covariance Network as an Endophenotype in Alzheimer’s Disease-Susceptible Single-Nucleotide Polymorphisms and the Correlations With Cognitive Outcomes. Front. Aging Neurosci. 2021, 13, 721217. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry J. Ment. Sci. 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Barbe, C.; Morrone, I.; Novella, J.L.; Dramé, M.; Wolak-Thierry, A.; Aquino, J.-P.; Ankri, J.; Jolly, D.; Mahmoudi, R. Predictive Factors of Rapid Cognitive Decline in Patients with Alzheimer Disease. Dement. Geriatr. Cogn. Dis. Extra 2016, 6, 549–558. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Lee, J.J.; Chen, H.H.; Wu, M.K.; Huang, C.W.; Chang, Y.T.; Lien, C.Y.; Wang, J.J.; Chang, H.I.; Chang, C.C. Adequacy of nutrition and body weight in patients with early stage dementia: The cognition and aging study. Clin. Nutr. 2019, 38, 2187–2194. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Dennison Himmelfarb, C.R.; Coke, L. New 2018 Cholesterol Guideline: Enhanced Risk Estimation and Therapeutic Options Drive Shared Decision Making. J. Cardiovasc. Nurs. 2019, 34, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Hsu, W.C.; Chang, C.C.; Lin, K.J.; Hsiao, I.T.; Fan, Y.C.; Bai, C.H. Everyday cognition scales are related to cognitive function in the early stage of probable Alzheimer’s disease and FDG-PET findings. Sci. Rep. 2017, 7, 1719. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48, S10–S16. [Google Scholar] [CrossRef]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Cahn-Weiner, D.; Jagust, W.; Baynes, K.; Decarli, C. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology 2008, 22, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sciences. J. Can. Des Sci. Neurol. 1997, 24, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.N.; Fischl, B. False positive rates in surface-based anatomical analysis. NeuroImage 2018, 171, 6–14. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Shaw, M.E.; Sachdev, P.S.; Abhayaratna, W.; Anstey, K.J.; Cherbuin, N. Body mass index is associated with cortical thinning with different patterns in mid- and late-life. Int. J. Obes. 2018, 42, 455–461. [Google Scholar] [CrossRef]
- Thirunavu, V.; McCullough, A.; Su, Y.; Flores, S.; Dincer, A.; Morris, J.C.; Cruchaga, C.; Benzinger, T.L.S.; Gordon, B.A. Higher Body Mass Index Is Associated with Lower Cortical Amyloid-beta Burden in Cognitively Normal Individuals in Late-Life. J. Alzheimer’s Dis. JAD 2019, 69, 817–827. [Google Scholar] [CrossRef]
- Lee, S.H.; Byun, M.S.; Lee, J.H.; Yi, D.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; Shin, S.A.; Sohn, C.H.; Lee, D.Y.; et al. Sex-Specific Association of Lifetime Body Mass Index with Alzheimer’s Disease Neuroimaging Biomarkers. J. Alzheimer’s Dis. JAD 2020, 75, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, A.; Choi, B.Y.; Nam, J.H.; Kim, M.K.; Oh, D.H.; Yang, Y.J. Dietary Patterns Derived by Cluster Analysis are Associated with Cognitive Function among Korean Older Adults. Nutrients 2015, 7, 4154–4169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, A.; Choi, B.; Nam, J.; Kim, M.; Oh, D.; Kim, K.; Yang, Y. Dietary patterns and cognitive function in Korean older adults. Eur. J. Nutr. 2015, 54, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Inagaki, H.; Gondo, Y.; Kamide, K.; Ikebe, K.; Masui, Y.; Arai, Y.; Ishizaki, T.; Sasaki, S.; Nakagawa, T.; et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: A cross-sectional analysis of the SONIC study. Nutr. J. 2017, 16, 56. [Google Scholar] [CrossRef]
- Pang, S.J.; Jia, S.S.; Man, Q.Q.; Song, S.; Li, Y.Q.; Song, P.K.; Zhao, W.H.; Zhang, J. Dietary Cholesterol in the Elderly Chinese Population: An Analysis of CNHS 2010–2012. Nutrients 2017, 9, 934. [Google Scholar] [CrossRef]
- Hsieh, S.W.; Chen, C.H.; Huang, L.C.; Chang, Y.H.; Yang, Y.H. Gender differences in presentation of behavioral and psychological symptoms in Alzheimer’s disease in Taiwan. Aging Ment. Health 2020, 24, 1342–1347. [Google Scholar] [CrossRef]
- Liu, T.; Luo, H.; Tang, J.Y.M.; Wong, G.H.Y. Does lifestyle matter? Individual lifestyle factors and their additive effects associated with cognitive function in older men and women. Aging Ment. Health 2020, 24, 405–412. [Google Scholar] [CrossRef]
- Spaccavento, S.; Del Prete, M.; Craca, A.; Fiore, P. Influence of nutritional status on cognitive, functional and neuropsychiatric deficits in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2009, 48, 356–360. [Google Scholar] [CrossRef]
- Sanders, C.L.; Wengreen, H.J.; Schwartz, S.; Behrens, S.J.; Corcoran, C.; Lyketsos, C.G.; Tschanz, J.T. Nutritional Status is Associated With Severe Dementia and Mortality: The Cache County Dementia Progression Study. Alzheimer Dis. Assoc. Disord. 2018, 32, 298–304. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Bio Med. Atenei Parm. 2018, 89, 276–290. [Google Scholar] [CrossRef]
- Li, C.P. Gender differences in nutrition knowledge, attitude, and practice among elderly people. Int. J. Manag. Econ. Soc. Sci. 2017, 6, 199–211. [Google Scholar]
- Food and Drug Administration, Ministry of Health and Welfare. Taiwan Food Nutrition Ingredient Database. 2017. Available online: https://consumer.fda.gov.tw/Food/TFND.aspx?nodeID=178 (accessed on 30 June 2022).
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed]

| Reduced Rank Regression | All (n = 248) | ||
|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | |
| Protein Group | Coffee/Tea Group | Lipid/Sugar Group | |
| Lean meat | 0.717 | ||
| Skimmed milk | 0.542 | ||
| Beans | 0.227 | ||
| Low-fat milk | 0.202 | ||
| Soy products | 0.177 | ||
| Oyster | 0.146 | ||
| Egg | −0.057 | ||
| Fish | −0.113 | ||
| Full-fat milk | −0.137 | ||
| Octopus | −0.145 | ||
| Poultry | −0.190 | ||
| Coffee/tea | 0.990 | ||
| Vegetable | 0.132 | ||
| Fruit | −0.040 | ||
| Mushroom | −0.218 | ||
| Entrails | 0.784 | ||
| Sugar | 0.537 | ||
| Fried food | 0.071 | ||
| Fatty meat | −0.098 | ||
| Sweet drink | −0.279 | ||
| Processed food | −0.290 | ||
| Dessert | −0.318 | ||
| Explained variation | |||
| Food Groups | 10.084 | 24.789 | 12.744 |
| MMSE score | 4.096 | 7.420 | 4.634 |
| All (n = 248) | Female (n = 139) | Male (n = 109) | p-Value | |
|---|---|---|---|---|
| Age (year) a | 74.8 ± 7.90 | 74.8 ± 7.68 | 74.8 ± 8.22 | 0.711 |
| Education (year) | 7.74 ± 4.70 | 6.40 ± 4.59 | 9.45 ± 4.28 | <0.001 *** |
| Apolipoprotein E4 carriers (n = 236) | 68 (28.8%) | 38 (29.2%) | 30 (28.3%) | 0.886 |
| Body mass index, BMI (kg/m2) | 23.99 ± 3.80 | 23.56 ± 4.06 | 24.53 ± 3.37 | 0.024 * |
| Underweight (BMI < 18.5) | 17.36 ± 0.98 | 17.24 ± 1.00 | 18.05 ± 0.50 | 0.352 |
| Normal (BMI 18.5~22.9) | 21.03 ± 1.20 | 21.04 ± 1.27 | 21.00 ± 1.09 | 0.656 |
| Overweight (BMI 23~24.9) | 23.89 ± 0.61 | 23.71 ± 0.56 | 24.03 ± 0.62 | 0.056 |
| Obese (BMI ≥ 25) | 27.81 ± 2.69 | 27.88 ± 2.98 | 27.73 ± 2.36 | 0.838 |
| Cases | ||||
| Underweight (BMI < 18.5) | 14 (5.6) | 12 (8.6) | 2 (1.8) | 0.011 * |
| Normal (BMI 18.5~22.9) | 87 (35.1) | 55 (39.6) | 32 (29.4) | |
| Overweight (BMI 23~24.9) | 54 (21.8) | 23 (16.5) | 31 (28.4) | |
| Obese (BMI ≥ 25) | 93 (37.5) | 49 (35.3) | 44 (40.4) | |
| MMSEa (n = 248) | 19.51 ± 7.86 | 17.5 ± 8.43 | 22.08 ± 6.22 | <0.001 *** |
| MMSEb (n = 226) | 20.03 ± 7.45 | 18.29 ± 7.94 | 22.25 ± 6.13 | <0.001 *** |
| Cortical thickness1 | 0.00 ± 1.00 | −0.025 ± 1.070 | 0.031 ± 0.912 | 0.432 |
| Cortical thickness2 | 0.00 ± 1.00 | 0.016 ± 1.004 | −0.012 ± 0.985 | 0.981 |
| Everyday cognition scale (0~228) | 118.6 ± 52.63 | 125.59 ± 53.99 | 109.68 ± 49.68 | 0.022 * |
| Neuropsychiatric inventory (0~144) | 3.57 ± 5.52 | 3.73 ± 5.93 | 3.37 ± 4.96 | 0.684 |
| Frontal behavior inventory (0~72) | 8.83 ± 12.04 | 9.94 ± 13.09 | 7.42 ± 10.44 | 0.166 |
| Blood data | ||||
| Glycated hemoglobin | 6.13 ± 0.87 | 6.18 ± 0.85 | 6.05 ± 0.90 | 0.111 |
| High density lipoprotein (mg/dL) | 52.45 ± 15.02 | 57.18 ± 15.47 | 45.63 ± 11.35 | <0.001 *** |
| Low density lipoprotein (mg/dL) | 104.42 ± 34.14 | 107 ± 35.34 | 100.01 ± 32.18 | 0.090 |
| Cholesterol (mg/dL) | 179.37 ± 39.84 | 185.37 ± 42.64 | 170.8 ± 33.90 | 0.005 ** |
| Triglyceride (mg/dL) | 112.28 ± 54.16 | 106.65 ± 45.64 | 120.41 ± 63.95 | 0.330 |
| B12 (pg/mL) | 867 ± 637.82 | 951.27 ± 715.46 | 749.44 ± 490.41 | 0.029 * |
| Folate (ng/mL) | 13.13 ± 8.28 | 13.92 ± 8.30 | 12.03 ± 8.17 | 0.041 * |
| Factor scores of 3 dietary pattern c | ||||
| Protein group | 0.00 ± 1.00 | −0.005 ± 1.11 | 0.006 ± 0.85 | 0.414 |
| Coffee/Tea group | 0.00 ± 1.00 | 0.123 ± 0.99 | 0.157 ± 1.00 | 0.042 * |
| Lipid/Sugar group | 0.00 ± 1.00 | −0.034 ± 0.86 | 0.043 ± 1.16 | 0.884 |
| Clinical Dementia Rating(CDR) b | 0.066 | |||
| 0.5 | 163 (65.7) | 84 (60.4) | 79 (73.4) | |
| 1 | 56 (22.6) | 32 (23) | 24 (22) | |
| 2 | 26 (10.5) | 20 (14.4) | 6 (5.5) | |
| ≥3 | 3 (1.2) | 3 (2.1) | 0 (0) | |
| Rapid cognitive decline d | 0.757 | |||
| No | 171 (75.3) | 95 (74.2) | 76 (76.8) | |
| Yes | 56 (24.7) | 33 (25.8) | 23 (23.2) | |
| Marital status | <0.001 *** | |||
| Married | 192 (77.4) | 88 (63.3) | 104 (95.4) | |
| Widowed | 51 (20.6) | 48 (34.5) | 3 (2.8) | |
| Single/divorced | 5 (2) | 3 (2.1) | 2 (1.8) | |
| Self-care ability | 0.055 | |||
| Independent | 119 (48) | 59 (42.4) | 60 (55) | |
| Dependent | 129 (52) | 80 (57.6) | 49 (45) | |
| Major Caregiver | <0.001 *** | |||
| Spouse | 153 (61.7) | 61 (43.9) | 92 (84.4) | |
| Others e | 95 (38.3) | 78 (56.1) | 17 (15.6) | |
| Comorbidity, cases (%) | 247 | |||
| Hypertension | 101 (40.9) | 55 (39.9) | 46 (42.2) | 0.794 |
| Diabetes Mellitus | 55 (22.3) | 32 (23.2) | 23 (21.1) | 0.759 |
| Hyperlipidemia | 54 (21.9) | 30 (21.7) | 24 (22.0) | 1.00 |
| Underweight a | Normal b | Overweight c | Obese d | p-Value | Post hoc | |
|---|---|---|---|---|---|---|
| Sample size | 14 (5.6) | 87 (35.1) | 54 (21.8) | 93 (37.5) | ||
| Body mass index, BMI | 17.36 ± 0.977 | 21.028 ± 1.20 | 23.89 ± 0.61 | 27.81 ± 2.69 | <0.001 *** | a < b; b < c; c < d |
| Blood data | ||||||
| Glycated hemoglobin | 5.74 ± 0.55 | 6.18 ± 1.14 | 6.02 ± 0.57 | 6.20 ± 0.72 | 0.047 * | |
| High density lipoprotein (mg/dL) | 66.15 ± 22.93 | 55.29 ± 14.50 | 50.79 ± 14.76 | 48.01 ± 11.87 | 0.003 ** | a > bcd; b > d |
| Low density lipoprotein (mg/dL) | 127.92 ± 25.61 | 105.57 ± 31.44 | 100.36 ± 37.04 | 101.33 ± 35.27 | 0.041 * | a > bcd |
| Cholesterol (mg/dL) | 212.77 ± 33.63 | 180.69 ± 36.51 | 174.40 ± 42.02 | 174.99 ± 40.62 | 0.008 ** | a > bcd |
| Triglyceride (mg/dL) | 93.23 ± 56.96 | 98.91 ± 44.34 | 117.44 ± 55.78 | 126.56 ± 58.56 | 0.005 ** | a < d; b < d |
| B12 (pg/mL) | 1003.77 ± 614.37 | 796.07 ± 449.39 | 861.07 ± 670.33 | 919.30 ± 776.53 | 0.560 | |
| Folate (ng/mL) | 17.54 ± 8.43 | 12.56 ± 8.10 | 13.76 ± 9.97 | 12.57 ± 7.20 | 0.018 | |
| Everyday cognition scale (0~228) | 146.21 ± 53.31 | 130.52 ± 58.15 | 118.17 ± 50.54 | 103.54 ± 43.87 | 0.003 ** | a > d; b > d |
| Neuropsychiatric inventory (0~144) | 6.07 ± 8.83 | 4.51 ± 6.35 | 3.70 ± 5.94 | 2.24 ± 3.03 | 0.11 | |
| Frontal behavior inventory (0~72) | 17.36 ± 24.76 | 10.8 ± 12.83 | 8.80 ± 10.11 | 5.72 ± 8.09 | 0.03 * | a > cd; b > d |
| MMSEa | 15.50 ± 9.01 | 17.57 ± 8.71 | 21.11 ± 7.51 | 21.00 ± 6.42 | 0.005 ** | a < cd; b < cd |
| MMSEb | 15.93 ± 9.56 | 18.62 ± 7.86 | 21.15 ± 7.57 | 21.46 ± 6.03 | 0.022 * | a < cd; b < cd |
| Cortical thickness1 | −0.697 ± 0.806 | −0.175 ± 0.964 | 0.128 ± 1.067 | 0.189 ± 0.957 | 0.005 ** | a < bc; b < d |
| Cortical thickness2 | −0.924 ± 1.024 | −0.062 ± 0.912 | 0.037 ± 1.087 | 0.150 ± 0.944 | 0.121 | |
| Factor score of 3 dietary pattern e | ||||||
| Protein group | −0.626 ± 0.555 | −0.080 ± 1.098 | −0.029 ± 0.759 | 0.186 ± 1.039 | 0.008 ** | a < cd; b < d |
| Coffee/Tea group | −0.185 ± 0.860 | −0.091 ± 1.048 | −0.072 ± 0.895 | 0.155 ± 1.025 | 0.344 | |
| Lipid/Sugar group | −0.157 ± 0.692 | −0.138 ± 0.809 | 0.271 ± 1.327 | −0.004 ± 0.962 | 0.359 | |
| Clinical Dementia Rating(CDR) | ||||||
| <1 | 6 (42.9) | 49 (56.3) | 40 (74.1) | 69 (74.2) | 0.010 * | |
| ≥1 | 8 (57.1) | 38 (43.7) | 14 (25.9) | 24 (25.8) | ||
| Self-care ability | 0.160 | |||||
| Independent | 4 (28.6) | 38 (43.7) | 25 (46.3) | 52 (55.9) | ||
| Dependent | 10 (71.4) | 49 (56.3) | 29 (53.7) | 41 (44.1) | ||
| Major Caregiver | ||||||
| Spouse | 7 (50.0) | 44 (50.6) | 38 (70.4) | 64 (68.8) | 0.028 * | |
| Others f | 7 (50.0) | 43 (49.4) | 16 (29.6) | 29 (31.2) | ||
| RCD | Stable Group | p-Value | |
|---|---|---|---|
| Sample size | 56 (24.7) | 171 (75.3) | |
| Age | 74.39 ± 9.08 | 75.29 ± 7.55 | 0.922 |
| Educational year | 7.59 ± 4.86 | 7.69 ± 4.74 | 0.804 |
| MMSEa | 15.86 ± 8.52 | 20.39 ± 7.41 | <0.001 ** |
| MMSEb | 19.00 ± 8.20 | 20.36 ± 7.18 | 0.339 |
| Factor score of 3 dietary pattern | |||
| Protein group | −0.03 ± 0.93 | −0.03 ± 1.26 | 0.316 |
| Coffee/Tea group | 0.01 ± 1.00 | −0.11 ± 0.96 | 0.342 |
| Lipid/Sugar group | 0.03 ± 1.05 | −0.16 ± 0.74 | 0.133 |
| Blood data | |||
| Glycated hemoglobin | 6.24 ± 1.16 | 6.09 ± 0.81 | 0.474 |
| High density lipoprotein (mg/dL) | 52.20 ± 15.64 | 52.46 ± 14.21 | 0.725 |
| Low density lipoprotein (mg/dL) | 104.98 ± 37.64 | 107.70 ± 34.29 | 0.323 |
| Cholesterol (mg/dL) | 182.71 ± 45.74 | 182.58 ± 39.07 | 0.681 |
| Triglyceride (mg/dL) | 130.05 ± 131.02 | 113.40 ± 55.94 | 0.687 |
| B12 (pg/mL) | 735.52 ± 483.32 | 862.76 ± 609.86 | 0.080 |
| Folate (ng/mL) | 11.48 ± 6.87 | 13.81 ± 8.50 | 0.100 |
| Everyday cognition scale (0~228) | 10.46 ± 13.92 | 8.46 ± 11.69 | 0.197 |
| Neuropsychiatric inventory (0~144) | 3.38 ± 6.01 | 3.49 ± 4.94 | 0.245 |
| Frontal behavior inventory (0~72) | 138.16 ± 58.47 | 114.72 ± 50.18 | 0.010 ** |
| Gender, cases | 0.757 | ||
| Male | 23 (41.1) | 76 (44.4) | |
| Female | 33 (58.9) | 95 (55.6) | |
| Marital status | 0.823 | ||
| Married | 43 (76.8) | 134 (78.4) | |
| Widowed | 12 (21.4) | 34 (19.9) | |
| Single/divorced | 1 (1.8) | 3 (1.8) | |
| Body mass index, BMI | 23.21 ± 3.68 | 23.95 ± 3.57 | 0.117 |
| BMI, cases | 0.039 * | ||
| Underweight (BMI < 18.5) | 4 (7.1) | 10 (5.8) | |
| Normal (BMI 18.5~22.9) | 27 (48.2) | 54 (31.6) | |
| Overweight (BMI 23~24.9) | 8 (14.3) | 44 (25.8) | |
| Obese (BMI ≥ 25) | 17 (30.4) | 63 (36.8) | |
| Self-care ability, cases | 0.001 ** | ||
| Independent | 15 (26.8) | 91 (53.2) | |
| Dependent | 41 (73.2) | 80 (46.5) | |
| Living status, cases | 0.638 | ||
| Spouse | 33 (58.9) | 107 (62.6) | |
| Others a | 23 (41.1) | 64 (37.4) | |
| Comorbidity, cases | |||
| Hypertension | 20 (35.7) | 71 (41.5) | 0.530 |
| Diabetes | 14 (25) | 33 (19.3) | 0.447 |
| Hyperlipidemia | 21 (37.5) | 30 (17.5) | 0.003 ** |
| Unstandardized Coefficients | z | p-Value | 95% Confidence Interval for B | |||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| All patients (n = 248, AIC = 1632.2) | ||||||
| (Constant) | −26.399 | 24.563 | −1.075 | 0.282 | −74.541 | 21.744 |
| Age | 1.337 | 0.660 | 2.027 | 0.043 * | 0.044 | 2.630 |
| Age*Age | −0.011 | 0.005 | −1.96 | 0.049 * | −0.021 | 0.000 |
| Male Gender | 3.016 | 0.871 | 3.463 | <0.001 *** | 1.309 | 4.723 |
| With Exercise habit | 2.553 | 0.829 | 3.078 | 0.002 ** | 0.927 | 4.178 |
| Education | 0.392 | 0.098 | 4.019 | <0.001 *** | 0.201 | 0.583 |
| Protein group | 1.262 | 0.411 | 3.067 | 0.002 ** | 0.456 | 2.068 |
| Coffee/tea group | 0.944 | 0.420 | 2.245 | 0.025 * | 0.120 | 1.768 |
| Lipid/sugar group | 0.977 | 0.411 | 2.375 | 0.018 * | 0.171 | 1.783 |
| Patients not living with a spouse (n = 95, AIC = 647.3) | ||||||
| (Constant) | −25.183 | 50.748 | −0.496 | 0.620 | −124.646 | 74.281 |
| Age | 1.237 | 1.329 | 0.931 | 0.352 | −1.368 | 3.843 |
| Age*Age | −0.010 | 0.009 | −1.1 | 0.271 | −0.027 | 0.008 |
| Male Gender | 2.093 | 1.835 | 1.141 | 0.254 | −1.503 | 5.689 |
| With Exercise habit | 2.058 | 1.442 | 1.427 | 0.154 | −0.768 | 4.885 |
| Education | 0.582 | 0.179 | 3.254 | 0.001 ** | 0.232 | 0.933 |
| Protein group | 0.820 | 0.608 | 1.348 | 0.178 | −0.372 | 2.012 |
| Coffee/tea group | 0.538 | 0.695 | 0.774 | 0.439 | −0.824 | 1.900 |
| Lipid/sugar group | 0.803 | 0.779 | 1.03 | 0.303 | −0.725 | 2.330 |
| Patients living with a spouse (n = 153, AIC = 999.7) | ||||||
| (Constant) | −22.079 | 29.166 | −0.757 | 0.449 | −79.244 | 35.086 |
| Age | 1.262 | 0.794 | 1.59 | 0.112 | −0.294 | 2.817 |
| Age*Age | −0.010 | 0.006 | −1.588 | 0.112 | −0.023 | 0.002 |
| Male Gender | 2.594 | 1.044 | 2.485 | 0.013 * | 0.548 | 4.639 |
| With Exercise habit | 2.747 | 1.028 | 2.671 | 0.008 ** | 0.731 | 4.762 |
| Education | 0.272 | 0.124 | 2.188 | 0.029 * | 0.028 | 0.516 |
| Protein group | 1.456 | 0.610 | 2.386 | 0.017 * | 0.260 | 2.651 |
| Coffee/tea group | 1.049 | 0.538 | 1.949 | 0.051 | −0.006 | 2.104 |
| Lipid/sugar group | 0.967 | 0.478 | 2.023 | 0.043 * | 0.030 | 1.903 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, H.-T.; Ma, M.-C.; Chang, H.-I.; Lin, C.-H.; Hsu, S.-W.; Huang, S.-H.; Lee, C.-C.; Huang, C.-W.; Chang, C.-C. Cognitive Decline Related to Diet Pattern and Nutritional Adequacy in Alzheimer’s Disease Using Surface-Based Morphometry. Nutrients 2022, 14, 5300. https://doi.org/10.3390/nu14245300
Hsiao H-T, Ma M-C, Chang H-I, Lin C-H, Hsu S-W, Huang S-H, Lee C-C, Huang C-W, Chang C-C. Cognitive Decline Related to Diet Pattern and Nutritional Adequacy in Alzheimer’s Disease Using Surface-Based Morphometry. Nutrients. 2022; 14(24):5300. https://doi.org/10.3390/nu14245300
Chicago/Turabian StyleHsiao, Hua-Tsen, Mi-Chia Ma, Hsin-I Chang, Ching-Heng Lin, Shih-Wei Hsu, Shu-Hua Huang, Chen-Chang Lee, Chi-Wei Huang, and Chiung-Chih Chang. 2022. "Cognitive Decline Related to Diet Pattern and Nutritional Adequacy in Alzheimer’s Disease Using Surface-Based Morphometry" Nutrients 14, no. 24: 5300. https://doi.org/10.3390/nu14245300
APA StyleHsiao, H.-T., Ma, M.-C., Chang, H.-I., Lin, C.-H., Hsu, S.-W., Huang, S.-H., Lee, C.-C., Huang, C.-W., & Chang, C.-C. (2022). Cognitive Decline Related to Diet Pattern and Nutritional Adequacy in Alzheimer’s Disease Using Surface-Based Morphometry. Nutrients, 14(24), 5300. https://doi.org/10.3390/nu14245300





