Neuroprotective Effects of Betulinic Acid Hydroxamate in Intraventricular Hemorrhage-Induced Brain Damage in Immature Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. IVH Induction
2.3. MRI Studies
2.4. Neurobehavioral Studies
2.5. Histologic Studies
2.6. Biochemical and Molecular Studies
2.7. Spectroscopy Studies
2.8. Statistical Analysis
3. Results
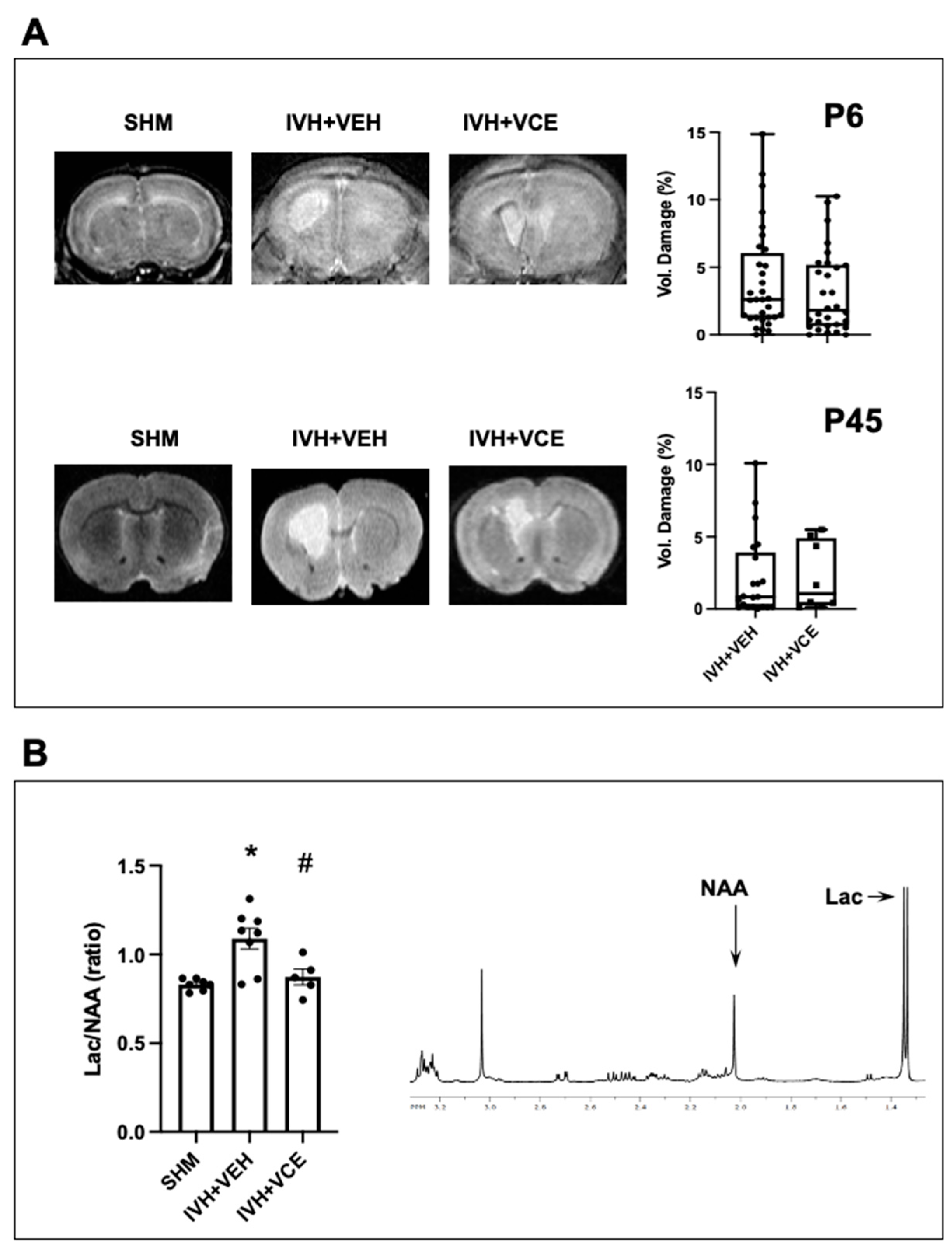
3.1. IVH-Induced Brain Damage
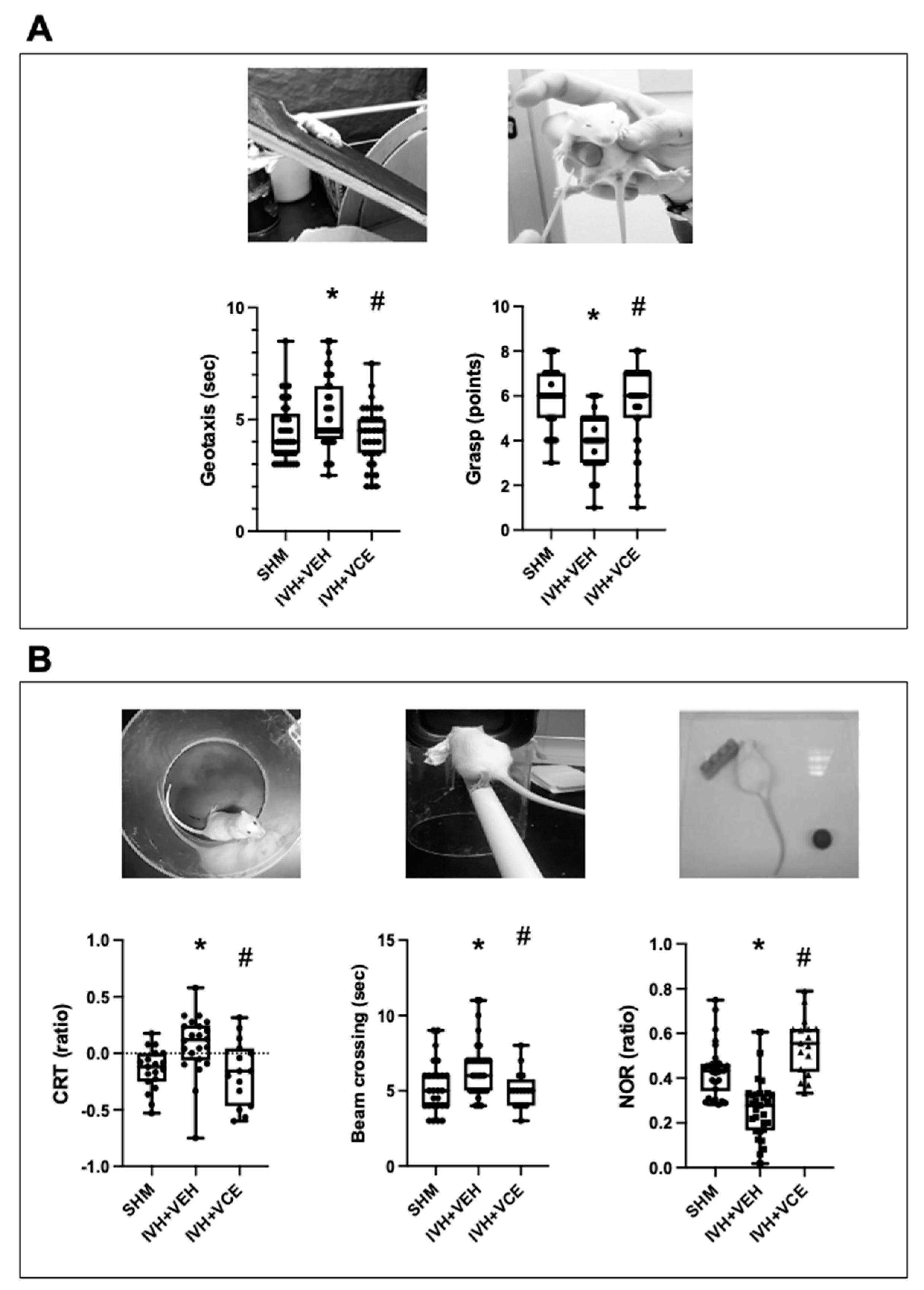
3.2. Functional Consequences of IVH
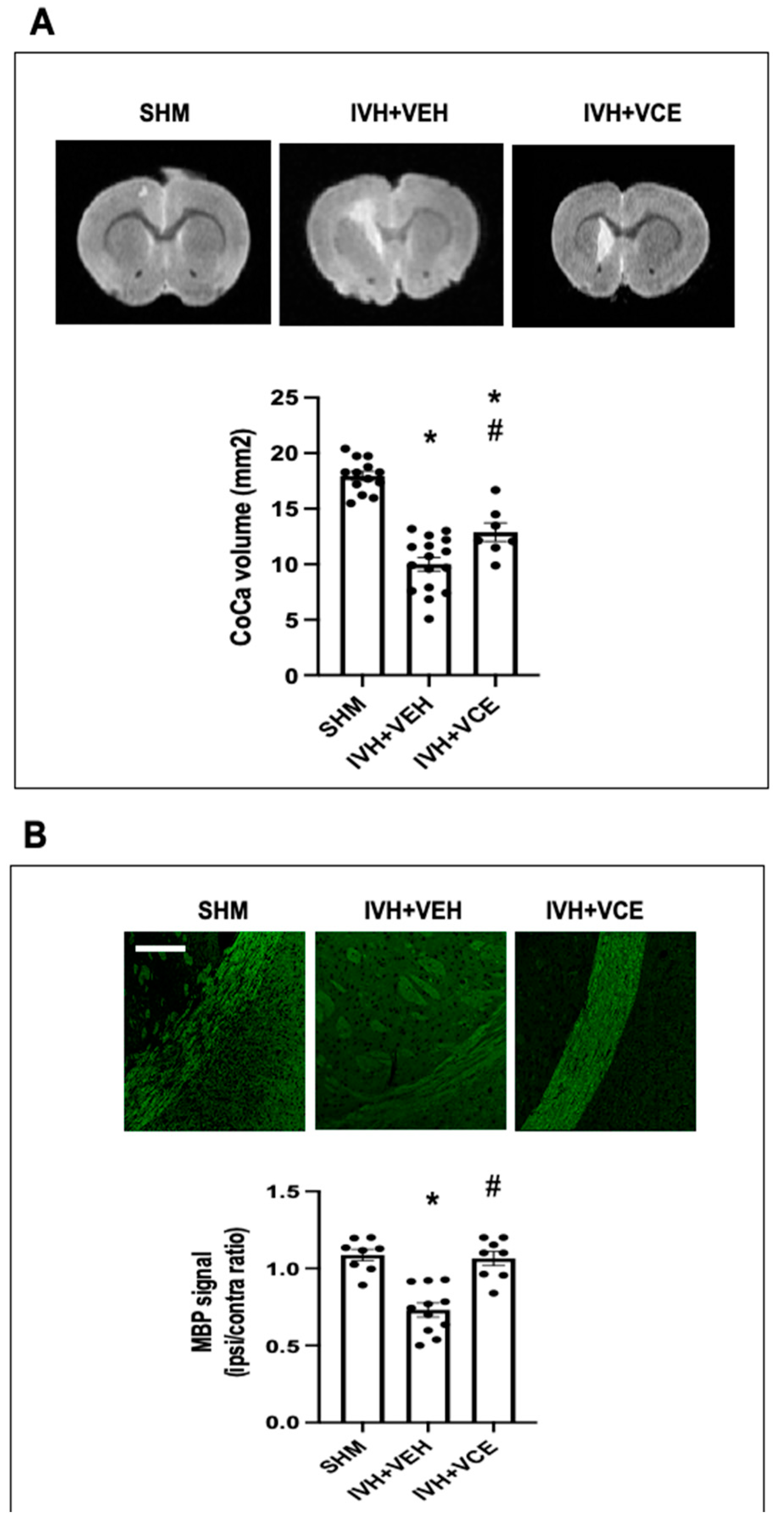
3.3. Long Term WMI Jury after IVH
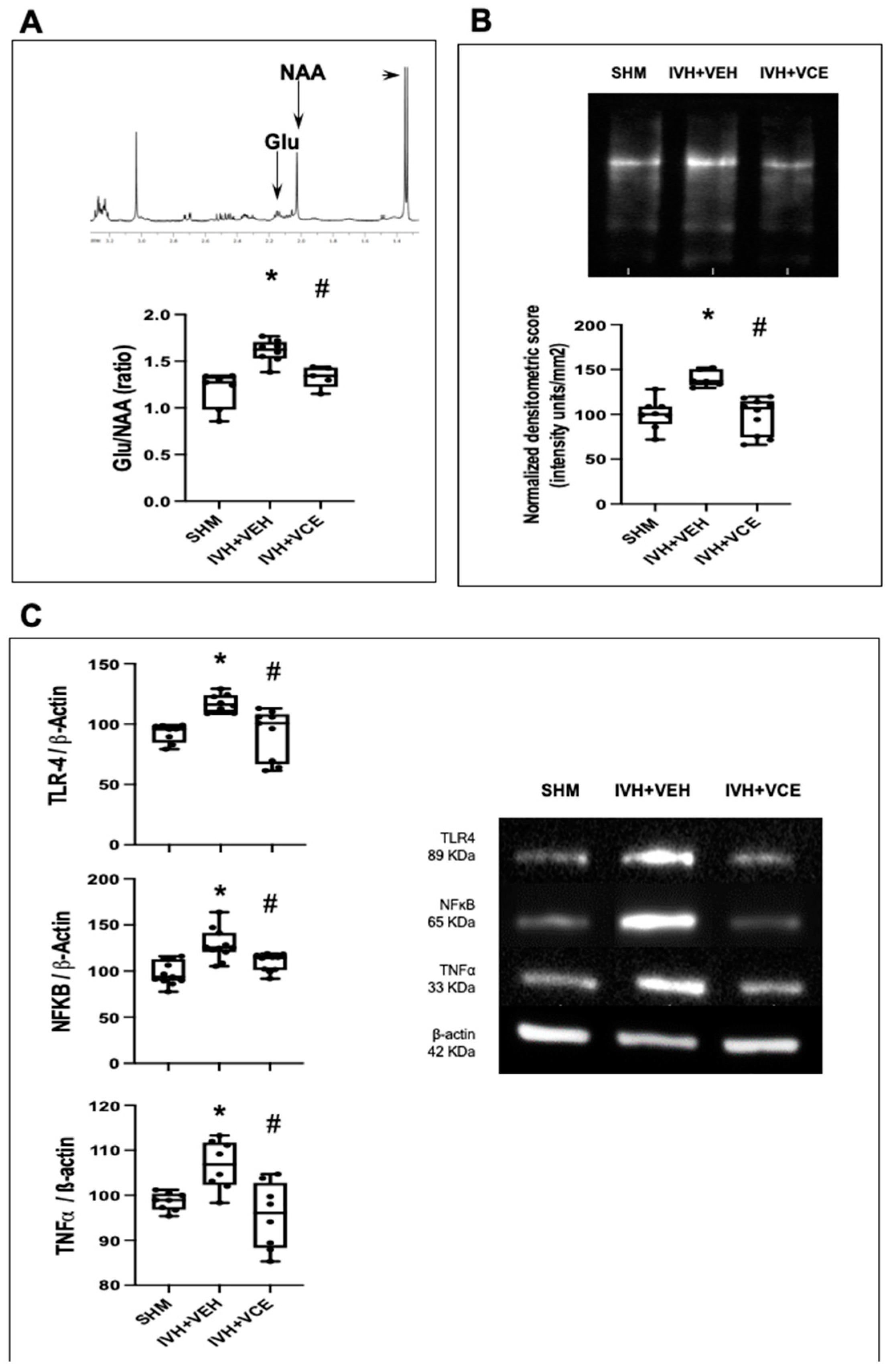
3.4. Mechanisms of Brain Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballabh, P. Pathogenesis and Prevention of Intraventricular Hemorrhage. Clin. Perinatol. 2014, 41, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.J.; de Vries, L.S.; Kersbergen, K.J.; van der Aa, N.E.; Brouwer, A.J.; Viergever, M.A.; Išgum, I.; Han, K.S.; Groenendaal, F.; Benders, M.J.N.L. Effects of Posthemorrhagic Ventricular Dilatation in the Preterm Infant on Brain Volumes and White Matter Diffusion Variables at Term-Equivalent Age. J. Pediatr. 2016, 168, 41–49.e1. [Google Scholar] [CrossRef] [PubMed]
- Garton, T.; Hua, Y.; Xiang, J.; Xi, G.; Keep, R.F. Challenges for Intraventricular Hemorrhage Research and Emerging Therapeutic Targets. Expert Opin. Ther. Targets 2017, 21, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Romantsik, O.; Bruschettini, M.; Ley, D. Intraventricular Hemorrhage and White Matter Injury in Preclinical and Clinical Studies. Neoreviews 2019, 20, e636–e652. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Cerebral Palsy and Motor Impairment in Children Born Very Preterm or Very Low Birthweight: A Systematic Review. Dev. Med. Child Neurol. 2016, 58, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.E.; Correa-Sáez, A.; Unciti-Broceta, J.D.; Garrido-Rodríguez, M.; Jimenez-Jimenez, C.; Mazzone, M.; Minassi, A.; Appendino, G.; Calzado, M.A.; Muñoz, E. Betulinic Acid Hydroxamate Is Neuroprotective and Induces Protein Phosphatase 2A-Dependent HIF-1α Stabilization and Post-Transcriptional Dephosphorylation of Prolyl Hydrolase 2. Neurotherapeutics 2021, 18, 1849–1861. [Google Scholar] [CrossRef]
- Silva, L.; Vargas, C.; Prados, M.E.; del Pozo, A.; Villa, M.; Martínez, M.; Alvarez, L.; Muñoz, E.; Unciti-Broceta, J.D.; Martínez-Orgado, J. Neuroprotective Efficacy of Betulinic Acid Hydroxamate, a B55α/PP2A Activator, in Acute Hypoxia–Ischemia-Induced Brain Damage in Newborn Rats. Transl. Stroke Res. 2022; ahead of print. [Google Scholar] [CrossRef]
- Del Pozo, A.; Villa, M.; Vargas, C.; Castejón, D.; Fernández-Valle, M.E.; Gutiérrez-Rodríguez, A.; Martínez-Orgado, J. Intraventricular Hemorrhage Induces Inflammatory Brain Damage with Blood–Brain Barrier Dysfunction in Immature Rats. Pediatr. Res. 2022; ahead of print. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol Administration after Hypoxia-Ischemia to Newborn Rats Reduces Long-Term Brain Injury and Restores Neurobehavioral Function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 9780123919496. [Google Scholar]
- Ceprián, M.; Vargas, C.; García-Toscano, L.; Penna, F.; Jiménez-Sánchez, L.; Achicallende, S.; Elezgarai, I.; Grandes, P.; Hind, W.; Ruth Pazos, M.; et al. Cannabidiol Administration Prevents Hypoxia-Ischemia-Induced Hypomyelination in Newborn Rats. Front. Pharmacol. 2019, 10, 1131. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Sung, D.K.; Kim, Y.E.; Sung, S.; Chang, Y.S.; Park, W.S. Brain-Derived Neurotropic Factor Mediates Neuroprotection of Mesenchymal Stem Cell-Derived Extracellular Vesicles against Severe Intraventricular Hemorrhage in Newborn Rats. Stem Cells Transl. Med. 2021, 10, 374–384. [Google Scholar] [CrossRef]
- Martins, C.A.; Neves, L.T.; de Oliveira, M.M.B.P.; Bagatini, P.B.; Barboza, R.; Mestriner, R.G.; Xavier, L.L.; Rasia-Filho, A.A. Neuroprotective Effect of ACTH on Collagenase-Induced Peri-Intraventricular Hemorrhage in Newborn Male Rats. Sci. Rep. 2020, 10, 17734. [Google Scholar] [CrossRef]
- Martínez-Orgado, J.; Villa, M.; del Pozo, A. Cannabidiol for the Treatment of Neonatal Hypoxic-Ischemic Brain Injury. Front. Pharmacol. 2021, 11, 584533. [Google Scholar] [CrossRef]
- Vetrovoy, O.; Rybnikova, E. Neuroprotective Action of PHD Inhibitors Is Predominantly HIF-1-Independent: An Editorial for ‘Sex Differences in Neonatal Mouse Brain Injury after Hypoxia-Ischemia and Adaptaquin Treatment’ on Page 759. J. Neurochem. 2019, 150, 645–647. [Google Scholar] [CrossRef]
- Trollmann, R.; Gassmann, M. The Role of Hypoxia-Inducible Transcription Factors in the Hypoxic Neonatal Brain. Brain Dev. 2009, 31, 503–509. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Wang, Y.; Xu, Y.; Zhang, S.; Culmsee, C.; Wang, X.; Zhu, C. Sex Differences in Neonatal Mouse Brain Injury after Hypoxia-Ischemia and Adaptaquin Treatment. J. Neurochem. 2019, 150, 759–775. [Google Scholar] [CrossRef]
- Fan, X.; Heijnen, C.J.; van der Kooij, M.A.; Groenendaal, F.; van Bel, F. The Role and Regulation of Hypoxia-Inducible Factor-1α Expression in Brain Development and Neonatal Hypoxic-Ischemic Brain Injury. Brain Res. Rev. 2009, 62, 99–108. [Google Scholar] [CrossRef]
- Chu, H.X.; Jones, N.M. Changes in Hypoxia-Inducible Factor-1 (HIF-1) and Regulatory Prolyl Hydroxylase (PHD) Enzymes Following Hypoxic–Ischemic Injury in the Neonatal Rat. Neurochem. Res. 2016, 41, 515–522. [Google Scholar] [CrossRef]
- Ehling, M.; Celus, W.; Martín-Pérez, R.; Alba-Rovira, R.; Willox, S.; Ponti, D.; Cid, M.C.; Jones, E.A.V.; Di Conza, G.; Mazzone, M. B55α/PP2A Limits Endothelial Cell Apoptosis during Vascular Remodeling: A Complementary Approach to Disrupt Pathological Vessels? Circ. Res. 2020, 127, 707–723. [Google Scholar] [CrossRef]
- Di Conza, G.; Trusso Cafarello, S.; Loroch, S.; Mennerich, D.; Deschoemaeker, S.; Di Matteo, M.; Ehling, M.; Gevaert, K.; Prenen, H.; Zahedi, R.P.; et al. The MTOR and PP2A Pathways Regulate PHD2 Phosphorylation to Fine-Tune HIF1α Levels and Colorectal Cancer Cell Survival under Hypoxia. Cell Rep. 2017, 18, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Mioc, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Coricovac, D.; Dehelean, C.; Avram, Ș.; Zamfir, A.D.; Munteanu, C.V.A.; et al. Structural Investigation of Betulinic Acid Plasma Metabolites by Tandem Mass Spectrometry. Molecules 2022, 27, 7359. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.-L.; Zhou, Y.; Li, J.-Q.; Wang, J.-Z.; Su, B.; Yang, Q.-W. Heme Activates TLR4-Mediated Inflammatory Injury via MyD88/TRIF Signaling Pathway in Intracerebral Hemorrhage. J. Neuroinflamm. 2012, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB System. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, M.A.; Nijboer, C.H.; Ohl, F.; Groenendaal, F.; Heijnen, C.J.; van Bel, F.; Kavelaars, A. NF-ΚB Inhibition after Neonatal Cerebral Hypoxia-Ischemia Improves Long-Term Motor and Cognitive Outcome in Rats. Neurobiol. Dis. 2010, 38, 266–272. [Google Scholar] [CrossRef]
- Zimmer, J.; Kristensen, B.W.; Jakobsen, B.; Noraberg, J. Excitatory Amino Acid Neurotoxicity and Modulation of Glutamate Receptor Expression in Organotypic Brain Slice Cultures. Amino Acids 2000, 19, 7–21. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of Cannabidiol Neuroprotection in Hypoxic-Ischemic Newborn Pigs: Role of 5HT(1A) and CB2 Receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Barata, L.; Arruza, L.; Rodríguez, M.-J.; Aleo, E.; Vierge, E.; Criado, E.; Sobrino, E.; Vargas, C.; Ceprián, M.; Gutiérrez-Rodríguez, A.; et al. Neuroprotection by Cannabidiol and Hypothermia in a Piglet Model of Newborn Hypoxic-Ischemic Brain Damage. Neuropharmacology 2019, 146, 1–11. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain Injury in Premature Infants: A Complex Amalgam of Destructive and Developmental Disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Dean, J.M.; Moravec, M.D.; Grafe, M.; Abend, N.; Ren, J.; Gong, X.; Volpe, J.J.; Jensen, F.E.; Hohimer, A.R.; Back, S.A. Strain-Specific Differences in Perinatal Rodent Oligodendrocyte Lineage Progression and Its Correlation with Human. Dev. Neurosci. 2011, 33, 251–260. [Google Scholar] [CrossRef]
- Thompson, D.K.; Inder, T.E.; Faggian, N.; Warfield, S.K.; Anderson, P.J.; Doyle, L.W.; Egan, G.F. Corpus Callosum Alterations in Very Preterm Infants: Perinatal Correlates and 2 year Neurodevelopmental Outcomes. Neuroimage 2012, 59, 3571–3581. [Google Scholar] [CrossRef]
- Bolisetty, S.; Dhawan, A.; Abdel-Latif, M.; Bajuk, B.; Stack, J.; Lui, K. Intraventricular Hemorrhage and Neurodevelopmental Outcomes in Extreme Preterm Infants. Pediatrics 2014, 133, 55–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Pozo, A.; Silva, L.; Romero, A.; De Hoz-Rivera, M.; Villa, M.; Martínez-Vega, M.; Prados, M.E.; Muñoz, E.; Martínez-Orgado, J. Neuroprotective Effects of Betulinic Acid Hydroxamate in Intraventricular Hemorrhage-Induced Brain Damage in Immature Rats. Nutrients 2022, 14, 5286. https://doi.org/10.3390/nu14245286
Del Pozo A, Silva L, Romero A, De Hoz-Rivera M, Villa M, Martínez-Vega M, Prados ME, Muñoz E, Martínez-Orgado J. Neuroprotective Effects of Betulinic Acid Hydroxamate in Intraventricular Hemorrhage-Induced Brain Damage in Immature Rats. Nutrients. 2022; 14(24):5286. https://doi.org/10.3390/nu14245286
Chicago/Turabian StyleDel Pozo, Aarón, Laura Silva, Angela Romero, María De Hoz-Rivera, María Villa, María Martínez-Vega, María Eugenia Prados, Eduardo Muñoz, and José Martínez-Orgado. 2022. "Neuroprotective Effects of Betulinic Acid Hydroxamate in Intraventricular Hemorrhage-Induced Brain Damage in Immature Rats" Nutrients 14, no. 24: 5286. https://doi.org/10.3390/nu14245286
APA StyleDel Pozo, A., Silva, L., Romero, A., De Hoz-Rivera, M., Villa, M., Martínez-Vega, M., Prados, M. E., Muñoz, E., & Martínez-Orgado, J. (2022). Neuroprotective Effects of Betulinic Acid Hydroxamate in Intraventricular Hemorrhage-Induced Brain Damage in Immature Rats. Nutrients, 14(24), 5286. https://doi.org/10.3390/nu14245286





