Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Extracts Preparation Procedure
2.2. Determination of Total Phenolic Content
2.3. Phytochemical Profile of S-EE by HPLC-ESI-QTOF-MS Methodology
2.4. Drosophila Treatments and Exposure to Hypoxia
2.5. Drosophila Locomotor Activity Assay
2.6. Animals and Transient Focal Cerebral Ischemia Model
2.7. Infarct Volume Assessment
2.8. Functional and Neurological Assessment
2.9. Preparation of Brain Homogenates
2.10. Quantification of Brain and Plasma Antioxidation Markers
2.11. Statistics
3. Results
3.1. Content of Phenolic Compounds in S. ramosissima Extracts
3.2. Tentative Characterization of S-EE by HPLC-ESI-QTOF-MS
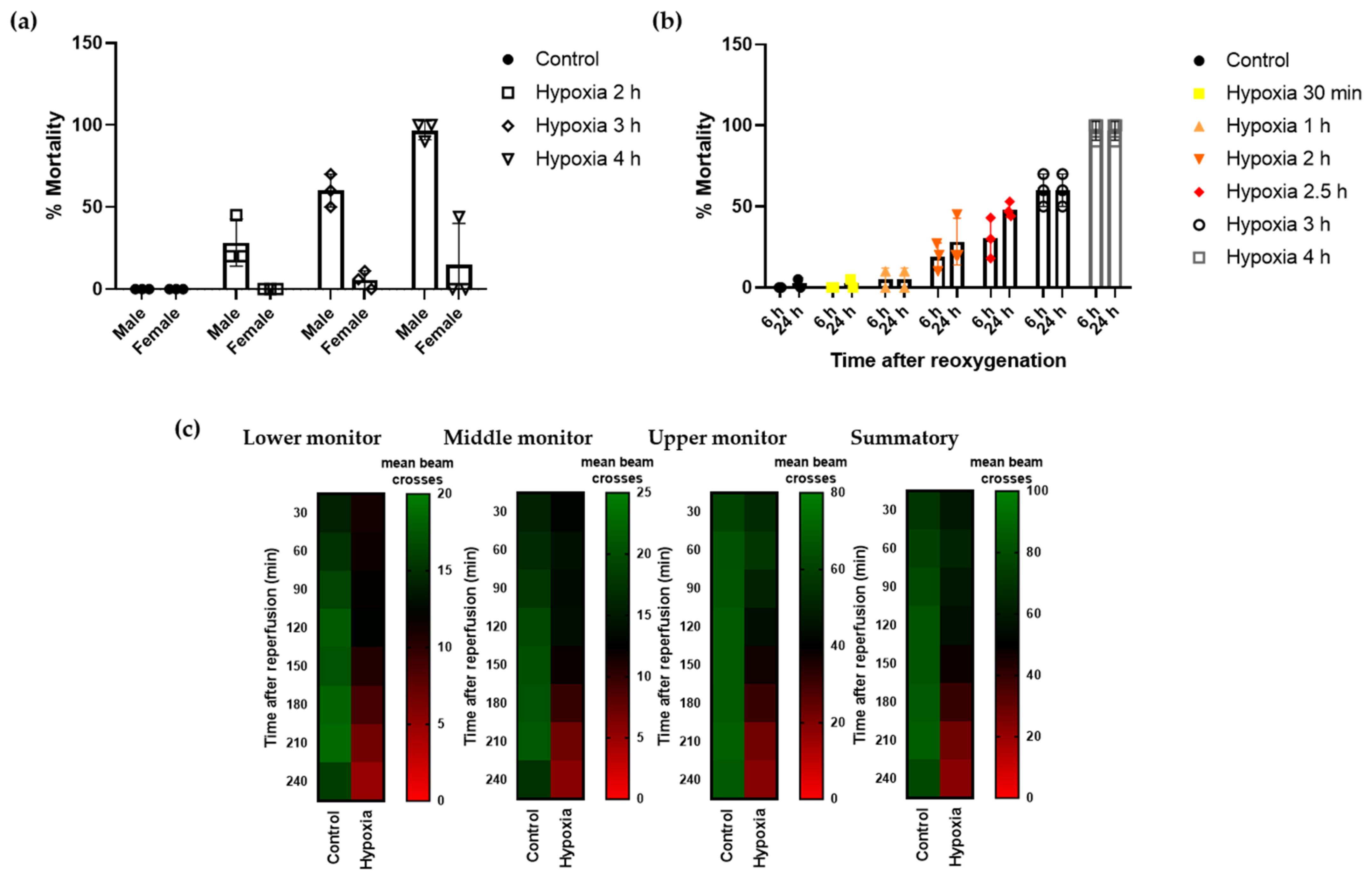
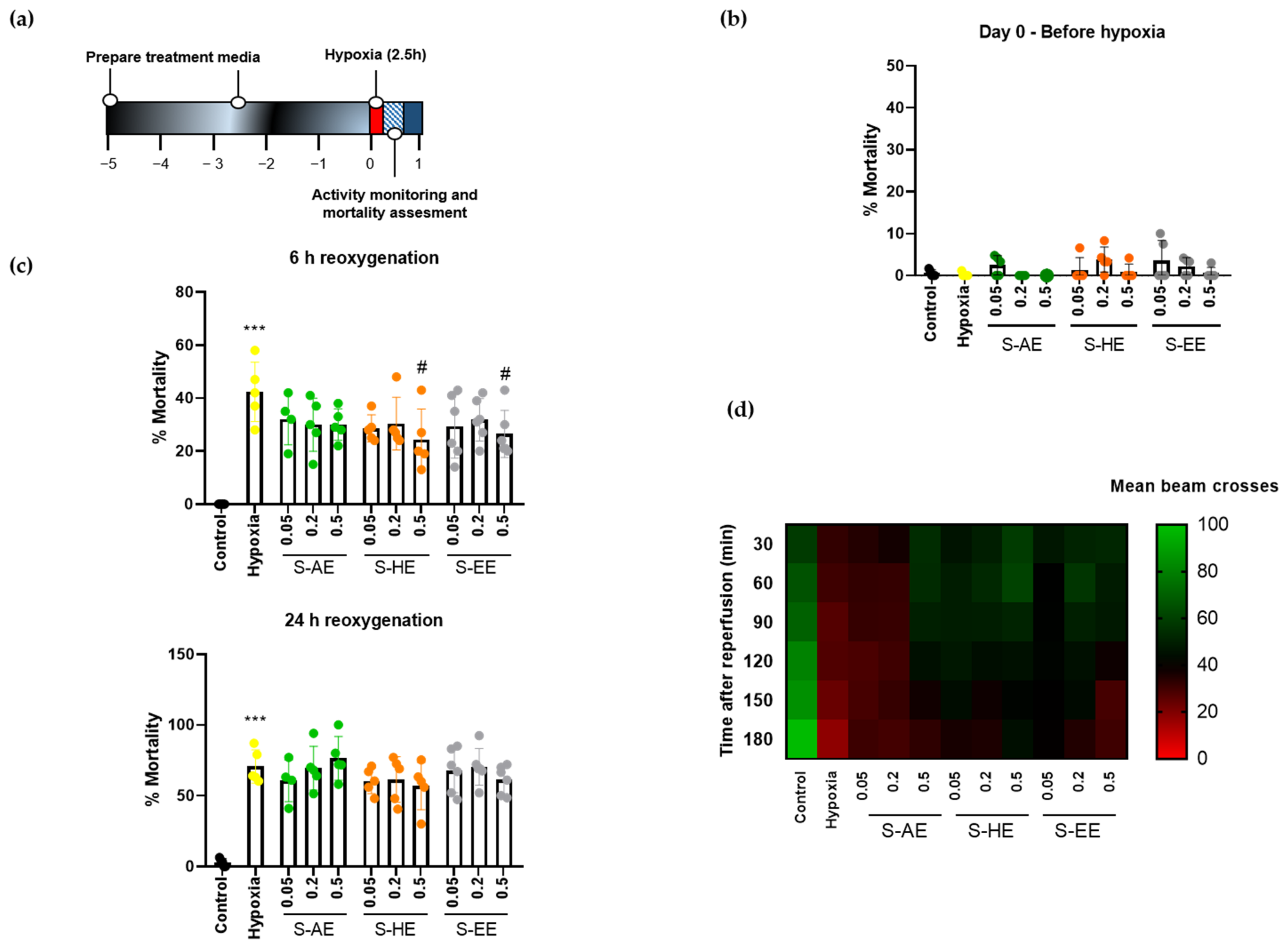
3.3. Effect of Supplementation with S. ramosissima Extracts on Drosophila Melanogaster Performance after Severe Hypoxia
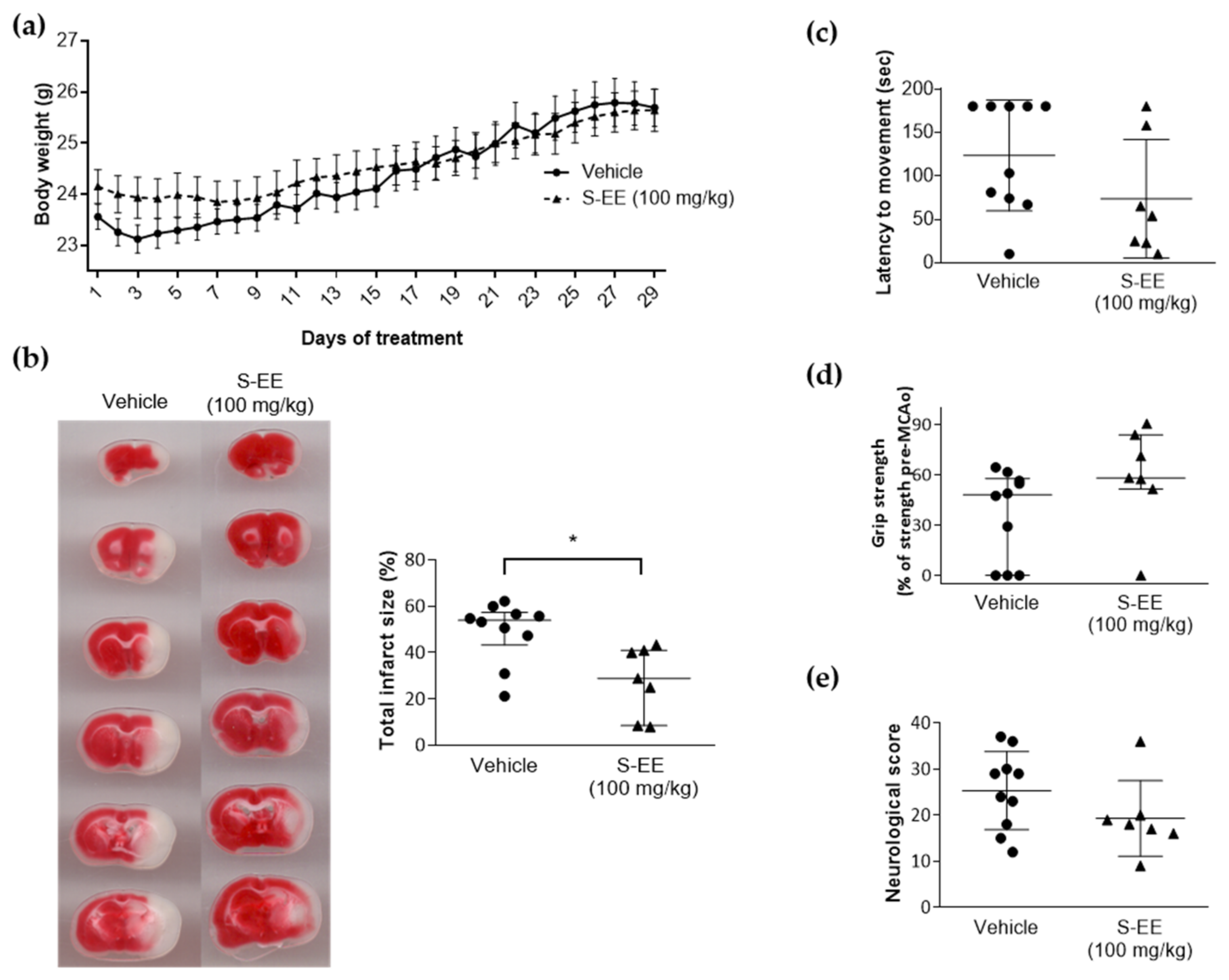
3.4. Oral Supplementation with S-EE Prevented Brain Damage after Experimental Stroke in Mice
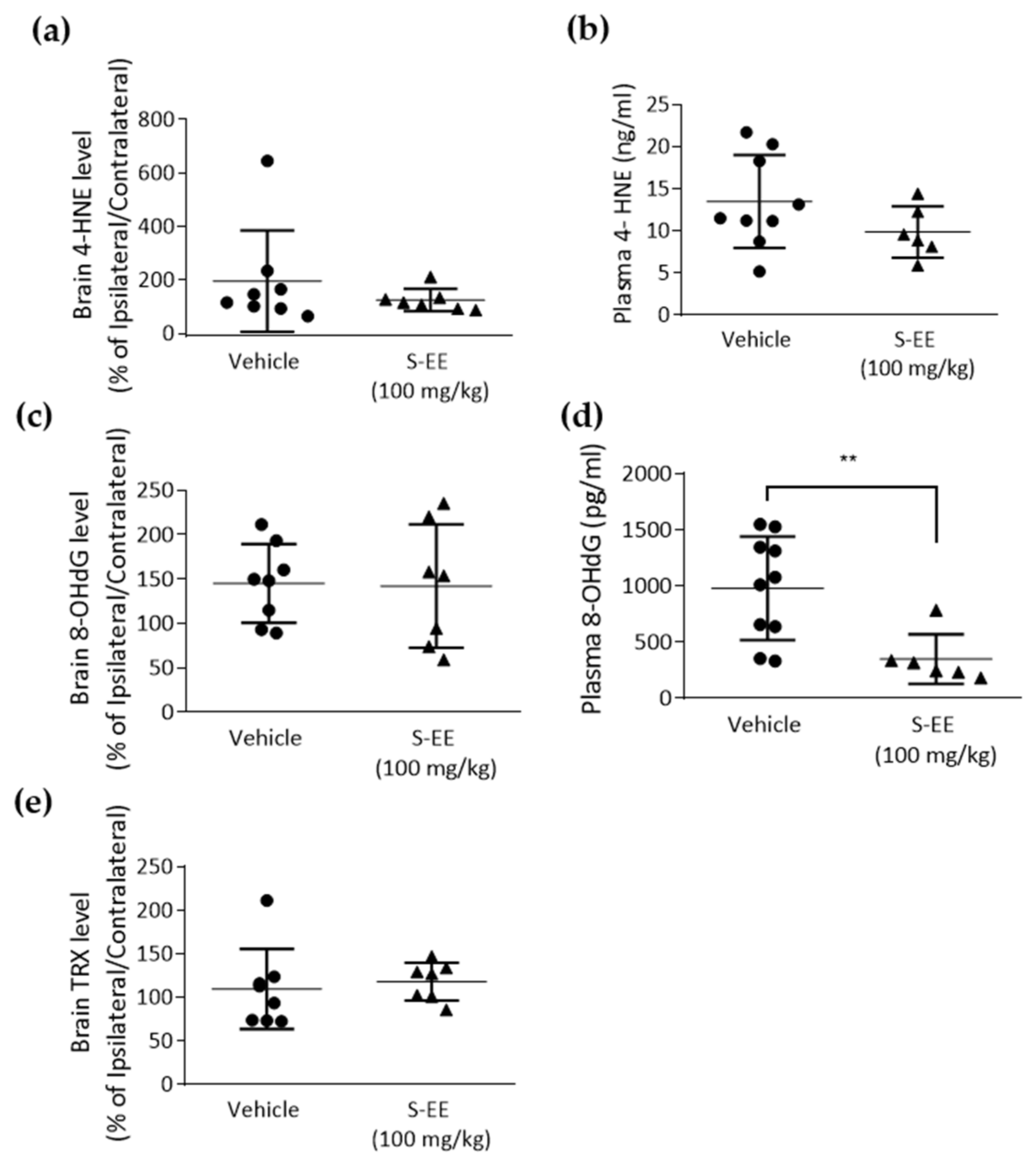
3.5. Effect of S-EE Supplementation Oxidative Stress Markers in Plasma and the Brain
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bejot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016, 45, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Chao, T.F.; Nedeljkovic, M.A.; Lip, G.Y.H.; Potpara, T.S. Stroke prevention in atrial fibrillation: Comparison of recent international guidelines. Eur. Heart J. Suppl. 2020, 22, O53–O60. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Montaner, J. Advanced neuroprotection for brain ischemia: An alternative approach to minimize stroke damage. Expert Opin. Investig. Drugs 2015, 24, 1137–1142. [Google Scholar] [CrossRef]
- Chelluboina, B.; Vemuganti, R. Therapeutic potential of nutraceuticals to protect brain after stroke. Neurochem. Int. 2021, 142, 104908. [Google Scholar] [CrossRef]
- Surget, G.; Stiger-Pouvreau, V.; Le Lann, K.; Kervarec, N.; Couteau, C.; Coiffard, L.J.; Gaillard, F.; Cahier, K.; Guerard, F.; Poupart, N. Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J. Photochem. Photobiol. B 2015, 143, 52–60. [Google Scholar] [CrossRef]
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From Preclinical Stroke Models to Humans: Polyphenols in the Prevention and Treatment of Stroke. Nutrients 2020, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Roberto, V.P.; Surget, G.; Le Lann, K.; Mira, S.; Tarasco, M.; Guerard, F.; Poupart, N.; Laize, V.; Stiger-Pouvreau, V.; Cancela, M.L. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front. Nutr. 2021, 8, 719438. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, J.Y.; Oh, J.; Jang, Y.K.; Lee, C.H.; Kim, J.S. Neuroprotective Effect of Halophyte Salicornia herbacea L. Is Mediated by Activation of Heme Oxygenase-1 in Mouse Hippocampal HT22 Cells. J. Med. Food 2017, 20, 140–151. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Chung, Y.C.; Choi, J.H.; Oh, K.N.; Chun, H.K.; Jeong, H.G. OhTungtungmadic acid isolated from Salicornia herbacea suppresses the progress of carbon tetrachloride-induced hepatic fibrosis. Off. J. Korean Soc. Toxicol. 2006, 22, 267–273. [Google Scholar]
- Clark, W.M.; Lessov, N.S.; Dixon, M.P.; Eckenstein, F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol. Res. 1997, 19, 641–648. [Google Scholar] [CrossRef]
- Ma, F.; Martinez-San Segundo, P.; Barcelo, V.; Morancho, A.; Gabriel-Salazar, M.; Giralt, D.; Montaner, J.; Rosell, A. Matrix metalloproteinase-13 participates in neuroprotection and neurorepair after cerebral ischemia in mice. Neurobiol. Dis. 2016, 91, 236–246. [Google Scholar] [CrossRef]
- Orsini, F.; Villa, P.; Parrella, S.; Zangari, R.; Zanier, E.R.; Gesuete, R.; Stravalaci, M.; Fumagalli, S.; Ottria, R.; Reina, J.J.; et al. Targeting mannose-binding lectin confers long-lasting protection with a surprisingly wide therapeutic window in cerebral ischemia. Circulation 2012, 126, 1484–1494. [Google Scholar] [CrossRef]
- De Simoni, M.G.; Storini, C.; Barba, M.; Catapano, L.; Arabia, A.M.; Rossi, E.; Bergamaschini, L. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 232–239. [Google Scholar] [CrossRef]
- Balkaya, M.G.; Trueman, R.C.; Boltze, J.; Corbett, D.; Jolkkonen, J. Behavioral outcome measures to improve experimental stroke research. Behav. Brain Res. 2018, 352, 161–171. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, ahead of print, 1–24. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Cacador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima J. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Ferreira, D.; Isca, V.M.S.; Leal, P.; Seca, A.M.L.; Silva, H.; de Lourdes Pereira, M.; Silva, A.M.S.; Pinto, D.C.G.A. Salicornia ramosissima: Secondary metabolites and protective effect against acute testicular toxicity. Arab. J. Chem. 2018, 11, 70–80. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Raheem, D.; Ahmed, F.; Alhodieb, F.S.; Alsharari, Z.D.; Alhaji, J.H.; BinMowyna, M.N.; Saraiva, A.; Raposo, A. Salicornia bigelovii, S. brachiata and S. herbacea: Their Nutritional Characteristics and an Evaluation of Their Potential as Salt Substitutes. Foods 2022, 11, 3402. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Gupta, M.L.; Tewari, J.P.; Khanna, S.N.; Gupta, P.C.; Srivastava, M.C.; Mishra, S.S. Phytopharmacologic studies of Ipomoea muricata seeds. J. Pharm. Sci. 1967, 56, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lv, Q.; Liu, L.; Zhang, Y.; Yang, X. New bakuchiol dimers from Psoraleae Fructus and their inhibitory activities on nitric oxide production. Chin. Med. 2021, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Choi, H.S.; Kim, J.H.; Kim, S.L.; Yun, B.S.; Lee, D.S. Coriolic Acid (13-(S)-Hydroxy-9Z, 11E-octadecadienoic Acid) from Glasswort (Salicornia herbacea L.) Suppresses Breast Cancer Stem Cell through the Regulation of c-Myc. Molecules 2020, 25, 4950. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, B.; Jiang, M.; Wang, H.; Hu, Y.; Wang, H.; Xu, X.; Gao, X.; Yang, W. Integrating Enhanced Profiling and Chemometrics to Unveil the Potential Markers for Differentiating among the Leaves of Panax ginseng, P. quinquefolius, and P. notoginseng by Ultra-High Performance Liquid Chromatography/Ion Mobility-Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2022, 27, 5549. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kinoshita, M.; Ohnishi, M. Chemical Characterization of Glycerolipids and Cerebrosides in a Halophytic Plant, Salicornia europaea L. J. Oleo Sci. 2004, 53, 337–341. [Google Scholar] [CrossRef][Green Version]
- Liu, P. Application of FT-ICR Mass Spectrometry in Hydrogen Deuterium Exchange and Lipidomics. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2018. [Google Scholar]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Awooda, H.A.; Lutfi, M.F.; Sharara, G.G.; Saeed, A.M. Oxidative/nitrosative stress in rats subjected to focal cerebral ischemia/reperfusion. Int. J. Health Sci. 2015, 9, 17–24. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kong, C.S.; Um, Y.R.; Lim, S.Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Ha, B.J.; Lee, S.H.; Kim, H.J.; Lee, J.Y. The role of Salicornia herbacea in ovariectomy-induced oxidative stress. Biol. Pharm. Bull. 2006, 29, 1305–1309. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, K.R.; Choi, S.W.; Woo, M.H.; Choi, J.H. Antioxidant and antithrombus activities of enzyme-treated Salicornia herbacea extracts. Ann. Nutr. Metab. 2007, 51, 119–125. [Google Scholar] [CrossRef]
- Giordano, R.; Aliotta, G.E.; Johannesen, A.S.; Voetmann-Jensen, D.; Laustsen, F.H.; Andersen, L.A.; Rezai, A.; Fredsgaard, M.; Vecchio, S.L.; Arendt-Nielsen, L.; et al. Effects of Salicornia-Based Skin Cream Application on Healthy Humans’ Experimental Model of Pain and Itching. Pharmaceuticals 2022, 15, 150. [Google Scholar] [CrossRef]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The Ameliorative Effects of the Ethyl Acetate Extract of Salicornia europaea L. and Its Bioactive Candidate, Irilin B, on LPS-Induced Microglial Inflammation and MPTP-Intoxicated PD-Like Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 6764756. [Google Scholar] [CrossRef]
- Park, D.J.; Kang, J.B.; Shah, M.A.; Koh, P.O. Quercetin alleviates the injury-induced decrease of protein phosphatase 2A subunit B in cerebral ischemic animal model and glutamate-exposed HT22 cells. J. Vet. Med. Sci. 2019, 81, 1047–1054. [Google Scholar] [CrossRef]
- Park, D.J.; Shah, F.A.; Koh, P.O. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci. 2018, 80, 676–683. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.M.; Hoda, M.N.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.E.; Safhi, M.M.; et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res. 2011, 36, 1360–1371. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, S.H.; Ye, Y.L.; Chu, L.S.; Zhang, W.P.; Wang, M.L.; Wei, E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006, 27, 1103–1110. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.O. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J. Food Sci. 2011, 76, C250–C256. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Ho, T.Y.; Lee, E.J.; Su, S.Y.; Tang, N.Y.; Hsieh, C.L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef]
- Ceprian, M.; Jimenez-Sanchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martinez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Sitlani, C.; Psaty, B.M.; Rimm, E.B.; Song, X.; McKnight, B.; Spiegelman, D.; King, I.B.; et al. Plasma phospholipid and dietary alpha-linolenic acid, mortality, CHD and stroke: The Cardiovascular Health Study. Br. J. Nutr. 2014, 112, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. Alpha-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Veno, S.K.; Schmidt, E.B.; Jakobsen, M.U.; Lundbye-Christensen, S.; Bach, F.W.; Overvad, K. Substitution of Linoleic Acid for Other Macronutrients and the Risk of Ischemic Stroke. Stroke 2017, 48, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Zhang, J.H.; Noble-Haeusslein, L.J. RIGOR guidelines: Escalating STAIR and STEPS for effective translational research. Transl. Stroke Res. 2013, 4, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lee, S.K.; Jo, J.R.; Kim, M.E.; So, H.A.; Cho, C.W.; Seo, Y.W.; Kim, J.I. Hypolipidemic effect of Salicornia herbacea in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2007, 1, 371–375. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Pires-Cabral, P.; Quintas, C. Salicornia ramosissima as a salt substitute in the fermentation of white cabbage. J. Food Sci. Technol. 2022, 59, 597–605. [Google Scholar] [CrossRef]
- Lee, W.J.; Shin, Y.W.; Kim, D.E.; Kweon, M.H.; Kim, M. Effect of desalted Salicornia europaea L. ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: A 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2020, 10, 19914. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Stompor-Goracy, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
| mg GAE/g DW | s.d. | |
|---|---|---|
| S-HE | 24 | ±2 |
| S-AE | 7 | ±2 |
| S-EE | 46 | ±3 |
| RT | m/z | Molecular Formula | Proposed Compounds | References |
|---|---|---|---|---|
| 1.00 | 157.0368 | C4H6N4O3 | Allantoin | |
| 1.03 | 191.0550 | C7H12O6 | Quinic acid | [26] |
| 1.09 | 191.0223 | C10H8O4 | Scopoletin | [27] |
| 6.33 | 355.1033 | C16H20O9 | Hydrocaffeoylquinic acid | [26] |
| 6.52 | 431.1932 | C20H32O10 | Sacranoside A | |
| 6.85 | 433.2087 | C20H34O10 | Benzyl trilactosylthreitol | |
| 6.99 | 353.0881 | C16H18O9 | Neochlorogenic acid | [26] |
| 7.21 | 165.0556 | C9H10O3 | Apocynin (caffeyl alcohol) | [28] |
| 9.00 | 193.0513 | C10H10O4 | Ferulic acid | [26] |
| 9.08 | 163.0393 | C9H8O3 | Coumaric acid | [26] |
| 9.44 | 305.0703 | C15H14O7 | (Epi)gallocatechin | [26] |
| 9.60 | 609.1458 | C27H30O16 | Quercetin-rhamnosyl-hexoside | [26] |
| 9.67 | 417.2131 | C20H34O9 | Maryal | |
| 9.85 | 463.0874 | C21H20O12 | Quercetin glucoside | [26] |
| 9.86 | 517.1358 | C25H26O12 | Tungtungmadic acid isomer 1 | [29] |
| 10.08 | 517.1352 | C25H26O12 | Tungtungmadic acid isomer 2 | [29] |
| 10.16 | 515.1208 | C25H24O12 | Dicaffeoylquinic acid isomer 1 | [30] |
| 10.24 | 549.0886 | C24H22O15 | Quercetin malonyglucoside | [26] |
| 10.30 | 515.1206 | C25H24O12 | Dicaffeoylquinic acid isomer 2 | [30] |
| 10.34 | 477.1018 | C22H22O12 | Isorhamnetin glucopyranoside | [29] |
| 10.51 | 517.1352 | C25H26O12 | Tungtungmadic acid isomer 3 | [29] |
| 10.61 | 515.1200 | C25H24O12 | Dicaffeoylquinic acid isomer 3 | [30] |
| 10.84 | 519.1158 | C24H24O13 | Luteolin glucosyllactate | |
| 10.89 | 515.1224 | C25H24O13 | Dicaffeoylquinic acid isomer 4 | [30] |
| 11.69 | 793.4033 | C42H66O14 | Calenduloside G isomer 1 | [30] |
| 12.04 | 327.2182 | C18H32O5 | Trihydroxyoctadecadienoic acid | |
| 12.52 | 329.2342 | C18H34O5 | Trihydroxyoctadecenoic acid | |
| 13.25 | 793.4375 | C42H66O14 | Calenduloside G isomer 2 | [30] |
| 13.33 | 289.1119 | - | Unknown 1 | |
| 13.99 | 293.1763 | C17H26O4 | Embelin | |
| 14.83 | 721.3693 | - | Unknown 2 | |
| 15.22 | 562.3167 | C28H52O11 | Glycoside muricatin | [31] |
| 15.71 | 559.3143 | C28H48O11 | Dirhamnosyl linolenic acid isomer 1 | |
| 15.82 | 293.2124 | C18H30O3 | Colneleic acid | |
| 15.91 | 559.3137 | C28H48O11 | Dirhamnosyl linolenic acid isomer 2 | |
| 16.32 | 540.3312 | C36H46O4 | Bakuchiol derivative | [32] |
| 16.51 | 295.2280 | C18H32O3 | Coriolic acid | [33] |
| 18.41 | 357.2075 | C22H30O4 | Cannabidiolic acid | |
| 18.59 | 277.2173 | C18H30O2 | Linolenic acid | |
| 18.93 | 997.5766 | C52H86O18 | Ginsenoside derivative | [34] |
| 19.29 | 279.2331 | C18H32O2 | Linoleic acid | |
| 19.83 | 835.5233 | C46H76O13 | Glycerolipid derivative | [35] |
| 19.96 | 255.2331 | C16H32O2 | Palmitic Acid | |
| 20.71 | 981.5809 | C52H86O17 | Spirastrellolide B | |
| 21.39 | 758.5436 | C45H76O9 | Decanedioic acid derivative isomer 1 | |
| 21.52 | 758.5438 | C45H76O9 | Decanedioic acid derivative isomer 2 | |
| 22.01 | 959.5975 | C50H88O17 | DGDG(33:3)acetate | [36] |
| 21.76 | 819.5277 | C46H76O12 | Salinomycin derivative | |
| 22.60 | 431.3177 | C29H52O2 | Tricosylresorcinol | |
| 23.11 | 797.5438 | - | Unknown 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rodríguez, P.; Ma, F.; Río, C.d.; Romero-Bernal, M.; Najar, A.M.; Cádiz-Gurrea, M.d.l.L.; Leyva-Jimenez, F.J.; Ramiro, L.; Menéndez-Valladares, P.; Pérez-Sánchez, S.; et al. Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia. Nutrients 2022, 14, 5077. https://doi.org/10.3390/nu14235077
García-Rodríguez P, Ma F, Río Cd, Romero-Bernal M, Najar AM, Cádiz-Gurrea MdlL, Leyva-Jimenez FJ, Ramiro L, Menéndez-Valladares P, Pérez-Sánchez S, et al. Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia. Nutrients. 2022; 14(23):5077. https://doi.org/10.3390/nu14235077
Chicago/Turabian StyleGarcía-Rodríguez, Paula, Feifei Ma, Carmen del Río, Marina Romero-Bernal, Ana M. Najar, María de la Luz Cádiz-Gurrea, Francisco Javier Leyva-Jimenez, Laura Ramiro, Paloma Menéndez-Valladares, Soledad Pérez-Sánchez, and et al. 2022. "Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia" Nutrients 14, no. 23: 5077. https://doi.org/10.3390/nu14235077
APA StyleGarcía-Rodríguez, P., Ma, F., Río, C. d., Romero-Bernal, M., Najar, A. M., Cádiz-Gurrea, M. d. l. L., Leyva-Jimenez, F. J., Ramiro, L., Menéndez-Valladares, P., Pérez-Sánchez, S., Segura-Carretero, A., & Montaner, J. (2022). Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia. Nutrients, 14(23), 5077. https://doi.org/10.3390/nu14235077









