Lower Fiber Consumption in Women with Polycystic Ovary Syndrome: A Meta-Analysis of Observational Studies
Abstract
1. Introduction
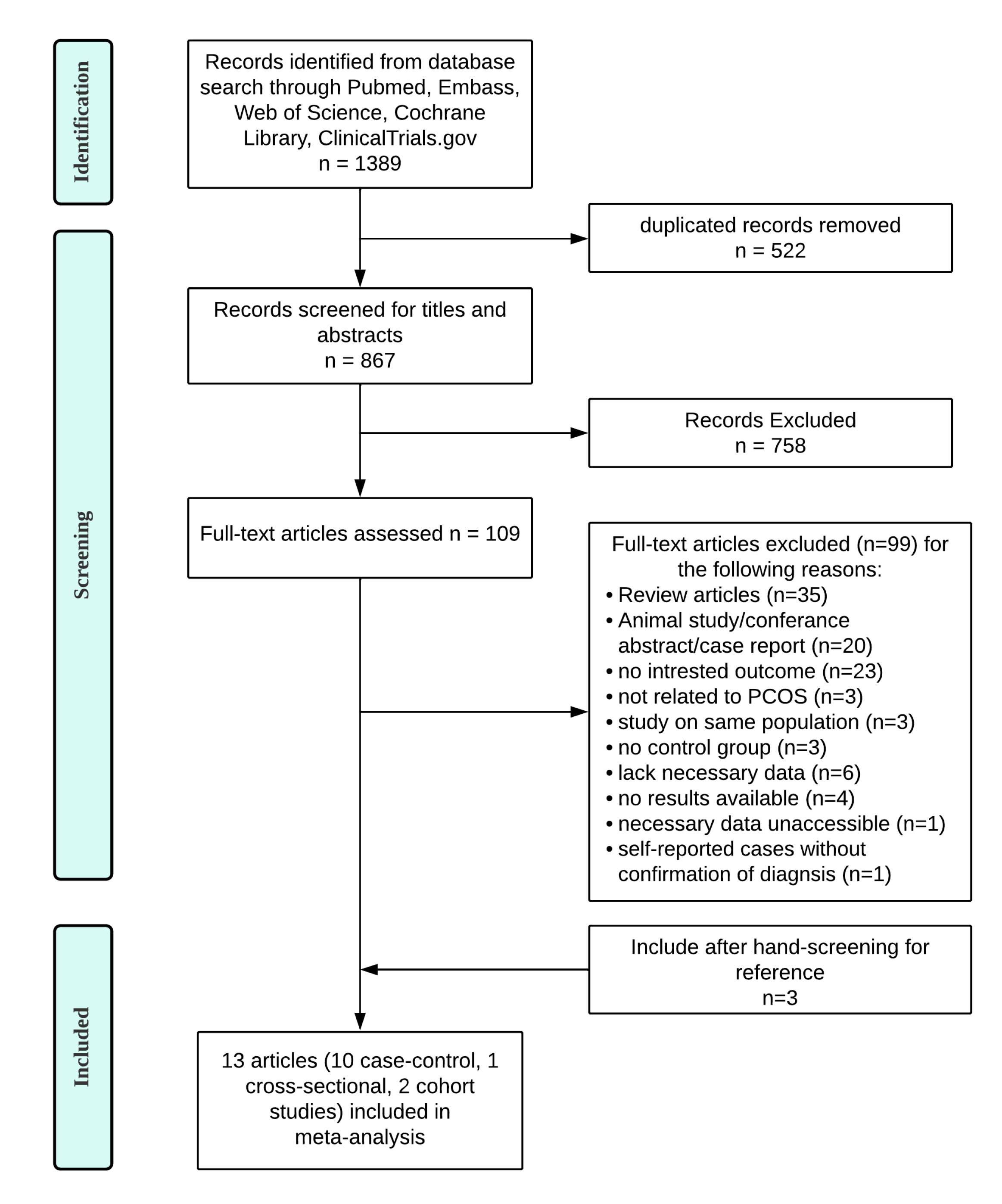
2. Materials and Methods
2.1. Data Sources and Searches Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of Included Studies
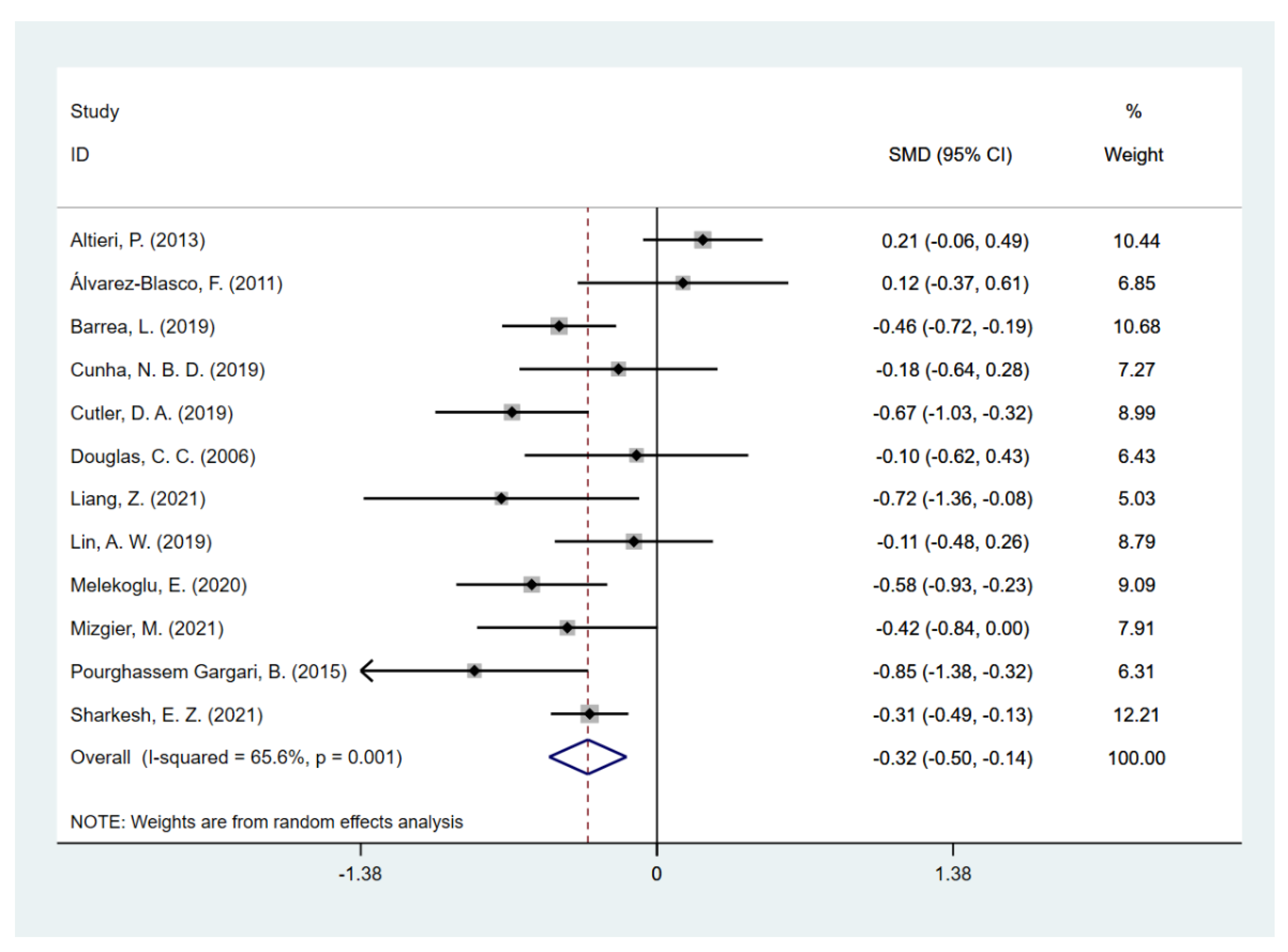
3.2. Daily Dietary Fiber Intake in PCOS and Controls
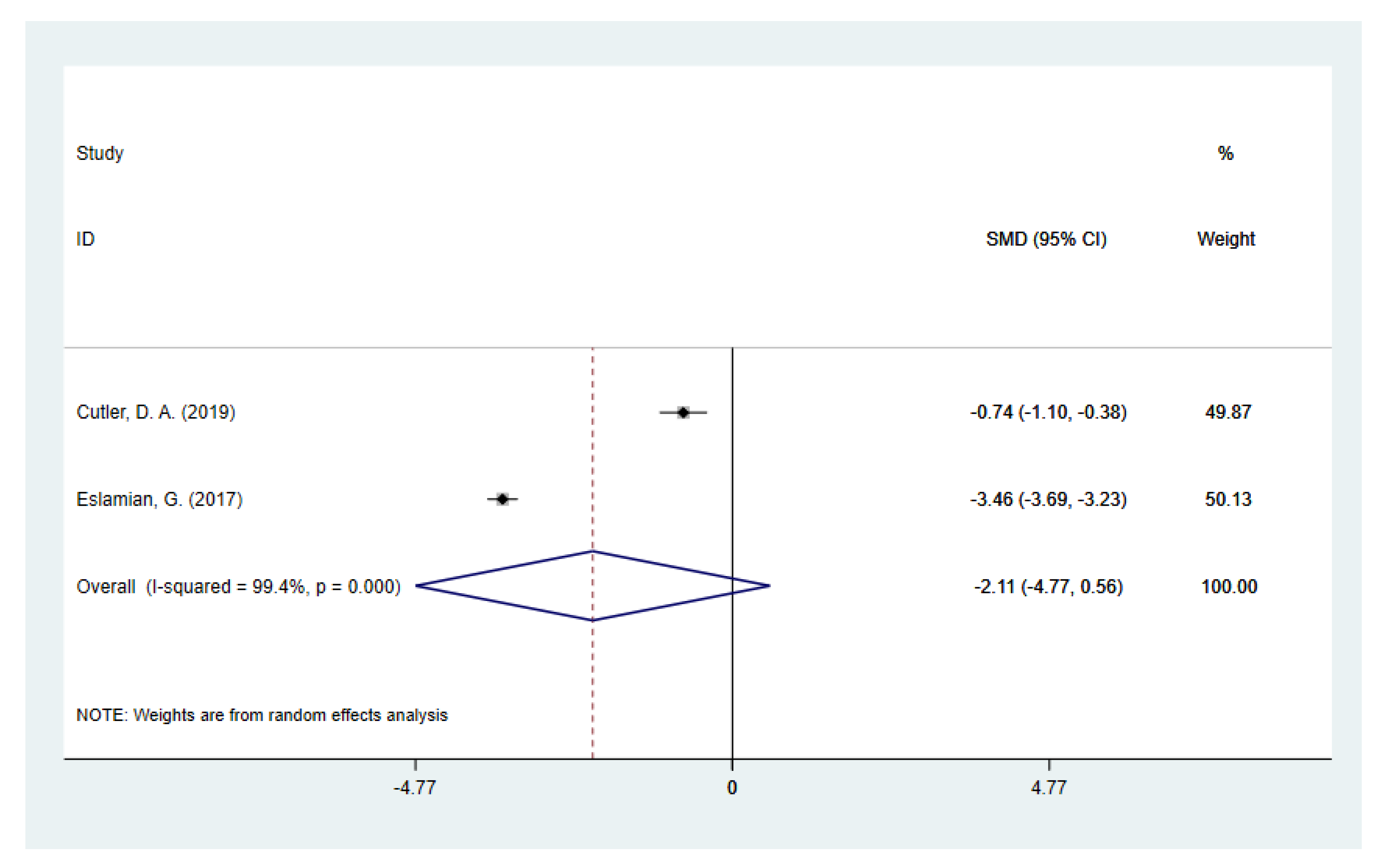
3.3. Subgroup Analysis
3.4. Meta-Regression
3.5. Influence Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists; Gynecologists’ Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.E25. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Paris, V.; Solon-Biet, S.M.; Senior, A.M.; Edwards, M.C.; Desai, R.; Tedla, N.; Cox, M.J.; Ledger, W.L.; Gilchrist, R.B.; Simpson, S.J.; et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nat. Commun. 2020, 11, 5262. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Deeks, A.A.; Moran, L.J.; Stuckey, B.G.; Wong, J.L.; Norman, R.J.; Costello, M.F.; Guideline Development, G. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med. J. Aust. 2011, 195, S65–S112. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Marshall, J.C. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Thomson, C.A.; Stefanick, M.L.; Newman, V.A.; Jones, L.A.; Natarajan, L.; Ritenbaugh, C.; Hollenbach, K.A.; Pierce, J.P.; et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J. Clin. Oncol. 2004, 22, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Wayne, S.J.; Neuhouser, M.L.; Ulrich, C.M.; Koprowski, C.; Baumgartner, K.B.; Baumgartner, R.N.; McTiernan, A.; Bernstein, L.; Ballard-Barbash, R. Dietary fiber is associated with serum sex hormones and insulin-related peptides in postmenopausal breast cancer survivors. Breast Cancer Res. Treat. 2008, 112, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Howe, G.R.; Benito, E.; Castelleto, R.; Cornee, J.; Esteve, J.; Gallagher, R.P.; Iscovich, J.M.; Deng-ao, J.; Kaaks, R.; Kune, G.A.; et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: Evidence from the combined analysis of 13 case-control studies. J. Natl. Cancer Inst. 1992, 84, 1887–1896. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Abreu, Y.A.A.T.; Milke-Garcia, M.P.; Arguello-Arevalo, G.A.; Calderon-de la Barca, A.M.; Carmona-Sanchez, R.I.; Consuelo-Sanchez, A.; Coss-Adame, E.; Garcia-Cedillo, M.F.; Hernandez-Rosiles, V.; Icaza-Chavez, M.E.; et al. Dietary fiber and the microbiota: A narrative review by a group of experts from the Asociacion Mexicana de Gastroenterologia. Rev. Gastroenterol. Mex. (Engl. Ed.) 2021, 86, 287–304. [Google Scholar] [CrossRef]
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q.; Yang, L.; Liu, Z.J.; Yuan, Y.Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, L.; Gutin, B.; Zhu, H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur. J. Clin. Nutr. 2019, 73, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Erthal Leinig, C.; Pecoits-Filho, R.; Kunii, L.; Claro, L.M.; Merlin, J.; Almeida, N.R.; Carvalho, C.R.S.; Moraes, T.P. Low-Fiber Intake Is Associated with High Production of Intraperitoneal Inflammation Biomarkers. J. Ren. Nutr. 2019, 29, 322–327. [Google Scholar] [CrossRef]
- Dietary fiber and health. Council on Scientific Affairs. JAMA 1989, 262, 542–546. [Google Scholar]
- Aune, D.; Sen, A.; Norat, T.; Riboli, E. Dietary fibre intake and the risk of diverticular disease: A systematic review and meta-analysis of prospective studies. Eur. J. Nutr. 2020, 59, 421–432. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 February 2022).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Altieri, P.; Cavazza, C.; Pasqui, F.; Morselli, A.M.; Gambineri, A.; Pasquali, R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin. Endocrinol. 2013, 78, 52–59. [Google Scholar] [CrossRef]
- Alvarez-Blasco, F.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol. Endocrinol. 2011, 27, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.B.D.; Ribeiro, C.T.; Silva, C.M.; Rosa, E.S.A.; De-Souza, D.A. Dietary intake, body composition and metabolic parameters in women with polycystic ovary syndrome. Clin. Nutr. 2019, 38, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, G.; Baghestani, A.R.; Eghtesad, S.; Hekmatdoost, A. Dietary carbohydrate composition is associated with polycystic ovary syndrome: A case-control study. J. Hum. Nutr. Diet. 2017, 30, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Di, N.; Li, L.; Yang, D. Gut microbiota alterations reveal potential gut-brain axis changes in polycystic ovary syndrome. J. Endocrinol. Investig. 2021, 44, 1727–1737. [Google Scholar] [CrossRef]
- Lin, A.W.; Kazemi, M.; Jarrett, B.Y.; Vanden Brink, H.; Hoeger, K.M.; Spandorfer, S.D.; Lujan, M.E. Dietary and Physical Activity Behaviors in Women with Polycystic Ovary Syndrome per the New International Evidence-Based Guideline. Nutrients 2019, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Melekoglu, E.; Goksuluk, D.; Akal Yildiz, E. Association between Dietary Glycaemic Index and Glycaemic Load and Adiposity Indices in Polycystic Ovary Syndrome. J. Am. Coll. Nutr. 2020, 39, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, M.; Jarzabek-Bielecka, G.; Formanowicz, D.; Jodlowska-Siewert, E.; Mruczyk, K.; Cisek-Wozniak, A.; Kedzia, W.; Opydo-Szymaczek, J. Dietary and Physical Activity Habits in Adolescent Girls with Polycystic Ovary Syndrome (PCOS)-HAstudy. J. Clin. Med. 2021, 10, 3469. [Google Scholar] [CrossRef]
- Pourghassem Gargari, B.; Houjeghani, S.; Farzadi, L.; Houjeghani, S.; Safaeiyan, A. Relationship between Serum Leptin, Ghrelin and Dietary Macronutrients in Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2015, 9, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zirak Sharkesh, E.; Keshavarz, S.A.; Nazari, L.; Abbasi, B. The dietary inflammatory index is directly associated with polycystic ovary syndrome: A case-control study. Clin. Endocrinol. 2021, 96, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Cutler, D.A.; Pride, S.M.; Cheung, A.P. Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: A cohort study. Food Sci. Nutr. 2019, 7, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.C.; Norris, L.E.; Oster, R.A.; Darnell, B.E.; Azziz, R.; Gower, B.A. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil. Steril. 2006, 86, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh Shamasbi, S.; Dehgan, P.; Mohammad-Alizadeh Charandabi, S.; Aliasgarzadeh, A.; Mirghafourvand, M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: A randomized, triple-blind, controlled, clinical trial. Eur. J. Nutr. 2019, 58, 629–640. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Munzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, H.; Yang, J.; Zhang, X.; Chen, Y.; Feng, R.; Qian, Y. Changes in Vaginal Microbiome Diversity in Women with Polycystic Ovary Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 755741. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Hayashi, T.; Kayama, H.; Motooka, D.; Hase, H.; Jingushi, K.; Yamamichi, G.; Yumiba, S.; Tomiyama, E.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021, 81, 4014–4026. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Dietary Assessment Primer, Learn More about Energy Adjustment. Available online: https://dietassessmentprimer.cancer.gov/ (accessed on 5 February 2022).
- Rhee, J.J.; Cho, E.; Willett, W.C. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014, 17, 1054–1060. [Google Scholar] [CrossRef] [PubMed]

| First Author, (Reference), Year, Country | Study Design (Period of Enrollment) | PCOS Definition | Dietary Assessment Method | Adjusted for Total Energy | Group | n | Mean Daily Fiber Intake (g/d) | SD (g/d) | p Value | Total Energy Intake * (kcal/d) | Age* (Year) | BMI* (kg/m2) | Matched/Adjusted Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altieri P [38], 2013, Italy | Case–control (2005–2010) | Rotterdam | 7-day food diary | No | overweight/obese PCOS | 100 | 19.30 | 5.00 | 0.025 | 2220.00 ± 457.00 | 27.7 ± 5.2 | 34.7 ± 5.5 | Age, BMI |
| overweight/obese controls | 100 | 18.20 | 5.30 | 2223.00 ± 405.00 | 28.4 ± 5.8 | 34.8 ± 5.4 | |||||||
| Álvarez-Blasco F [39], 2011, Spain | Case–control (2002–2005) | AES | FFQ/ not stated | No | overweight/obese PCOS | 22 | 23.00 | 11.00 | 0.361 | 2374.00 ± 681.00 | 26.3 ±7.6 | 35.2 ± 6.7 | Age |
| overweight/obese controls | 59 | 22.00 | 7.00 | 2368.00 ± 702.00 | 32.2 ± 7.5 | 34.8 ± 6.1 | |||||||
| Barrea L [48], 2019, Italy | Cross-sectional (2014–2019) | Rotterdam | 7-day food record | No | PCOS | 112 | 15.43 | 3.66 | 0.001 | 2245.31 ± 290.75 | 24.21 ± 5.47 | 30.95 ± 5.66 | Age, BMI |
| controls | 112 | 17.22 | 4.19 | 2254.84 ± 272.37 | 24.07 ± 5.05 | 30.76 ± 5.60 | |||||||
| Cunha NBD, [40], 2019, Brazil | Case–control (2015–2017) | Rotterdam | 7-day food report | No | all PCOS | 39 | 11.50 | 5.38 | 0.580 | 1651.42 (1184.19–1949.22) | 25.17 ± 3.86 | 24.43 (20.90–33.84) | Age, BMI |
| all controls | 34 | 12.65 | 7.46 | 1487.88 (1240.79–1903.91) | 25.67 ± 4.42 | 23.95 (21.62–31.01) | |||||||
| lean PCOS | 20 | 15.31 | 11.29 | NA | 1683.64 (1415.49–2156.04) | (18–35) | NA | ||||||
| lean controls | 19 | 12.48 | 5.96 | 1704.98 (1120.49–2120.01) | NA | ||||||||
| overweight/obese PCOS | 19 | 9.50 | 3.18 | NA | 1479.12 (1030.53–1922.42) | NA | |||||||
| overweight/obese controls | 15 | 12.87 | 8.68 | 1372.38 (1258.75–1665.95) | NA | ||||||||
| Cutler DA [49], 2019, Canada | Cohort (2014–2016) | Rotterdam | 3-day food record | No | PCOS | 87 | 19.80 | 6.03 | 0.01 | 1783.00 (1516.00–1966.00) | 30.7 ± 4.6 | 29.0 ± 7.1 | Unmatch |
| Yes | PCOS | 87 | 19.77 | 6.26 | |||||||||
| No | controls | 50 | 24.83 | 9.46 | 1815.00 (1578.00–2083.00) | 35.7 ± 5.2 | 24.1 ± 5.1 | ||||||
| Yes | controls | 50 | 25.03 | 8.40 | |||||||||
| Douglas CC [50], 2006, USA | Cohort (not specified) | NIH (1990) | 4-day food record | No | PCOS | 30 | 14.90 | 3.30 | 0.761 | 1781.50 ± 444.80 | 28.9 ± 6.3 | 29.7 ± 4.8 | Age, race, BMI |
| controls a | 27 | 15.40 | 6.80 | 1783.90 ± 379.30 | 28.9 ± 6.5 | 29.1 ± 4.8 | |||||||
| Eslamian G [41], 2017, Iran | Case–control (2012–2014) | Rotterdam | FFQ/stated | Yes | PCOS | 281 | 12.00 | 5.30 | 0.001 | 3215.00 ± 721.00 | 28.8 ± 7.6 | 31.2 ± 7.5 | age |
| controls | 472 | 29.50 | 4.90 | 2489.00 ± 561.00 | 29.4 ± 7.5 | 25.9 ± 3.8 | |||||||
| Liang Z [42], 2021, China | Case–control (not stated) | Rotterdam | 24-h food recall | No | all PCOS | 20 | 8.99 | 2.10 | 0.05 | 1578.75 ± 334.98 | 26.54 ± 5.17 | 23.90 ± 4.41 | Age, BMI |
| all controls | 20 | 11.43 | 4.28 | 1780.00 ± 379.44 | 27.60 ± 5.06 | 23.24 ± 3.69 | |||||||
| lean PCOS | 10 | 9.04 | 2.43 | NS | 1568.80 ± 351.01 | 24.13 ± 2.45 | 20.46 ± 1.58 | ||||||
| lean controls | 10 | 10.78 | 4.27 | 1728.50 ± 417.00 | 25.08 ± 3.59 | 20.43 ± 1.19 | |||||||
| overweight/obese PCOS | 10 | 8.94 | 1.84 | 0.05 | 1588.70 ± 336.84 | 28.94 ± 6.13 | 27.34 ± 3.51 | ||||||
| overweight/obese controls | 10 | 12.08 | 4.42 | 1831.50 ± 352.37 | 30.12 ± 5.20 | 26.05 ± 3.13 | |||||||
| Lin AW, [43] 2019, USA | Case–control (2013–2018) | Rotterdam | FFQ | No | PCOS | 80 | 24.00 | 8.99 | 0.49 | 2218.00 (2017.00–2419.00) | 26.8 (25.4–28.1) | 31.5 (29.5–33.4) | Unmatch |
| controls | 44 | 25.00 | 9.87 | 2180.00 (1866.00–2494.00) | 29.5 (27.5–31.4) | 28.0 (26.1–29.8) | |||||||
| Melekoglu E [44], 2020, Turkey | Case–control (2013–2013) | Rotterdam | 3-day food record | No | PCOS | 65 | 20.70 | 7.70 | 0.001 | 1732.70 ± 474.00 | 26.45 ± 7.42 | 29.7 ± 9.13 | age |
| controls | 65 | 25.80 | 9.70 | 1854.40 ± 452.80 | 26.52 ± 8.90 | 22.6 ± 6.60 | |||||||
| Mizgier M [45], 2021, Poland | Case–control (not stated) | Rotterdam | 3-day food record | No | PCOS | 61 | 15.53 | 6.91 | 0.069 | 1663.50 (1444.70–1788.40) | 16 (15–17) | NA | Age |
| controls | 35 | 18.27 | 5.93 | 1474.01 (1189.44–1746.39) | 15 (15–17) | NA | |||||||
| Pourghassem Gargari B [46], 2015, Iran | Case–control (2009–2010) | Rotterdam | 3-day food recall and FFQ | No | PCOS | 30 | 6.00 | 1.00 | NS | 1334.90 ± 143.40 | 25.83 ± 4.00 | 25.00 ± 3.61 | BMI |
| controls | 30 | 6.70 | 0.60 | 1716.10 ± 142.07 | 26.06 ± 4.44 | 23.68 ± 3.07 | |||||||
| Sharkesh EZ [47], 2021, Iran | Case–control (2019–2020) | Rotterdam | FFQ | No | PCOS | 203 | 38.01 | 18.21 | 0.001 | 2500.07 ± 696.19 | 28.98 ± 5.43 | 25.74 ±5.44 | Unmatch |
| controls | 291 | 44.73 | 23.47 | 2388.03 ± 657.88 | 30.15 ± 6.21 | 23.65 ±3.90 |
| Subgroup | N | SMD (95% CI) | Test of SMD = 0 | Heterogeneity | Articles Included | ||
|---|---|---|---|---|---|---|---|
| Z | p for Z | I2 (%) | p for I2 | ||||
| Geographic location | |||||||
| Asia | 4 | −0.53 (−0.78, −0.27) | 4.03 | <0.001 | 46 | 0.135 | [42,44,46,47] |
| North America | 3 | −0.31 (−0.71, 0.09) | 1.52 | 0.128 | 65.2 | 0.056 | [43,49,50] |
| Europe | 4 | −0.14 (−0.52, 0.24) | 0.72 | 0.470 | 79.1 | 0.002 | [38,39,45,48] |
| South America | 1 | −0.18 (0.64, 0.28) | 0.76 | 0.447 | - | - | [40] |
| Dietary assessment | |||||||
| Food diary/records | 7 | −0.32 (−0.58, −0.05) | 2.35 | 0.019 | 73.1 | 0.001 | [38,40,44,45,48,49,50] |
| FFQ | 3 | −0.18 (−0.41, 0.05) | 1.51 | 0.131 | 37.7 | 0.201 | [39,43,47] |
| Food recall | 2 | −0.73 (−1.07, −0.39) | 3.83 | <0.001 | 0.0 | 0.768 | [42,46] |
| Study design | |||||||
| Case–control | 9 | −0.28 (−0.50, −0.06) | 2.51 | 0.012 | 68.1 | 0.001 | [38,39,40,42,43,44,45,46,47] |
| Cohort | 2 | −0.42 (−0.98, 0.15) | 1.45 | 0.147 | 69.1 | 0.072 | [49,50] |
| Cross-sectional | 1 | −0.46 (−0.72, −0.19) | 3.36 | 0.001 | - | - | [48] |
| Adult or Adolescent | |||||||
| Adult | 11 | −0.31 (−0.51, −0.12) | 3.17 | 0.002 | 68.5 | 0.000 | [38,39,40,42,43,44,46,47,48,49,50] |
| Adolescent | 1 | −0.42 (−0.84, 0.00) | 1.95 | 0.052 | - | - | [45] |
| PCOS definition | |||||||
| Rotterdam | 10 | −0.37 (−0.57, −0.18) | 3.77 | <0.001 | 68.1 | 0.001 | [38,40,42,43,44,45,46,47,48,49] |
| AES | 1 | 0.12 (−0.37, 0.61) | 0.48 | 0.628 | - | - | [39] |
| NIH | 1 | −0.10 (−0.62, 0.43) | 0.36 | 0.720 | - | - | [50] |
| Adjustment or match for confounders | |||||||
| age | |||||||
| Yes | 7 | −0.25 (−0.50, −0.00) | 1.99 | 0.047 | 67.8 | 0.003 | [38,40,42,44,45,48,50] |
| No | 5 | −0.44 (−0.72, −0.16) | 3.10 | 0.002 | 63.7 | 0.041 | [39,43,46,47,49] |
| BMI | |||||||
| Yes | 6 | −0.31 (−0.65, 0.03) | 1.81 | 0.071 | 75.5 | 0.001 | [38,40,42,46,48,50] |
| No | 6 | −0.35 (−0.55, −0.15) | 3.43 | 0.001 | 51.6 | 0.066 | [39,43,44,45,47,49] |
| Covariates for Meta-Regression | p Values |
|---|---|
| Continent (Asia, North America, Europe, South America) | 0.060 |
| Age group (adult, adolescent) | 0.777 |
| Study design (case–control, cross-sectional, cohort) | 0.498 |
| Individual age match (yes, no) | 0.317 |
| Individual BMI match (yes, no) | 0.817 |
| PCOS definition (Rotterdam, AES, physician-confirmed but criteria not stated) | 0.234 |
| Dietary assessment method (FFQ, food diary/record, food recall) | 0.058 |
| Publication year (2000s, 2010s, 2020s) | 0.221 |
| Country (Italy, Spain, Brazil, Canada, USA, Iran, China, Turkey, Poland, Australia) | 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, W.T.; Tang, Z.; Feng, Y.; Guan, H.; Huang, Z.; Zhang, W. Lower Fiber Consumption in Women with Polycystic Ovary Syndrome: A Meta-Analysis of Observational Studies. Nutrients 2022, 14, 5285. https://doi.org/10.3390/nu14245285
Leung WT, Tang Z, Feng Y, Guan H, Huang Z, Zhang W. Lower Fiber Consumption in Women with Polycystic Ovary Syndrome: A Meta-Analysis of Observational Studies. Nutrients. 2022; 14(24):5285. https://doi.org/10.3390/nu14245285
Chicago/Turabian StyleLeung, Wing Ting, Zhijing Tang, Yuanyuan Feng, Haiyun Guan, Zengshu Huang, and Wei Zhang. 2022. "Lower Fiber Consumption in Women with Polycystic Ovary Syndrome: A Meta-Analysis of Observational Studies" Nutrients 14, no. 24: 5285. https://doi.org/10.3390/nu14245285
APA StyleLeung, W. T., Tang, Z., Feng, Y., Guan, H., Huang, Z., & Zhang, W. (2022). Lower Fiber Consumption in Women with Polycystic Ovary Syndrome: A Meta-Analysis of Observational Studies. Nutrients, 14(24), 5285. https://doi.org/10.3390/nu14245285





