Assessment of Dietary Adequacy and Quality in a Sample of Patients with Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
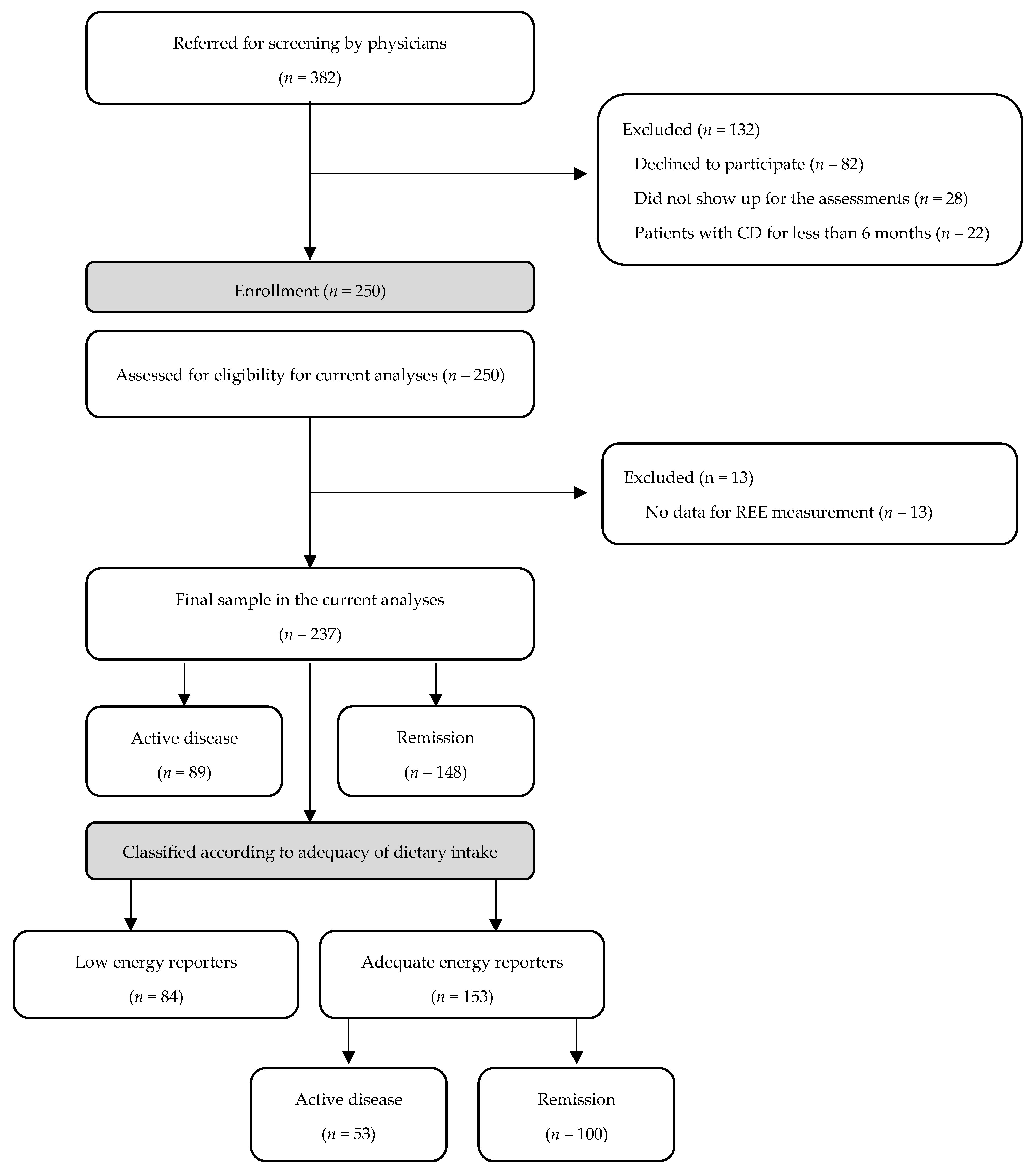
2.1. Study Sample
2.2. Medical Assessment
2.3. Anthropometry, Body Composition and Assessment of Malnutrition and Sarcopenia
2.4. Energy Expenditure
2.5. Dietary Intake Assessment
2.6. Statistical Analysis
3. Results
3.1. Descriptive Characteristics and Differences between Low and Adequate Energy Reporters
3.2. Energy and Macronutrients’ Intake
3.3. Food Groups Consumption and Adherence to Mediterranean Diet and European Dietary Guidelines for CVD Prevention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, C.G.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.L.; Limketkai, B.; Medici, V.; Mendoza, M.S.; Palmer, L.; Bechtold, M. Nutritional Strategies in the Management of Adult Patients with Inflammatory Bowel Disease: Dietary Considerations from Active Disease to Disease Remission. Curr. Gastroenterol. Rep. 2016, 18, 55. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Waish, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.N.; Langham, S.; Severac, H.C.; Levesque, B.G. The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: A systematic review. Dig. Dis. Sci. 2015, 60, 299–312. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M. Symposium on ‘The challenge of translating nutrition research into public health nutrition’. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’. Nutrition in Crohn’s disease. Proc. Nutr. Soc. 2009, 68, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N. Clinical nutrition: 6. Management of nutritional problems of patients with Crohn’s disease. CMAJ 2002, 166, 913–918. [Google Scholar]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Kalla, R.; Ventham, N.T.; Satsangi, J.; Arnot, I.D.R. Crohn’s disease. BMJ 2014, 349, g6670. [Google Scholar] [CrossRef] [PubMed]
- Sandall, M.A.; Wall, C.L.; Lomer, M.C.E. Nutrition Assessment in Crohn’s Disease using Anthropometric, Biochemical, and Dietary Indexes: A Narrative Review. J. Acad. Nutr. Diet. 2019, 120, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Gibson, P.R. Dietary management of IBD—Insights and advice. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 133–146. [Google Scholar] [CrossRef]
- De Vries, J.H.M.; Dijkhuizen, M.j; Tap, P.; Witteman, B.J.M. Patient’s Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, A.; Anastasiou, C.A.; Bletsa, M.; Mantzaris, G.J.; Archavlis, E.; Karampekos, G.; Tzouvala, M.; Zacharopoulou, E.; Veimou, C.; Bamias, G.; et al. Poor performance of predictive equations to estimate resting energy expenditure in patients with Crohn’s disease. Br. J. Nutr. 2022, 1–31. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. SA), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Colombel, J.F.; D’haens, G.; Lee, W.-J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2020, 14, 254–266. [Google Scholar] [CrossRef]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Glynn, C.C.; Greene, G.W.; Winkler, M.F.; Albina, J.E. Predictive versus measured energy expenditure using limits-of-agreement analysis in hospitalized, obese patients. JPEN J. Parenter. Enteral Nutr. 1999, 23, 147–154. [Google Scholar] [CrossRef]
- Alves, V.G.; da Rocha, E.E.; Gonzalez, M.C.; da Fonseca, R.B.; Silva, M.H.; Chiesa, C.A. Assessement of resting energy expenditure of obese patients: Comparison of indirect calorimetry with formulae. Clin. Nutr. 2009, 28, 299–304. [Google Scholar] [CrossRef]
- Wright, M.; Jones, C. Clinical Practice Guidelines: Nutrition in CKD, 5th ed.; UK Renal Association: Bristol, UK, 2010. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. JPEN J. Parenter. Enteral Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Harris, J.A.; Benedict, F.G. Biometric Studies of Basal Metabolism in Man; Publication No. 279; Carnegie Institution: Washington, DC, USA, 1919. [Google Scholar]
- Kavouras, S.A.; Maraki, M.; Kollia, M.; Gioxari, A.; Jansen, L.T.; Sidossis, L.S. Development, reliability and validity of a physical activity questionnaire for estimating energy expenditure in Greek adults. Sci. Sport. 2016, 31, e47–e53. [Google Scholar]
- Kollia, M.; Gioxari, A.; Maraki, M.; Kavouras, S.A.A. Development, validity and reliability of the Harokopio Physical Activity Questionnaire in Greek adults. In Proceedings of the 8th Panhellenic Congress on Nutrition and Dietetics, Athens, Greece, 9–11 December 2005; Beta Medical Publishing: Athens, Greece, 2006. [Google Scholar]
- Moshfegh, A.J.L.; Borrud, B.P.; LaComb, R. Improved method for the 24-hour dietary recall for use in national surveys. FASEB J. 1999, 13, A603. [Google Scholar]
- Trichopoulou, A. Composition Tables of Foods and Greek Dishes, 3rd ed.; Parisianou Publications: Athens, Greece, 2004. [Google Scholar]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr 1991, 45, 569–581. [Google Scholar]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary reference values for nutrients: Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stafanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Dietary guidelines for adults in Greece. Arch. Hellenic Med. 1999, 16, 516–524. [Google Scholar]
- Karfopoulou, E. Dietary Behaviors in Weight Loss Maintenance; Harokopio University: Athens, Greece, 2016. [Google Scholar]
- Tselefa, V. Dietary Behaviors and Early Markers of Vascular Dysfunction; Harokopio University: Athens, Greece, 2018. [Google Scholar]
- Greek National Dietary Guidelines for Adults. 2014. Available online: http://diatrofikoiodigoi.gr/?page=summaryadults (accessed on 18 March 2022).
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; 916, i–viii, 1–149, backcover. [Google Scholar]
- Massironi, S.; Rossi, R.E.; Cavalcoli, F.A.; Valle, S.D.; Fraquelli, M.; Conte, D. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin. Nutr. 2013, 32, 904–910. [Google Scholar] [CrossRef]
- Steed, H.; Walsh, S.; Reynolds, N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts 2009, 2, 370–372. [Google Scholar] [CrossRef]
- Sousa Guerreiro, C.; Cravo, M.; Raimundo Costa, A.; Miranda, A.; Tavares, L.; Moura-Santos, P.; MarquesVidal, P.; Nobre Leitao, C. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Am. J. Gastroenterol. 2007, 102, 2551–2556. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development; European Union. Health at a Glance: Europe 2020—State of Health in the EU Cycle; Organisation for Economic Co-operation and Development: Paris, France, 2020. [Google Scholar]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef]
- Aghdassi, E.; Wendland, B.E.; Stapleton, M.; Raman, M.; Allard, J.P. Adequacy of nutritional intake in a Canadian population of patients with Crohn’s disease. J. Am. Diet. Assoc. 2007, 107, 1575–1580. [Google Scholar] [CrossRef]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Pagano, M.C.; Santarpia, L.; Pellegrini, L.; Testa, A.; Marra, M.; Contaldo, F.; Castiglione, F.; et al. Evaluation of nutritional adequacy in adult patients with Crohn’s disease: A cross-sectional study. Eur. J. Nutr. 2020, 59, 3647–3658. [Google Scholar] [CrossRef]
- Principi, M.; Losurdo, G.; Iannone, A.; Contaldo, A.; Deflorio, V.; Ranaldo, N.; Pisani, A.; Ierardi, E.; Di Leo, A.; Barone, M. Differences in dietary habits between patients with inflammatory bowel disease in clinical remission and a healthy population. Ann. Gastroenterol. 2018, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Starz, E.; Wzorek, K.; Folwarski, M.; Kazmierczak-Siedlecka, K.; Stachowska, L.; Przewlocka, K.; Stachowska, E.; Skonieczna-Zydecka, K. The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease. Nutrients 2021, 13, 2125. [Google Scholar] [CrossRef]
- Jakobsen, L.M.A.; Sundekilde, U.K.; Andersen, H.J.; Nielsen, D.S.; Bertram, H.C. Lactose and Bovine Milk Oligosaccharides Synergistically Stimulate B. longum subsp. longum Growth in a Simplified Model of the Infant Gut Microbiome. J. Proteome Res. 2019, 18, 3086–3098. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Ronveaux, C.C.; Rust, B.M.; Newman, J.W.; Hawley, M.; Barile, D.; Mills, D.A.; Raybould, H.E. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am. J. Physiol. Gastrointest Liver Physiol. 2017, 312, G474–G487. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; von Martels, J.H.Z.; Gabriëls, R.Y.; Blokzijl, T.; Buist-Homan, M.; Heegsma, J.; Jansen, B.H.; van Dullemen, H.M.; Festen, E.A.M.; Ter Steege, R.W.F.; et al. A Combined Set of Four Serum Inflammatory Biomarkers Reliably Predicts Endoscopic Disease Activity in Inflammatory Bowel Disease. Front. Med. 2019, 6, 251. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–196. [Google Scholar] [CrossRef]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. MBio 2020, 11, e02179-19. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, D.H.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Magriplis, E.; Panagiotakos, D.; Mitsopoulou, A.-V.; Karageorgou, D.; Bakogianni, I.; Dimakopoulos, I.; Micha, R.; Michas, G.; Chourdakis, M.; Chrousos, G.P.; et al. Prevalence of hyperlipidaemia in adults and its relation to the Mediterranean diet: The Hellenic National Nutrition and Health Survey (HNNHS). Eur. J. Prev. Cardiol. 2019, 26, 1957–1967. [Google Scholar] [CrossRef]
- Marsh, A.; Radford-Smith, G.; Banks, M.; Lord, A.; Chachay, V. Dietary intake of patients with inflammatory bowel disease aligns poorly with traditional Mediterranean diet principles. Nutr. Diet. 2022, 79, 229–237. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vidra, N.; Farmaki, A.-E.; Koinaki, S.; Belogianni, K.; Sofrona, S.; Magkanari, F.; Yannakoulia, M. Adherence rates to the Mediterranean diet are low in a representative sample of Greek children and adolescents. J. Nutr. 2008, 138, 1951–1956. [Google Scholar] [CrossRef]
- Georgoulis, M.; Georgousopoulou, E.N.; Chrysohoou, C.; Pitsavos, C.; Panagiotakos, D.B. Longitudinal Trends, Determinants, and Cardiometabolic Impact of Adherence to the Mediterranean Diet among Greek Adults. Foods 2022, 11, 2389. [Google Scholar] [CrossRef]
- Moss, A.C. The meaning of low-grade inflammation in clinically quiescent inflammatory bowel disease. Curr. Opin. Gastroenterol. 2014, 30, 365–369. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (n = 237) | Active Disease (n = 89) | Remission (n = 148) | p | |
|---|---|---|---|---|
| Age (years) | 41.3 ± 14.1 | 42.2 ± 14.7 | 40.8 ± 13.7 | 0.52 |
| Sex | 0.01 | |||
| Male | 130 (54.9) | 55 (61.8) | 75 (50.7) | |
| BMI (kg/m2) | 27.3 ± 6.0 | 27.6 ± 6.4 | 27.1 ± 5.8 | 0.84 |
| BMI categories (n, %) | 0.80 | |||
| <18.5 kg/m2 | 7 (3.0) | 2 (2.2) | 5 (3.3) | |
| 18.5–24.9 kg/m2 | 84 (35.4) | 34 (38.2) | 50 (33.8) | |
| 25.0–29.9 kg/m2 | 82 (34.6) | 28 (31.5) | 54 (36.5) | |
| ≥30.0 kg/m2 | 64 (27.0) | 25 (28.1) | 39 (26.4) | |
| Educational level (n, %) | 0.19 | |||
| <6 years | 23 (9.7) | 11 (12.4) | 12 (8.1) | |
| 6–12 years | 94 (39.7) | 39 (43.8) | 55 (37.2) | |
| >12 years | 120 (50.6) | 39 (43.8) | 81 (54.7) | |
| Work status (n, %) | 0.88 | |||
| Full time job | 150 (63.3) | 56 (62.9) | 94 (63.5) | |
| Part time job | 21 (8.9) | 7 (7.9) | 14 (9.5) | |
| Unemployment | 66 (27.8) | 26 (29.2) | 40 (27.0) | |
| Family status (n, %) | 0.28 | |||
| Married | 115 (48.5) | 42 (47.2) | 73 (49.4) | |
| Unmarried | 106 (44.7) | 38 (42.7) | 68 (45.9) | |
| Widowed/divorced | 16 (6.8) | 9 (10.1) | 7 (4.7) | |
| Disease duration (years) | 7.0 (3.0–13.2) | 6.0 (2.0, 14.0) | 8.0 (3.0, 13.0) | 0.24 |
| Age at diagnosis (n, %) | 0.02 | |||
| <16 years, A1 | 22 (9.3) | 2 (2.2) | 20 (13.5) | |
| 17–40 years, A2 | 151 (63.7) | 62 (69.7) | 89 (60.1) | |
| >40 years, A3 | 64 (27.0) | 25 (28.1) | 39 (26.4) | |
| Disease location (n, %) | 0.10 | |||
| Ileal, L1 | 111 (46.8) | 44 (50.0) | 67 (44.8) | |
| Colonic, L2 | 23 (9.9) | 4 (4.5) | 19 (13.1) | |
| Ileocolonic, L3 | 103 (43.3) | 41 (45.5) | 62 (42.1) | |
| Isolated upper GI disease, L4 (n, %) | 27 (11.4) | 11 (12.4) | 16 (10.8) | 0.72 |
| Disease behavior (n, %) | 0.28 | |||
| Non-stricturing, non-penetrating, B1 | 134 (56.6) | 46 (51.7) | 88 (59.2) | |
| Stricturing, B2 | 71 (30.0) | 32 (36.0) | 39 (26.5) | |
| Penetrating, B3 | 22 (9.2) | 6 (6.7) | 16 (10.9) | |
| B2 + B3 | 10 (4.2) | 5 (5.6) | 5 (3.4) | |
| Perianal disease, p (n, %) | 33 (14.0) | 12 (13.5) | 21 (14.3) | 0.86 |
| HBI | 1.0 (1.0–4.0) | 4.0 (2.0, 6.0) | 1.0 (0.0, 2.0) | <0.001 |
| Presence of malnutrition (n, %) | 27 (11.4) | 20 (23.5) | 7 (4.8) | <0.001 |
| Presence of sarcopenia (n, %) | 5 (2.2) | 3 (3.5) | 2 (1.4) | 0.29 |
| Guidelines | Total Sample (n = 237, 100%) | Active Disease (n = 89) | Remission (n = 148) | p * | Adequate Energy Reporters (AERs) and Not following a Weight Loss Diet (Ν = 153, 64.6%) | Active Disease (n = 53) | Remission (n = 100) | p ** | |
|---|---|---|---|---|---|---|---|---|---|
| Total energy intake (TEI) (kcal/day) | 1933 (1556, 2559) | 1921 (1377, 2437) | 1966 (1590, 2596) | 0.27 | 2295 (1879, 2899) | 2369 (1920, 2983) | 2280 (1871, 2884) | 0.79 | |
| Per kg actual BW (kcal/kg/day) | 26.2 (19.2, 34.7) | 24.4 (18.2, 32.6) | 26.9 (20.0, 35.7) | 0.13 | 31.6 (25.8, 39.1) | 30.2 (24.3, 39.3) | 31.6 (26.5, 38.7) | 0.54 | |
| Per kg desired BW (kcal/kg/day) | 28.2 (22.8, 37.4) | 27.0 (21.7, 37.7) | 28.7 (23.9, 37.1) | 0.17 | 34.2 (28.0, 41.6) | 33.2 (27.9, 41.4) | 34.3 (28.2, 41.6) | 0.97 | |
| Protein intake (% TEI) | 18.2 ± 4.6 | 19.1 ± 5.0 | 17.6 ± 4.3 | 0.01 | 17.5 ± 4.0 | 17.8 ± 3.8 | 17.3 ± 4.1 | 0.47 | |
| Animal protein (%) | 65.3 | 67.4 | 64.0 | 0.02 | 64.1 | 64.9 | 63.8 | 0.37 | |
| Plant protein (%) | 34.7 | 32.6 | 36.0 | 0.63 | 35.9 | 35.1 | 36.2 | 0.85 | |
| Per kg actual BW (g/kg/day) | ESPEN: Active disease: 1.2–1.5 g/kg BW Remission: 1 g/kg BW | 1.14 (0.85, 1.46) | 1.07 (0.83, 1.57) | 1.15 (0.91, 1.44) | 0.63 | 1.36 (1.05, 1.78) | 1.30 (0.96, 1.94) | 1.37 (1.09, 1.68) | 0.89 |
| Per kg desired BW (g/kg/day) | 1.26 (1.03, 1.63) | 1.22 (1.01, 1.76) | 1.28 (1.04, 1.60) | 0.91 | 1.44 (1.17, 1.85) | 1.52 (1.10, 1.94) | 1.43 (1.18, 1.76) | 0.53 | |
| Lower than recommended (n, %) | 96 (40.5) | 56 (62.9) | 40 (27.1) | <0.001 | 39 (25.5) | 25 (47.1) | 14 (14.0) | <0.001 | |
| Carbohydrates (% TEI) | EFSA: 45–60% | 41.2 ± 8.5 | 39.5 ± 8.6 | 42.3 ± 8.3 | 0.01 | 41.4 ± 8.7 | 39.4 ± 8.0 | 42.3 ± 8.9 | 0.05 |
| Sugars (%TEI) | EFSA: As low as possible | 12.8 (9.2, 16.3) | 12.0 (8.9, 15.6) | 13.1 (9.3, 16.4) | 0.29 | 11.7 (8.8, 15.5) | 10.7 (7.5, 15.0) | 12.6 (9.1, 15.8) | 0.11 |
| Dietary fiber (g) | Active disease, ESPEN: Elimination Remission, general population-EFSA: 25 g/day | 14.1 (10.5, 19.6) | 12.7 (8.4, 15.8) | 15.7 (11.5, 21.2) | <0.001 | 16.1 (12.4, 22.2) | 14.9 (11.7, 17.9) | 17.7 (13.0, 22.8) | 0.01 |
| Fat (% TEI) | EFSA: 20–35% | 39.6 ± 8.0 | 40.1 ± 8.8 | 39.3 ± 7.5 | 0.45 | 40.1 ± 7.7 | 41.6 ± 7.2 | 39.3 ± 7.9 | 0.08 |
| SFA (% TEI) | EFSA: As low as possible | 12.8 (10.6, 14.9) | 13.4 (10.8, 15.0) | 12.7 (10.5, 14.6) | 0.23 | 12.8 (10.6, 14.8) | 13.7 (11.3, 15.0) | 12.7 (10.0, 14.6) | 0.09 |
| MUFA (% TEI) | 17.1 (14.3, 20.7) | 16.7 (14.5, 21.5) | 17.3 (14.3, 20.2) | 0.79 | 17.4 (15.1, 20.8) | 18.2 (15.8, 21.5) | 17.3 (14.2, 20.5) | 0.11 | |
| PUFA (% TEI) | 5.6 (4.6, 7.0) | 5.7 (4.6, 6.9) | 5.5 (4.6, 7.1) | 0.80 | 5.6 (4.8, 7.2) | 5.8 (4.8, 6.9) | 5.6 (4.8, 7.3) | 0.91 | |
| Trans fatty acids (g) | EFSA: As low as possible | 0.16 (0.03, 0.50) | 0.09 (0.03, 0.48) | 0.24 (0.03, 0.50) | 0.30 | 0.28 (0.05, 0.58) | 0.15 (0.04, 0.56) | 0.30 (0.07, 0.59) | 0.42 |
| Greek Dietary Guidelines [38] | Total Sample (n = 237, 100%) | Active Disease (n = 89) | Remission (n = 148) | p * | Adequate Energy Reporters (AERs) and Not following a Weight Loss Diet (Ν = 153, 64.6%) | Active Disease (n = 53) | Remission (n = 100) | p ** | |
|---|---|---|---|---|---|---|---|---|---|
| Full fat dairy (portions/day) | 2 portions/day Preference for low-fat | 1.21 (0.64, 2.00) | 1.14 (0.42, 2.00) | 1.21 (0.64, 2.00) | 0.70 | 1.21 (0.63, 2.00) | 1.28 (0.42, 2.00) | 1.21 (0.64, 2.00) | 0.84 |
| Low fat dairy (portions/day) | 0.00 (0.00, 0.64) | 0.00 (0.00, 0.64) | 0.00 (0.00, 0.64) | 0.94 | 0.00 (0.00, 0.21) | 0.00 (0.00, 0.07) | 0.00 (0.00, 0.64) | 0.17 | |
| Total dairy products (portions/day) | 1.49 (0.89, 2.28) | 1.42 (0.71, 2.56) | 1.60 (0.96, 2.28) | 0.54 | 1.42 (0.85, 2.28) | 1.28 (0.56, 2.56) | 1.57 (0.96, 2.28) | 0.28 | |
| Refined cereals (portions/day) | 5–8 portions/day Preference for non-refined | 4.98 (2.86, 6.41) | 5.03 (2.89, 6.31) | 4.98 (2.86, 6.47) | 0.84 | 5.56 (3.31, 6.68) | 5.61 (3.70, 6.54) | 5.47 (3.05, 6.68) | 0.55 |
| Non refined cereals (portions/day) | 0.14 (0.02, 0.64) | 0.07 (0.00, 0.33) | 0.21 (0.02, 0.70) | 0.11 | 0.12 (0.02, 0.42) | 0.07 (0.00, 0.28) | 0.21 (0.02, 0.61) | 0.01 | |
| Total cereals (portions/day) | 5.33 ± 2.18 | 5.13 ± 2.21 | 5.45 ± 2.16 | 0.29 | 5.60 ± 2.11 | 5.55 ± 1.93 | 5.63 ± 2.21 | 0.83 | |
| Fruits (portions/day) | 3 portions/day | 1.00 (0.35, 1.71) | 0.85 (0.21, 1.35) | 1.06 (0.42, 1.92) | 0.02 | 1.06 (0.42, 1.77) | 0.92 (0.21, 1.35) | 1.31 (0.63, 2.00) | 0.01 |
| Fruits and fruit juice ± (portions/day) | Preference for fruits rather than fruit juice | 1.35 (0.63, 2.49) | 1.20 (0.49, 2.21) | 1.46 (0.70, 2.74) | 0.01 | 1.53 (0.70, 2.56) | 1.38 (0.46, 2.25) | 1.60 (0.84, 2.83) | 0.12 |
| Vegetables (portions/day) | 4 portions/day | 1.00 (0.53, 1.76) | 0.89 (0.35, 1.55) | 1.11 (0.64, 1.86) | 0.02 | 0.96 (0.49, 1.78) | 0.75 (0.35, 1.33) | 1.27 (0.61, 2.00) | 0.01 |
| Total fruits and vegetables (portions/day) | 2.65 (1.45, 4.21) | 2.08 (1.30, 3.76) | 2.83 (1.72, 4.53) | 0.01 | 2.70 (1.46, 4.47) | 2.16 (1.10, 3.72) | 2.89 (1.76, 4.78) | 0.01 | |
| Legumes (portions/week) | 3 portions/week | 1.26 (0.00, 3.71) | 0.00 (0.00, 1.26) | 1.26 (0.00, 3.71) | <0.001 | 1.26 (0.00, 3.71) | 0.00 (0.00, 1.26) | 1.26 (0.00, 3.71) | <0.001 |
| Fish and fisheries (portions/week) | 2–3 portions/week | 1.48 (0.50, 2.94) | 1.48 (0.50, 2.94) | 1.48 (0.50, 2.94) | 0.39 | 1.48 (0.50, 2.94) | 1.48 (0.98, 2.94) | 1.48 (0.50, 2.94) | 0.27 |
| Poultry (portions/week) | 1–2 portions/week | 1.48 (1.48, 4.48) | 1.48 (1.48, 4.48) | 1.48 (1.48, 4.48) | 0.51 | 1.48 (1.48, 4.48) | 1.48 (1.48, 4.48) | 1.48 (1.48, 4.48) | 0.54 |
| Red meat (portions/week) | Up to 1 portion/week | 3.13 (2.16, 4.62) | 3.14 (2.16, 4.56) | 3.39 (2.35, 4.62) | 0.43 | 3.64 (2.35, 4.62) | 3.64 (2.35, 4.62) | 1.30 (3.64, 5.07) | 0.65 |
| Cold cuts (portions/week) | Avoidance | 1.47 (0.49, 2.73) | 1.26 (0.28, 2.73) | 1.96 (0.77, 2.73) | 0.15 | 1.96 (0.49, 3.01) | 1.26 (0.28, 2.52) | 2.24 (0.63, 3.50) | 0.20 |
| Total red meat (portions/week) | 4.20 (2.80, 5.99) | 4.12 (2.66, 5.71) | 4.23 (2.94, 6.22) | 0.39 | 4.62 (3.14, 6.36) | 4.62 (2.94, 5.94) | 4.68 (3.22, 6.50) | 0.62 | |
| Alcohol (portions/day) | Males: 2 portions/day, Females: 1 portion/day | 0.07 (0.00, 0.28) | 0.07 (0.00, 0.21) | 0.07 (0.00, 0.28) | 0.36 | 0.07 (0.00, 0.28) | 0.07 (0.00, 0.21) | 0.07 (0.00, 0.28) | 0.18 |
| Sweets (portions/week) | Avoidance | 4.48 (1.47, 8.40) | 3.92 (1.47, 7.91) | 4.97 (1.96, 8.96) | 0.05 | 5.46 (2.24, 10.22) | 4.97 (1.96, 10.43) | 5.95 (2.45, 9.94) | 0.78 |
| Soft drinks (portions/week) | Avoidance | 0.63 (0.00, 5.88) | 1.26 (0.00, 5.88) | 0.63 (0.00, 5.88) | 0.87 | 0.28 (0.00, 0.84) | 0.28 (0.00, 0.84) | 0.18 (0.00, 0.84) | 0.21 |
| Coffee and tea (portions/day) | 2.00 (0.85, 2.00) | 1.07 (0.71, 2.00) | 2.00 (1.00, 2.00) | 0.11 | 2.00 (0.85, 2.00) | 1.07 (0.71, 2.00) | 2.00 (1.00, 2.00) | 0.39 | |
| Total MedDietScore (0–55) | 28.0 ± 5.5 | 26.8 ± 5.2 | 28.7 ± 5.5 | 0.01 | 28.4 ± 5.5 | 27.0 ± 5.0 | 29.1 ± 5.7 | 0.03 | |
| Total score for adherence to European dietary guidelines for CVD prevention (0–11) | 5.25 ± 1.36 | 4.98 ± 1.26 | 5.42 ± 1.39 | 0.30 | 5.35 ± 1.42 | 4.70 ± 1.29 | 5.52 ± 1.55 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachaliou, A.; Yannakoulia, M.; Bletsa, M.; Mantzaris, G.J.; Archavlis, E.; Karampekos, G.; Tzouvala, M.; Bamias, G.; Kokkotis, G.; Kontogianni, M.D. Assessment of Dietary Adequacy and Quality in a Sample of Patients with Crohn’s Disease. Nutrients 2022, 14, 5254. https://doi.org/10.3390/nu14245254
Karachaliou A, Yannakoulia M, Bletsa M, Mantzaris GJ, Archavlis E, Karampekos G, Tzouvala M, Bamias G, Kokkotis G, Kontogianni MD. Assessment of Dietary Adequacy and Quality in a Sample of Patients with Crohn’s Disease. Nutrients. 2022; 14(24):5254. https://doi.org/10.3390/nu14245254
Chicago/Turabian StyleKarachaliou, Alexandra, Mary Yannakoulia, Maria Bletsa, Gerassimos J. Mantzaris, Emmanuel Archavlis, George Karampekos, Maria Tzouvala, Giorgos Bamias, George Kokkotis, and Meropi D. Kontogianni. 2022. "Assessment of Dietary Adequacy and Quality in a Sample of Patients with Crohn’s Disease" Nutrients 14, no. 24: 5254. https://doi.org/10.3390/nu14245254
APA StyleKarachaliou, A., Yannakoulia, M., Bletsa, M., Mantzaris, G. J., Archavlis, E., Karampekos, G., Tzouvala, M., Bamias, G., Kokkotis, G., & Kontogianni, M. D. (2022). Assessment of Dietary Adequacy and Quality in a Sample of Patients with Crohn’s Disease. Nutrients, 14(24), 5254. https://doi.org/10.3390/nu14245254







