Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
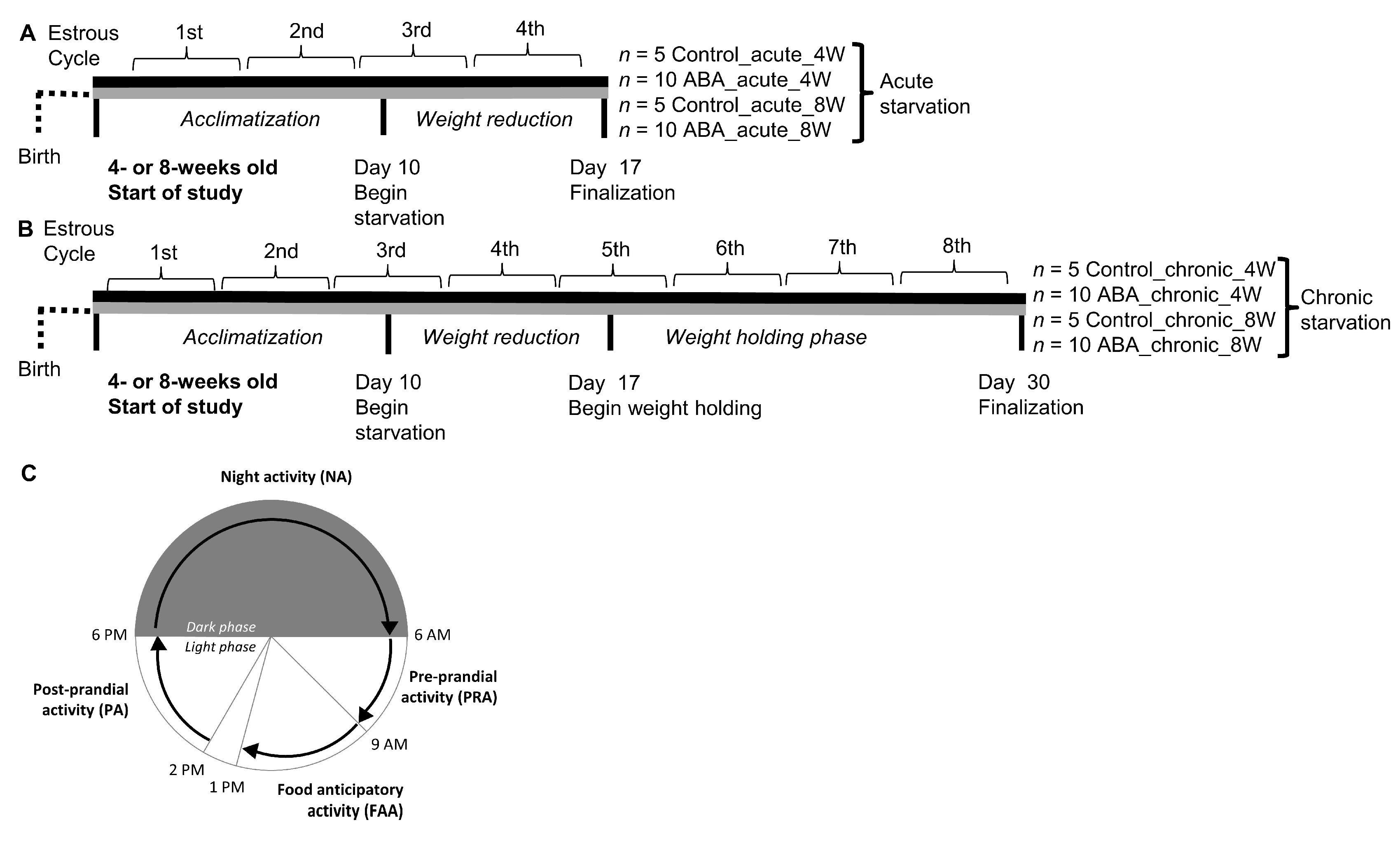
2.2. Study Design
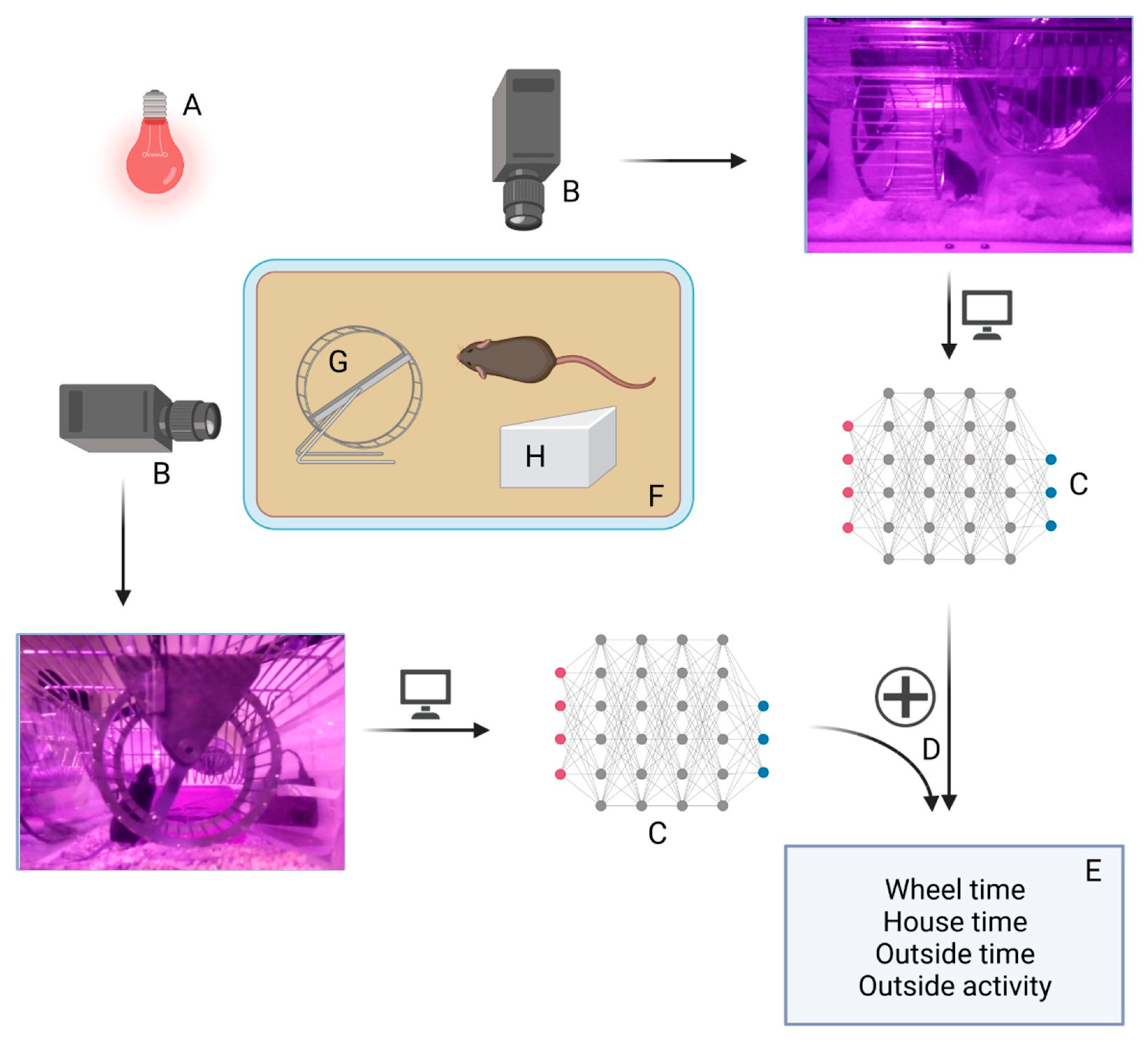
2.3. Locomotor Activity Determination
2.4. Determination of the Estrous Cycle
2.5. Goblotrop System
2.6. Statistics
3. Results
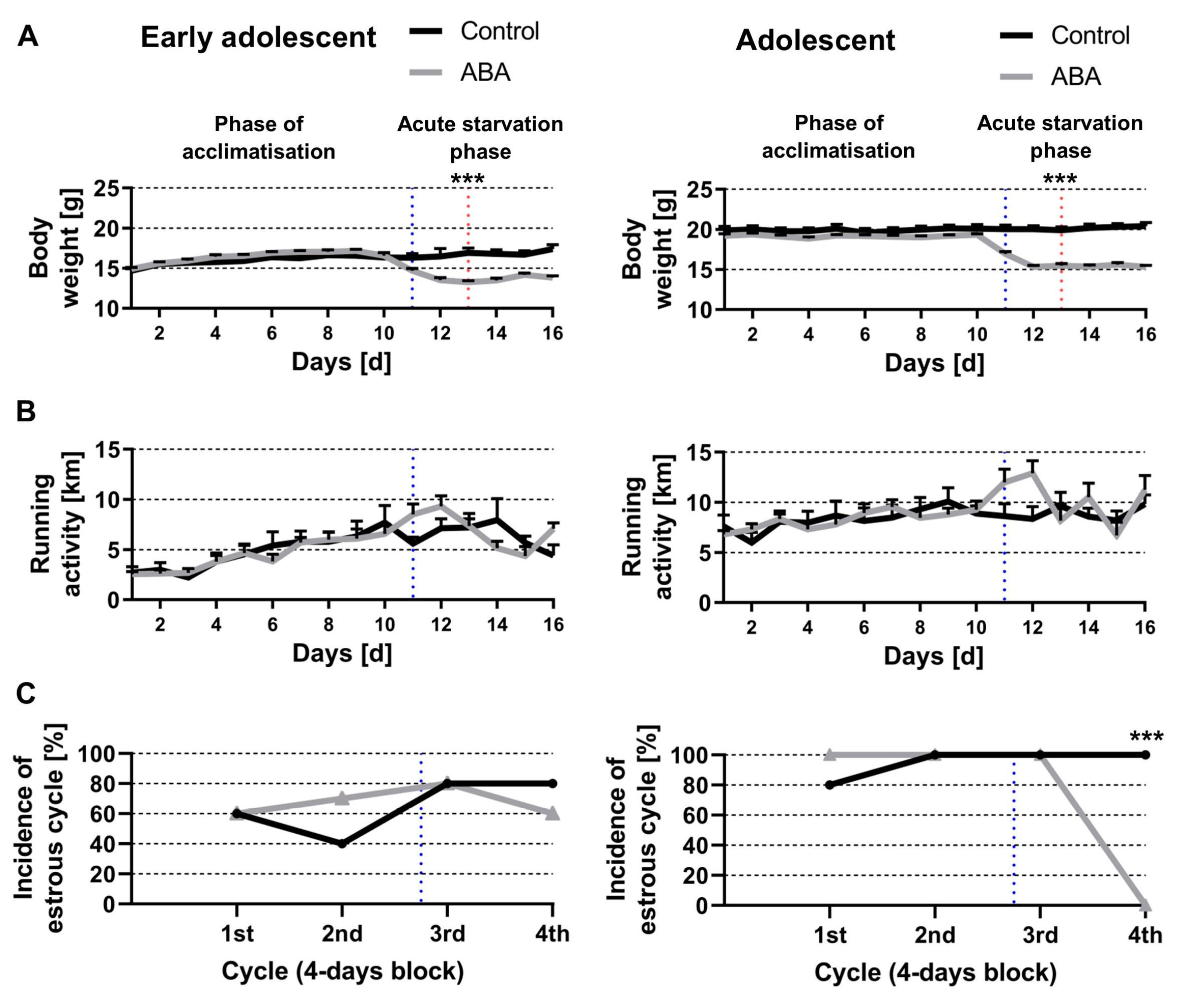
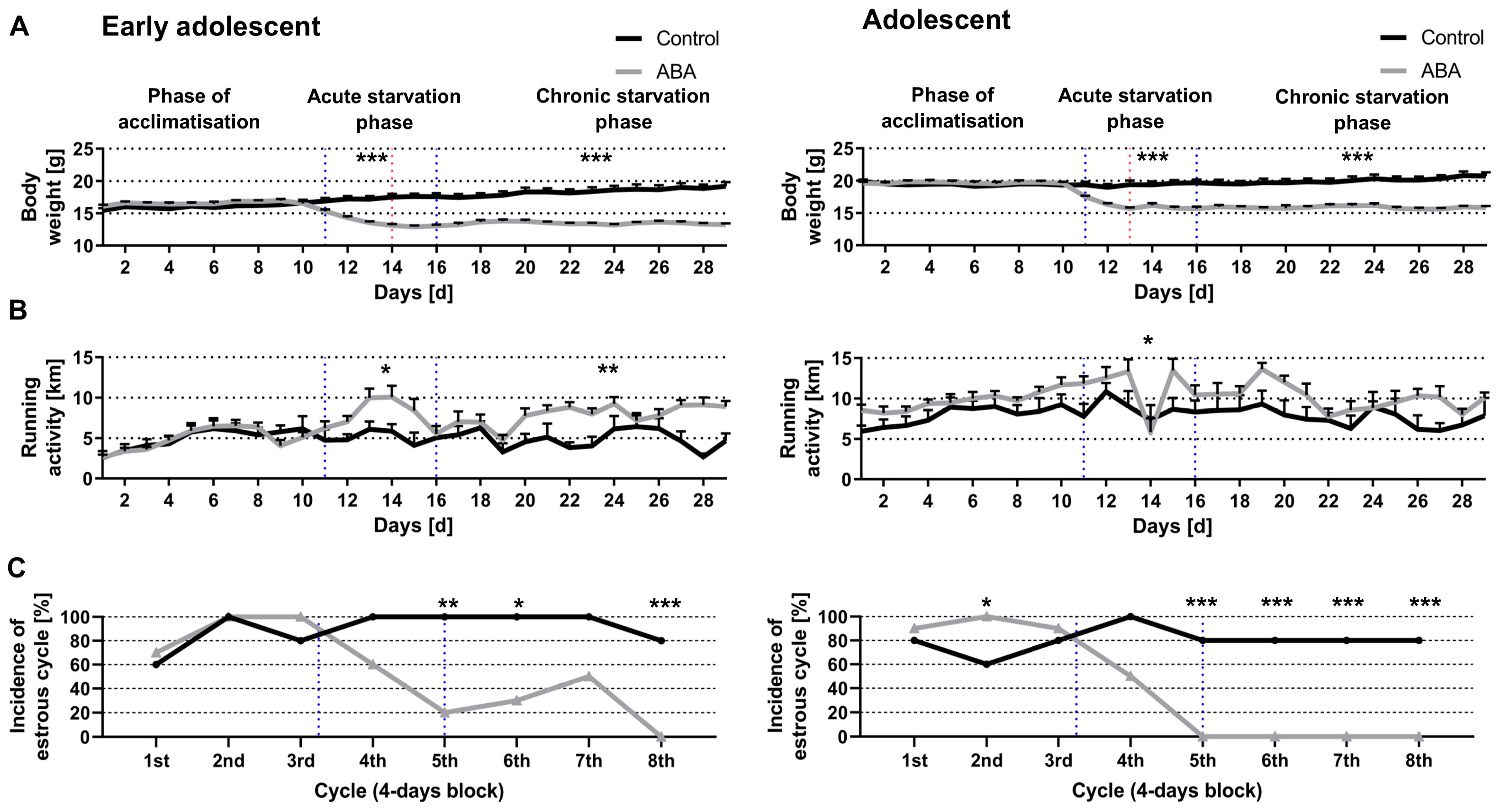
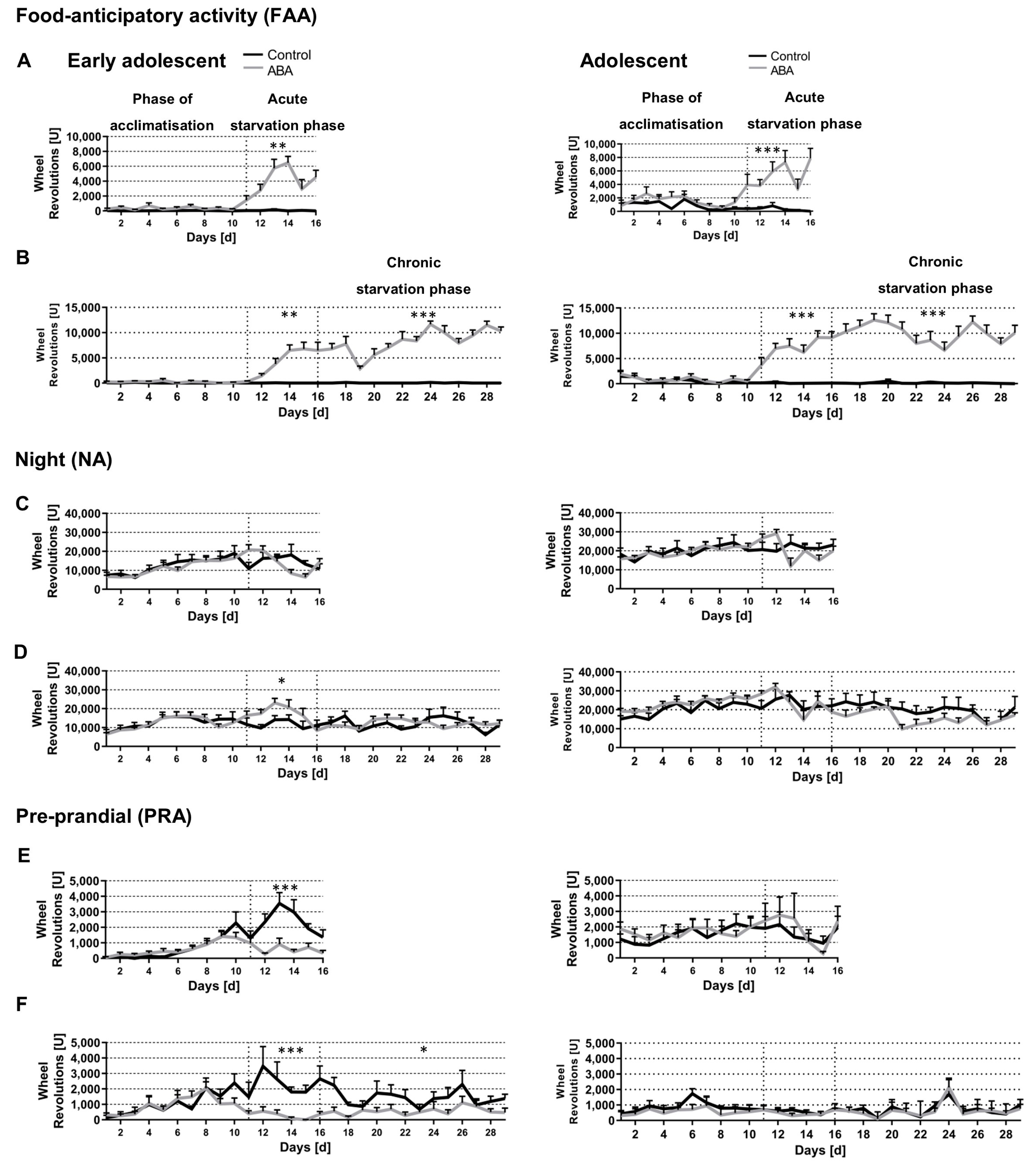
3.1. Chronic Starvation Induced AN-Related Symptoms of Hyperactivity and Amenorrhea
3.2. Acute and Chronic Starvation Induced Food-Anticipatory Activity
3.3. Starvation Decreased Circadian-Rhythm-Related Activity in Adolescent ABA Mice at Night
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herpertz-Dahlmann, B. Adolescent eating disorders: Update on definitions, symptomatology, epidemiology, and comorbidity. Child. Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.C.; Voderholzer, U.; Naab, S.; Schlegl, S. Increased urge for movement, physical and mental restlessness, fundamental symptoms of restricting anorexia nervosa? Brain Behav. 2020, 10, e01556. [Google Scholar] [CrossRef] [PubMed]
- Melissa, R.; Lama, M.; Laurence, K.; Sylvie, B.; Jeanne, D.; Odile, V.; Nathalie, G. Physical Activity in Eating Disorders: A Systematic Review. Nutrients 2020, 12, 183. [Google Scholar] [CrossRef]
- Solenberger, S.E. Exercise and eating disorders: A 3-year inpatient hospital record analysis. Eat. Behav. 2001, 2, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Taranis, L.; Meyer, C. Associations between specific components of compulsive exercise and eating-disordered cognitions and behaviors among young women. Int. J. Eat. Disord. 2011, 44, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Menculini, G.; Brufani, F.; Del Bello, V.; Moretti, P.; Tortorella, A. Circadian rhythms disruptions and eating disorders: Clinical impact and possible psychopathological correlates. Psychiatr. Danub. 2019, 31 (Suppl. 3), 497–502. [Google Scholar]
- Allison, K.C.; Spaeth, A.; Hopkins, C.M. Sleep and Eating Disorders. Curr. Psychiatry Rep. 2016, 18, 92. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Activity Based Anorexia as an Animal Model for Anorexia Nervosa-A Systematic Review. Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef]
- Scharner, S.; Stengel, A. Animal Models for Anorexia Nervosa—A Systematic Review. Front. Hum. Neurosci. 2020, 14, 596381. [Google Scholar] [CrossRef]
- Routtenberg, A.; Kuznesof, A.W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 1967, 64, 414–421. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Paulukat, L.; Kas, M.J.; Tolba, R.; Konrad, K.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J. Establishment of a chronic activity-based anorexia rat model. J. Neurosci. Methods 2018, 293, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Paulukat, L.; Frintrop, L.; Liesbrock, J.; Heussen, N.; Johann, S.; Exner, C.; Martien, K.J.; Tolba, R.; Neulen, J.; Konrad, K.; et al. Memory impairment is associated with the loss of regular oestrous cycle and plasma oestradiol levels in an activity-based anorexia animal model. World J. Biol. Psychiatry 2016, 17, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Adan, R.A.H.; Hillebrand, J.J.G.; Danner, U.N.; Cano, S.C.; Kas, M.J.H.; Verhagen, L.A.W. Neurobiology driving hyperactivity in activity-based anorexia. Curr. Top. Behav. Neurosci. 2011, 6, 229–250. [Google Scholar] [PubMed]
- Mistlberger, R.E. Food-anticipatory circadian rhythms: Concepts and methods. Eur. J. Neurosci. 2009, 30, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J. Comp. Physiol. B 2010, 180, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.W.; Harbour, V.; Barry-Shaw, J.; Diaz, L.R.; Robinson, B.; Stewart, J.; Amir, S. Restricted access to food, but not sucrose, saccharine, or salt, synchronizes the expression of Period2 protein in the limbic forebrain. Neuroscience 2007, 144, 402–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, D.Y.; Brett, R.R. Activity-based anorexia in C57/BL6 mice: Effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. Eur. Neuropsychopharmacol. 2010, 20, 622–631. [Google Scholar] [CrossRef]
- Beeler, J.A.; Mourra, D.; Zanca, R.M.; Kalmbach, A.; Gellman, C.; Klein, B.Y.; Ravenelle, R.; Serrano, P.; Moore, H.; Rayport, S.; et al. Vulnerable and Resilient Phenotypes in a Mouse Model of Anorexia Nervosa. Biol. Psychiatry. 2021, 90, 829–842. [Google Scholar] [CrossRef]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef]
- Silen, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef]
- Singhal, V.; Misra, M.; Klibanski, A. Endocrinology of anorexia nervosa in young people: Recent insights. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 64–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoving, R.K. Mechanisms in Endocrinology: Anorexia nervosa and endocrinology: A clinical update. Eur. J. Endocrinol. 2019, 180, R9–R27. [Google Scholar] [CrossRef]
- Clarke, J.D.; Coleman, G.J. Persistent meal-associated rhythms in SCN-lesioned rats. Physiol. Behav. 1986, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Marchant, E.G.; Mistlberger, R.E. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997, 765, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Honma, S.; Mitome, M.; Honma, K.-I. Independence of feeding-associated circadian rhythm from light conditions and meal intervals in SCN lesioned rats. Neurosci. Lett. 1997, 222, 95–98. [Google Scholar] [CrossRef]
- Wu, H.; Van Kuyck, K.; Tambuyzer, T.; Luyten, L.; Aerts, J.-M.; Nuttin, B. Rethinking food anticipatory activity in the activity-based anorexia rat model. Sci. Rep. 2014, 4, 3929. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Zhang, Z.; Silveyra, E.; Jin, N.; Ribelayga, C.P. A congenic line of the C57BL/6J mouse strain that is proficient in melatonin synthesis. J. Pineal. Res. 2018, 65, e12509. [Google Scholar] [CrossRef]
- Kasahara, T.; Abe, K.; Mekada, K.; Yoshiki, A.; Kato, T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 6412–6417. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Halter, K.A.; Prosser, R.A. Circadian rhythm and sleep-wake systems share the dynamic extracellular synaptic milieu. Neurobiol. Sleep Circadian Rhythm. 2018, 5, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Mong, J.A. Neuroendocrine Control of Sleep. Curr. Top. Behav. Neurosci. 2019, 43, 353–378. [Google Scholar] [PubMed]
- MacDonald, K.J.; Lustig, K.A.; Geniole, S.N.; McCormick, C.M.; Cote, K.A. Sleep restriction alters reactive aggressive behavior and its relationship with sex hormones. Aggress. Behav. 2019, 45, 193–205. [Google Scholar] [CrossRef]
- Truglia, E.; Mannucci, E.; Lassi, S.; Rotella, C.M.; Faravelli, C.; Ricca, V. Aggressiveness, anger and eating disorders: A review. Psychopathology 2006, 39, 55–68. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Leunissen, C.; Kempermann, J.; Etdöger, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; et al. The reduction of astrocytes and brain volume loss in anorexia nervosa-the impact of starvation and refeeding in a rodent model. Transl. Psychiatry 2019, 9, 159. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Seitz, J.; Kipp, M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. J. Clin. Med. 2021, 11, 186. [Google Scholar] [CrossRef]
- Frintrop, L.; Liesbrock, J.; Paulukat, L.; Johann, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; Konrad, K.; Herpertz-Dahlmann, B.; et al. Reduced astrocyte density underlying brain volume reduction in activity-based anorexia rats. World J. Biol. Psychiatry 2018, 19, 225–235. [Google Scholar] [CrossRef]
- Blutstein, T.; Haydon, P.G. The Importance of astrocyte-derived purines in the modulation of sleep. Glia 2013, 61, 129–139. [Google Scholar] [CrossRef]
- Costa, R.; Montagnese, S. The role of astrocytes in generating circadian rhythmicity in health and disease. J. Neurochem. 2021, 157, 42–52. [Google Scholar] [CrossRef]
- Seitz, J.; Bühren, K.; Von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. A meta-analysis and qualitative review. Z. Kinder Jugendpsychiatr. Psychother. 2014, 42, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Spadini, S.; Ferro, M.; Lamanna, J.; Malgaroli, A. Activity-based anorexia animal model: A review of the main neurobiological findings. J. Eat. Disord. 2021, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Mandelblat-Cerf, Y.; Verstegen, A.M. Verstegen, Interacting Neural Processes of Feeding, Hyperactivity, Stress, Reward, and the Utility of the Activity-Based Anorexia Model of Anorexia Nervosa. Harv. Rev. Psychiatry 2016, 24, 416–436. [Google Scholar] [CrossRef] [PubMed]
- Exner, C.; Hebebrand, J.; Remschmidt, H.; Wewetzer, C.; Ziegler, A.; Herpertz, S.; Schweiger, U.; Blum, W.F.; Preibisch, G.; Heldmaier, G.; et al. Leptin suppresses semi-starvation induced hyperactivity in rats: Implications for anorexia nervosa. Mol. Psychiatry 2000, 5, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Milos, G.; Antel, J.; Kaufmann, L.-K.; Barth, N.; Koller, A.; Tan, S.; Wiesing, U.; Hinney, A.; Libuda, L.; Wabitsch, M.; et al. Short-term metreleptin treatment of patients with anorexia nervosa: Rapid on-set of beneficial cognitive, emotional, and behavioral effects. Transl. Psychiatry 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabloffsky, T.; Gill, S.; Staffeld, A.; Salomon, R.; Power Guerra, N.; Joost, S.; Hawlitschka, A.; Kipp, M.; Frintrop, L. Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes. Nutrients 2022, 14, 5252. https://doi.org/10.3390/nu14245252
Gabloffsky T, Gill S, Staffeld A, Salomon R, Power Guerra N, Joost S, Hawlitschka A, Kipp M, Frintrop L. Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes. Nutrients. 2022; 14(24):5252. https://doi.org/10.3390/nu14245252
Chicago/Turabian StyleGabloffsky, Theo, Sadaf Gill, Anna Staffeld, Ralf Salomon, Nicole Power Guerra, Sarah Joost, Alexander Hawlitschka, Markus Kipp, and Linda Frintrop. 2022. "Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes" Nutrients 14, no. 24: 5252. https://doi.org/10.3390/nu14245252
APA StyleGabloffsky, T., Gill, S., Staffeld, A., Salomon, R., Power Guerra, N., Joost, S., Hawlitschka, A., Kipp, M., & Frintrop, L. (2022). Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes. Nutrients, 14(24), 5252. https://doi.org/10.3390/nu14245252






