Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding
Abstract
1. Introduction
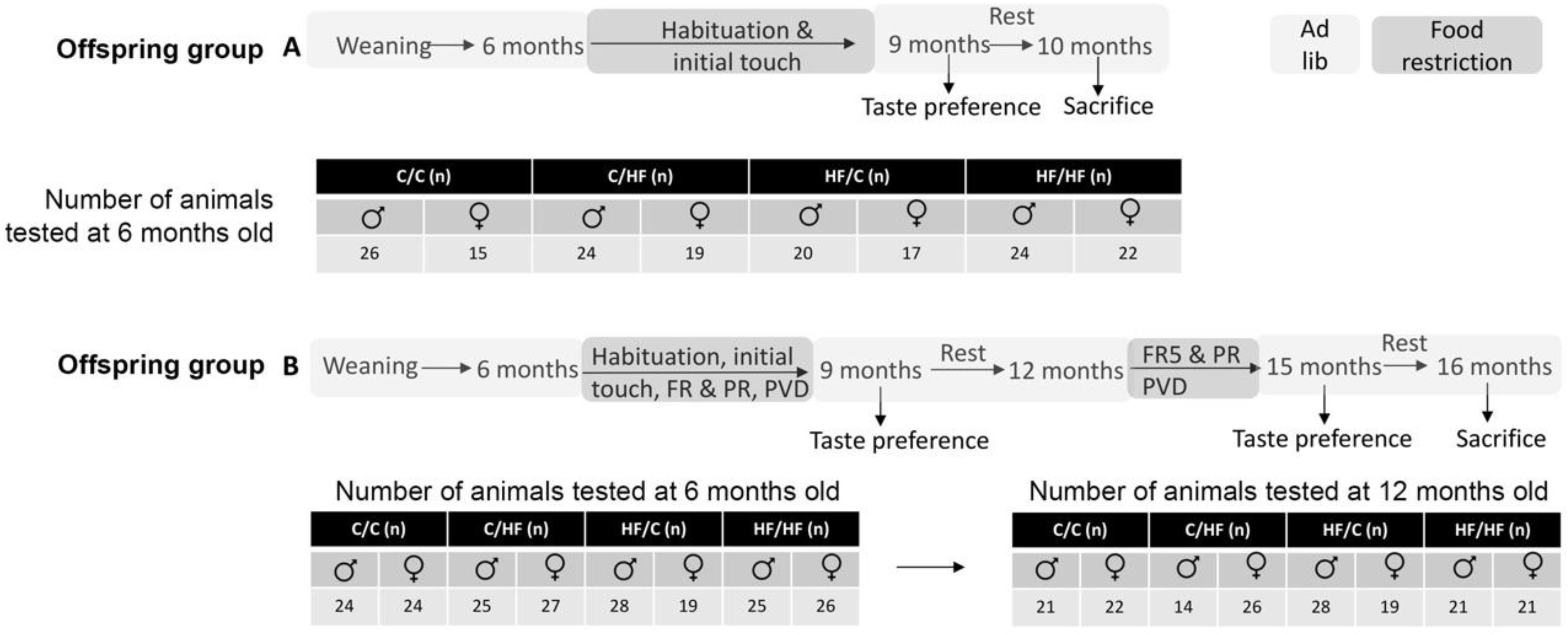
2. Materials and Methods
3. Results
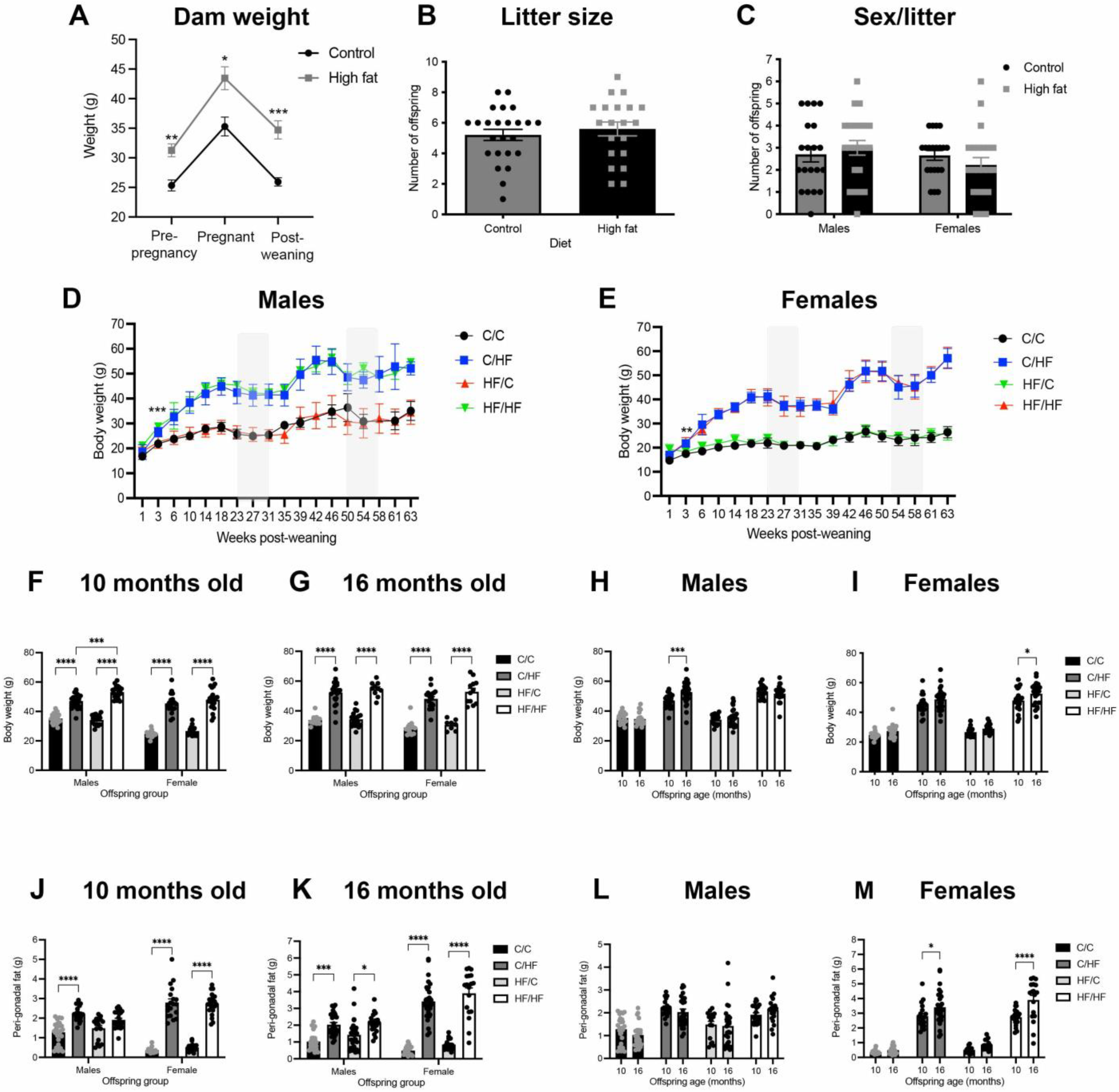
3.1. High Fat Diet Induced an Obese Phenotype in Dams and Offspring
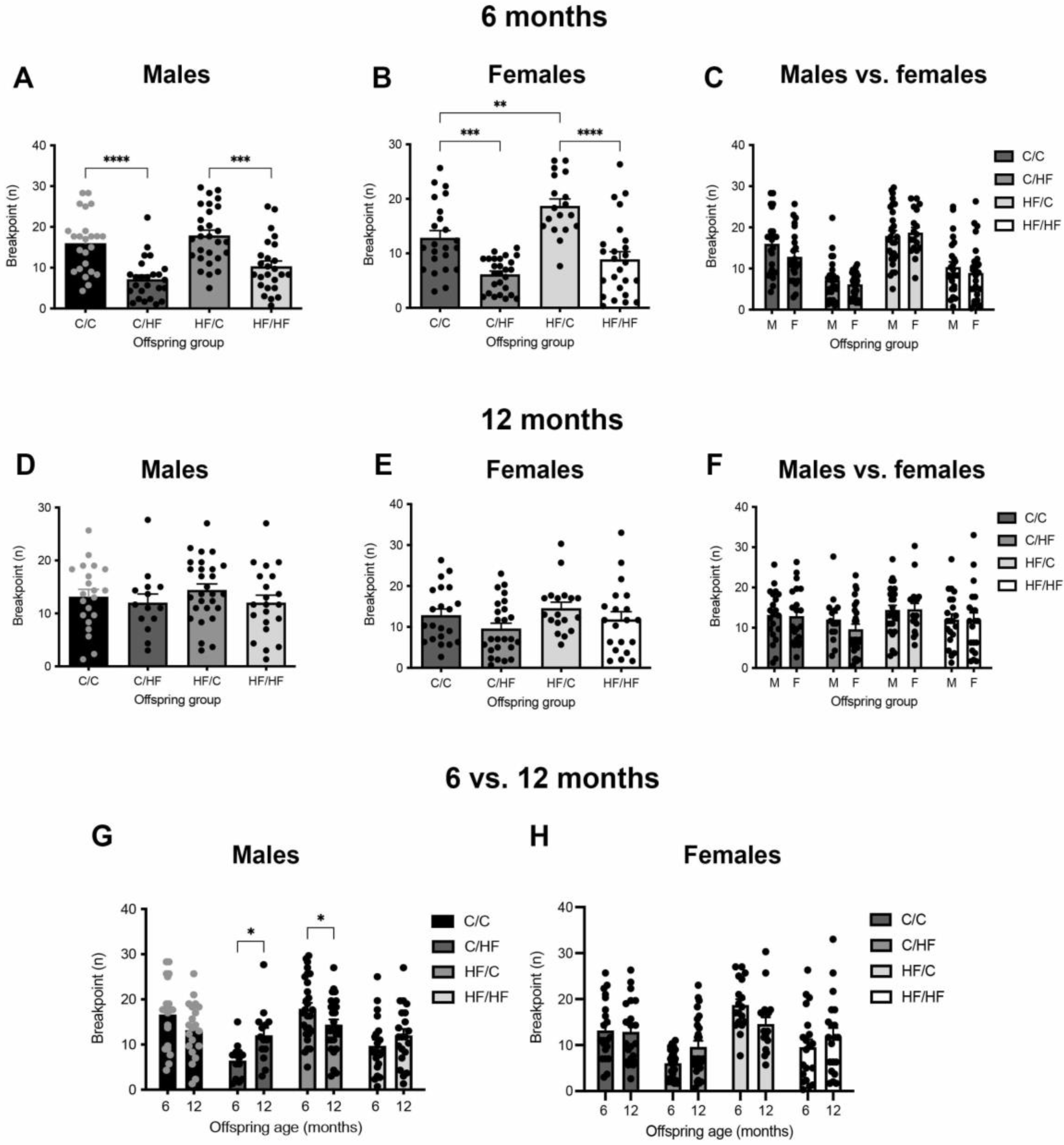
3.2. Young HF-Fed Offspring Were Less Motivated for Milkshake Than C-Fed Offspring
3.3. Motivation for Milkshake Reward Did Not Differ between Aged C- and HF-Fed Offspring
3.4. Motivation Decreased over Time in C-Fed Offspring but Increased in HF-Fed Animals
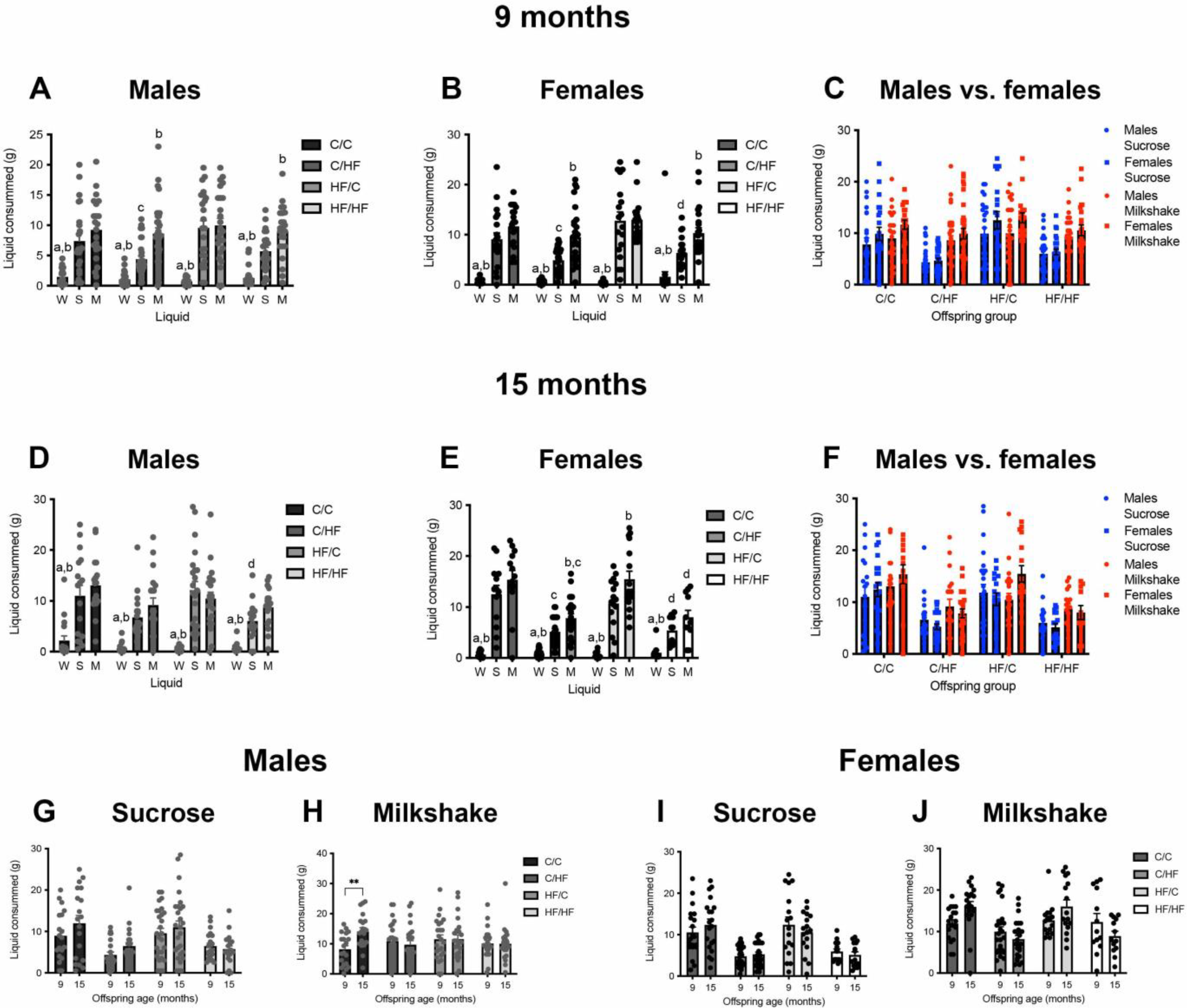
3.5. HF-Fed Mice Showed Preference for Milkshake over Sucrose
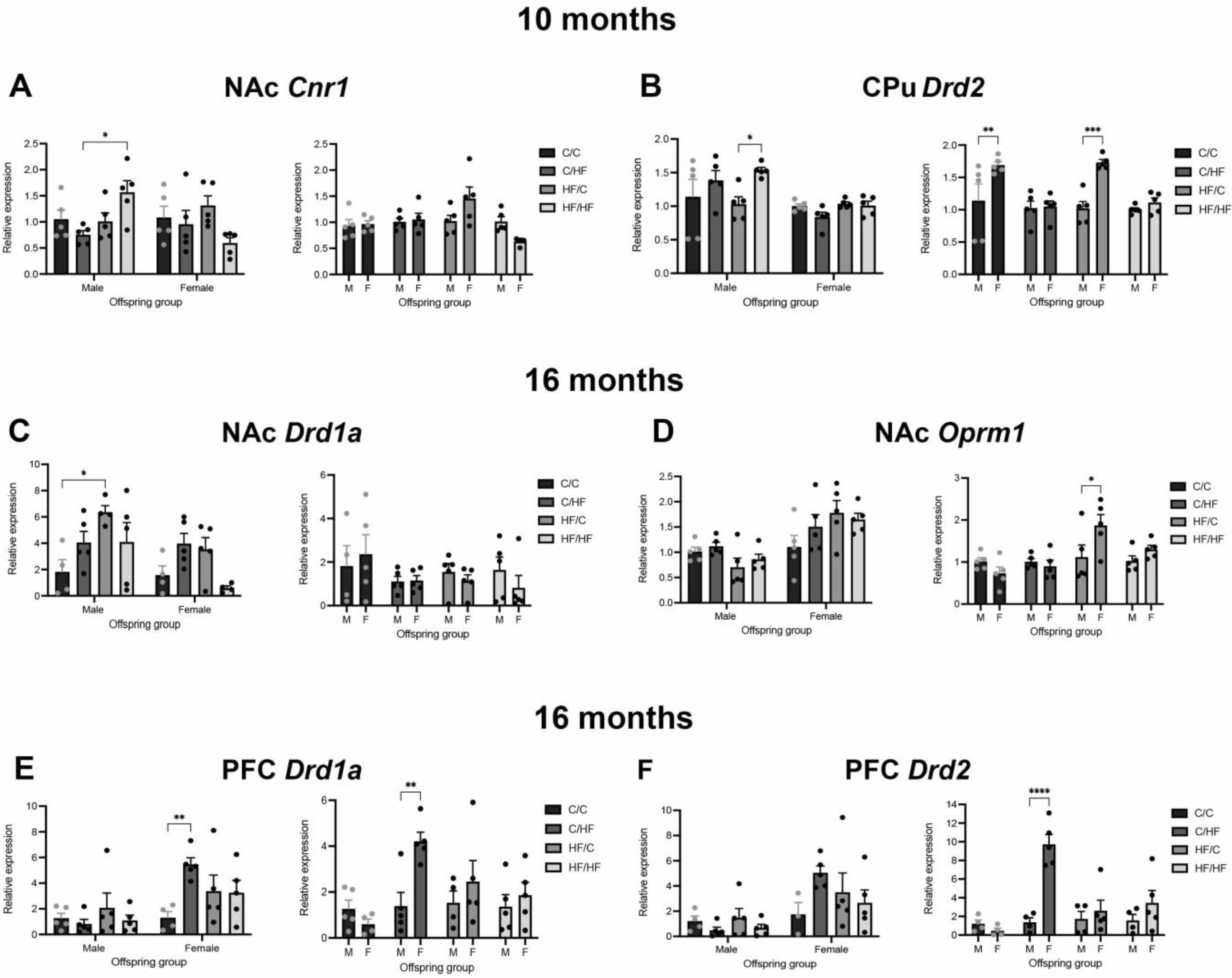
3.6. Expression of Markers of DA, μ- Opioid and CB1 Receptors between Diet Groups in 10-Month-Old Offspring
3.7. Expression of Markers of DA, μ-Opioid and CB1 Receptors between Diet Groups in 16-Month-Old Offspring
3.8. Expression of Markers of DA, μ-Opioid and CB1 Receptors between Male and Female Offspring at Both Ages
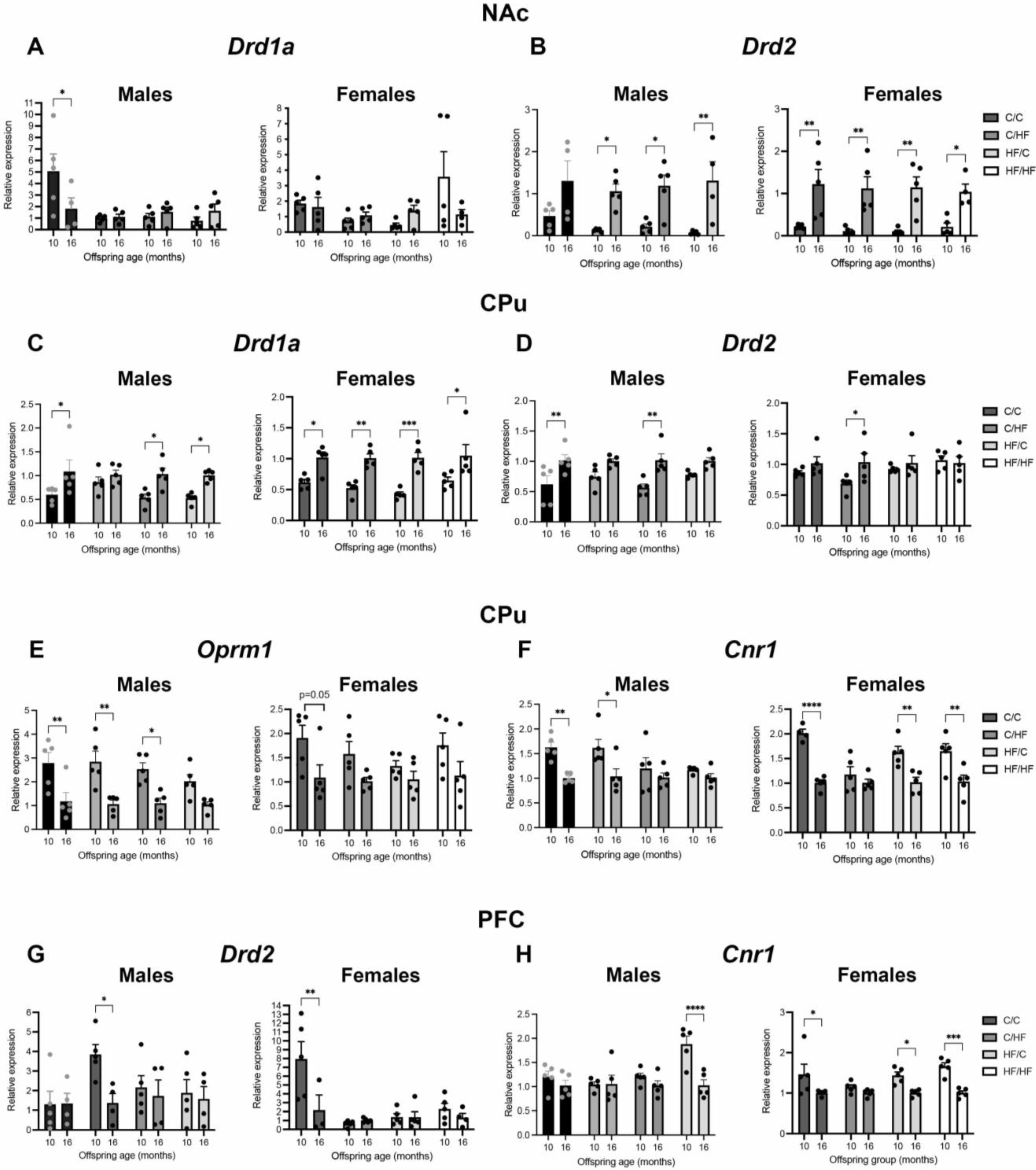
3.9. Expression of DA, μ-Opioid and CB1 Receptors Is Altered between Young and Aged Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int. J. Obes. 2010, 34, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Dietz, P.M.; England, L.; Morrow, B.; Callaghan, W.M. Trends in Pre-pregnancy Obesity in Nine States, 1993–2003*. Obesity 2007, 15, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Gregor, L.; Remington, P.L.; Lindberg, S.; Ehrenthal, D. Prevalence of Pre-pregnancy Obesity, 2011–2014. WMJ Off. Publ. State Med. Soc. Wis. 2016, 115, 228–232. [Google Scholar]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Anderson, C.; Pillai, P.; Tandias, A.; Arndt, B.; Hanrahan, L. Prevalence and Predictors of Unhealthy Weight Gain in Pregnancy. WMJ Off. Publ. State Med. Soc. Wis. 2016, 115, 233–237. [Google Scholar]
- Siega-Riz, A.M.; Gray, G.L. Gestational weight gain recommendations in the context of the obesity epidemic. Nutr. Rev. 2013, 71, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; MacDougald, O.A. Maternal nutrition and risk of obesity in offspring: The Trojan horse of developmental plasticity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 495–506. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Allan, K.M.; A Raja, E.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Norman, J. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1 323 275 person years. Bmj 2013, 347, f4539. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Linabery, A.; Nahhas, R.W.; Johnson, W.; Choh, A.C.; Towne, B.; Odegaard, A.O.; Czerwinski, S.A.; Demerath, E.W. Stronger influence of maternal than paternal obesity on infant and early childhood body mass index: The Fels Longitudinal Study. Pediatr. Obes. 2012, 8, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Næss, M.; Holmen, T.L.; Langaas, M.; Bjørngaard, J.H.; Kvaløy, K. Intergenerational Transmission of Overweight and Obesity from Parents to Their Adolescent Offspring—The HUNT Study. PLoS ONE 2016, 11, e0166585. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Sandboge, S.; Salonen, M.; Kajantie, E.; Osmond, C. Maternal weight in pregnancy and offspring body composition in late adulthood: Findings from the Helsinki Birth Cohort Study (HBCS). Ann. Med. 2015, 47, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hochner, H.; Friedlander, Y.; Calderon-Margalit, R.; Meiner, V.; Sagy, Y.; Avgil-Tsadok, M.; Burger, A.; Savitsky, B.; Siscovick, D.S.; Manor, O. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: The Jerusalem Perinatal Family Follow-up Study. Circulation 2012, 125, 1381–1389. [Google Scholar] [CrossRef]
- Eilander, A.; Harika, R.K.; Zock, P.L. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur. J. Lipid. Sci. Technol. 2015, 117, 1370–1377. [Google Scholar] [CrossRef]
- Shan, Z.; Rehm, C.D.; Rogers, G. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality among US Adults, 1999–2016. JAMA J. Am. Med. Assoc. 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Fu, O.; Minokoshi, Y.; Nakajima, K.-I. Recent Advances in Neural Circuits for Taste Perception in Hunger. Front. Neural Circuits 2021, 15, 609824. [Google Scholar] [CrossRef]
- Adams, R.C.; Sedgmond, J.; Maizey, L.; Chambers, C.D.; Lawrence, N.S. Food Addiction: Implications for the Diagnosis and Treatment of Overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Jay, J.L.; Acheson, M.A.; Magrisso, I.J.; West, C.H.; Zavosh, A.; Benoit, S.C.; Davis, J.F. Moderate high fat diet increases sucrose self-administration in young rats. Appetite 2013, 61, 19–29. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Thompson, A.C.; A Cassis, L.; Bardo, M.T.; Dwoskin, L.P. Diet-induced obesity: Dopamine transporter function, impulsivity and motivation. Int. J. Obes. 2013, 37, 1095–1103. [Google Scholar] [CrossRef]
- Harb, M.R.; Almeida, O.F.X. Altered motivation masks appetitive learning potential of obese mice. Front. Behav. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Arcego, D.M.; Krolow, R.; Lampert, C.; Toniazzo, A.P.; Garcia, E.D.S.; Lazzaretti, C.; Costa, G.; Scorza, C.; Dalmaz, C. Chronic high-fat diet affects food-motivated behavior and hedonic systems in the nucleus accumbens of male rats. Appetite 2020, 153, 104739. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Rodríguez-González, G.; Reyes-Castro, L.; Ibáñez, C.; Ramírez, A.; Chavira, R.; Larrea, F.; Nathanielsz, P.; Zambrano, E. Maternal obesity in the rat programs male offspring exploratory, learning and motivation behavior: Prevention by dietary intervention pre-gestation or in gestation. Int. J. Dev. Neurosci. 2012, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Naef, L.; Moquin, L.; Bo, G.D.; Giros, B.; Gratton, A.; Walker, C.-D. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 2011, 176, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology 2015, 40, 1353–1363. [Google Scholar] [CrossRef]
- McKee, S.E.; Grissom, N.M.; Herdt, C.T.; Reyes, T.M. Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet—fed dams. FASEB J. 2017, 31, 2352–2363. [Google Scholar] [CrossRef]
- Fisher, J.O.; Birch, L.L. Fat Preferences and Fat Consumption of 3- to 5-year-old Children are Related to Parental Adiposity. J. Am. Diet. Assoc. 1995, 95, 759–764. [Google Scholar] [CrossRef]
- Wardle, J.; Guthrie, C.; Sanderson, S.; Birch, L.; Plomin, R. Food and activity preferences in children of lean and obese parents. Int. J. Obes. 2001, 25, 971–977. [Google Scholar] [CrossRef]
- Movassagh, E.Z.; Baxter-Jones, A.D.G.; Kontulainen, S.; Whiting, S.J.; Vatanparast, H. Tracking Dietary Patterns over 20 Years from Childhood through Adolescence into Young Adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients 2017, 9, 990. [Google Scholar] [CrossRef]
- Baldo, B.A.; Kelley, A.E. Discrete neurochemical coding of distinguishable motivational processes: Insights from nucleus accumbens control of feeding. Psychopharmacology 2007, 191, 439–459. [Google Scholar] [CrossRef]
- E Kelley, A.; A Baldo, B.; Pratt, W.; Will, M. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.R.; Simpson, E.; Kellendonk, C.; Herzberg, W.G.; Lipatova, O.; Fairhurst, S.; Kandel, E.R.; Malapani, C.; Balsam, P.D. Transient Overexpression of Striatal D2 Receptors Impairs Operant Motivation and Interval Timing. J. Neurosci. 2007, 27, 7731–7739. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, L.; Talani, G.; Biggio, F.; Utzeri, C.; Lallai, V.; Licheri, V.; Lutzu, S.; Mostallino, M.C.; Secci, P.P.; Biggio, G.; et al. Involvement of the Cannabinoid CB1 Receptor in Modulation of Dopamine Output in the Prefrontal Cortex Associated with Food Restriction in Rats. PLoS ONE 2014, 9, e92224. [Google Scholar] [CrossRef]
- Melis, T.; Succu, S.; Sanna, F.; Boi, A.; Argiolas, A.; Melis, M.R. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci. Lett. 2007, 419, 231–235. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.A.; Solinas, M.; Bimpisidis, Z.; Goldberg, S.R.; Di Chiara, G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 2012, 63, 161–168. [Google Scholar] [CrossRef]
- Covey, D.P.; Yocky, A.G. Endocannabinoid Modulation of Nucleus Accumbens Microcircuitry and Terminal Dopamine Release. Front. Synaptic Neurosci. 2021, 13, 734975. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Caref, K.; Nicola, S.M. Endogenous opioids in the nucleus accumbens promote approach to high-fat food in the absence of caloric need. eLife 2018, 7, 34955. [Google Scholar] [CrossRef]
- Carlin, J.; George, R.; Reyes, T.M. Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS ONE 2013, 8, e63549. [Google Scholar] [CrossRef]
- Pérez, M.R.; Lépinay, A.L.; Alonso, L.; Rincel, M.; Xia, L.; Fanet, H.; Caille-Garnier, S.; Cador, M.; Layé, S.; Vancassel, S.; et al. Impact of perinatal exposure to high-fat diet and stress on responses to nutritional challenges, food-motivated behaviour and mesolimbic dopamine function. Int. J. Obes. 2017, 41, 502–509. [Google Scholar] [CrossRef]
- Vucetic, Z.; Kimmel, J.; Totoki, K.; Hollenbeck, E.; Reyes, T.M. Maternal High-Fat Diet Alters Methylation and Gene Expression of Dopamine and Opioid-Related Genes. Endocrinology 2010, 151, 4756–4764. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011, 25, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Contu, L.; Nizari, S.; Heath, C.J.; Hawkes, C.A. Pre- and Post-natal High Fat Feeding Differentially Affects the Structure and Integrity of the Neurovascular Unit of 16-Month Old Male and Female Mice. Front. Neurosci. 2019, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.J.; Phillips, B.U.; Bussey, T.J.; Saksida, L.M. Measuring Motivation and Reward-Related Decision Making in the Rodent Operant Touchscreen System. Curr. Protoc. Neurosci. 2016, 74, 8.34.1–8.34.20. [Google Scholar] [CrossRef]
- Edlow, A.G.; Guedj, F.; Pennings, J.L.; Sverdlov, D.; Neri, C.; Bianchi, D.W. Males are from Mars, and females are from Venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 2016, 214, 623.e1–623.e10. [Google Scholar] [CrossRef]
- Gemici, A.; Sinen, O.; Bülbül, M. Sexual dimorphism in rats exposed to maternal high fat diet: Alterations in medullary sympathetic network. Metab. Brain Dis. 2021, 36, 1305–1314. [Google Scholar] [CrossRef]
- Bordeleau, M.; Lacabanne, C.; de Cossío, L.F.; Vernoux, N.; Savage, J.C.; González-Ibáñez, F.; Tremblay, M. Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet. J. Neuroinflammation 2020, 17, 264. [Google Scholar] [CrossRef]
- Lippert, R.; Hess, S.; Klemm, P.; Burgeno, L.; Jahans-Price, T.; Walton, M.; Kloppenburg, P.; Brüning, J. Maternal high-fat diet during lactation reprograms the dopaminergic circuitry in mice. J. Clin. Investig. 2020, 130, 3761–3776. [Google Scholar] [CrossRef]
- Chavez, C.; Hollaus, M.; Scarr, E.; Pavey, G.; Gogos, A.; Buuse, M.V.D. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: An autoradiography study. Brain Res. 2010, 1321, 51–59. [Google Scholar] [CrossRef]
- Sinclair, D.; Purves-Tyson, T.D.; Allen, K.M.; Weickert, C.S. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014, 231, 1581–1599. [Google Scholar] [CrossRef]
- Ford, C. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, B.-J.; Hsieh, T.-H.; Kuo, T.-T.; Miller, J.; Chou, Y.-C.; Huang, E.Y.-K.; Hoffer, B.J. Differences in Nicotine Encoding Dopamine Release between the Striatum and Shell Portion of the Nucleus Accumbens. Cell Transplant. 2018, 28, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Monteleone, E. Food Preferences and Obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, L.; Flores-Barrantes, P.; Moreno, L.; Manios, Y.; Gonzalez-Gil, E. The Influence of Parental Dietary Behaviors and Practices on Children’s Eating Habits. Nutrients 2021, 13, 1138. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Kim, E.; Seo, K.Y.; Kang, Y.J.; Kim, J.Y.; Kim, C.H.; Song, H.-T.; Saksida, L.M.; Lee, J.E. Assessment of Cognitive Impairment in a Mouse Model of High-Fat Diet-Induced Metabolic Stress with Touchscreen-Based Automated Battery System. Exp. Neurobiol. 2018, 27, 277–286. [Google Scholar] [CrossRef]
- Pandit, R.; Mercer, J.G.; Overduin, J.; la Fleur, S.E.; Adan, R.A. Dietary Factors Affect Food Reward and Motivation to Eat. Obes. Facts 2012, 5, 221–242. [Google Scholar] [CrossRef]
- Tellez, L.A.; Medina, S.; Han, W.; Ferreira, J.G.; Licona-Limón, P.; Ren, X.; Lam, T.T.; Schwartz, G.J.; De Araujo, I.E. A Gut Lipid Messenger Links Excess Dietary Fat to Dopamine Deficiency. Science 2013, 341, 800–802. [Google Scholar] [CrossRef]
- Geiger, B.; Haburcak, M.; Avena, N.; Moyer, M.; Hoebel, B.; Pothos, E. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef]
- Appannah, G.; Murray, K.; Trapp, G.; Dymock, M.; Oddy, W.H.; Ambrosini, G.L. Dietary pattern trajectories across adolescence and early adulthood and their associations with childhood and parental factors. Am. J. Clin. Nutr. 2020, 113, 36–46. [Google Scholar] [CrossRef]
- Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Zaragoza-Jordana, M.; Ferré, N.; Grote, V.; Koletzko, B.; Totzauer, M.; Verduci, E.; ReDionigi, A.; et al. Unhealthy Dietary Patterns Established in Infancy Track to Mid-Childhood: The EU Childhood Obesity Project. J. Nutr. 2018, 148, 752–759. [Google Scholar] [CrossRef]
- Mikkilä, V.; Räsänen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2007, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.C.; Samanez-Larkin, G.R.; Smith, C.T.; Castrellon, J.J.; Perkins, S.F.; Cowan, R.L.; Claassen, D.O.; Zald, D.H. FTO affects food cravings and interacts with age to influence age-related decline in food cravings. Physiol. Behav. 2018, 192, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, B.L.; Özalay, Ö.; Mediavilla, T.; Ericsson, M.; Axelsson, J.; Rieckmann, A.; Sultan, F.; Marcellino, D. The Aged Striatum: Evidence of Molecular and Structural Changes Using a Longitudinal Multimodal Approach in Mice. Front. Aging Neurosci. 2022, 14, 795132. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hatano, K.; Sakiyama, Y.; Kawasumi, Y.; Kato, T.; Ito, K. Age-related changes of dopamine D1-like and D2-like receptor binding in the F344/N rat striatum revealed by positron emission tomography and in vitro receptor autoradiography. Synapse 2001, 41, 285–293. [Google Scholar] [CrossRef]
- Jackson, M.G.; Lightman, S.L.; Gilmour, G.; Marston, H.; Robinson, E.S.J. Evidence for deficits in behavioural and physiological responses in aged mice relevant to the psychiatric symptom of apathy. Brain Neurosci. Adv. 2021, 5, 23982128211015110. [Google Scholar] [CrossRef]
- Bordner, K.A.; Kitchen, R.R.; Carlyle, B.; George, E.D.; Mahajan, M.C.; Mane, S.M.; Taylor, J.R.; Simen, A.A. Parallel declines in cognition, motivation, and locomotion in aging mice: Association with immune gene upregulation in the medial prefrontal cortex. Exp. Gerontol. 2011, 46, 643–659. [Google Scholar] [CrossRef][Green Version]
- Tracy, A.L.; Wee, C.J.; Hazeltine, G.E.; Carter, R.A. Characterization of attenuated food motivation in high-fat diet-induced obesity: Critical roles for time on diet and reinforcer familiarity. Physiol. Behav. 2015, 141, 69–77. [Google Scholar] [CrossRef]
- Altherr, E.; Rainwater, A.; Kaviani, D.; Tang, Q.; Güler, A.D. Long-term high fat diet consumption reversibly alters feeding behavior via a dopamine-associated mechanism in mice. Behav. Brain Res. 2021, 414, 113470. [Google Scholar] [CrossRef]
- Capper-Loup, C.; Canales, J.J.; Kadaba, N.; Graybiel, A.M. Concurrent Activation of Dopamine D1and D2Receptors Is Required to Evoke Neural and Behavioral Phenotypes of Cocaine Sensitization. J. Neurosci. 2002, 22, 6218–6227. [Google Scholar] [CrossRef]
- Dobbs, L.K.; Kaplan, A.R.; Bock, R.; Phamluong, K.; Shin, J.H.; Bocarsly, M.E.; Eberhart, L.; Ron, D.; Alvarez, V.A. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 2018, 44, 805–816. [Google Scholar] [CrossRef]
- Baladi, M.G.; France, C.P. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur. J. Pharmacol. 2009, 610, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Branch, S.Y.; Goertz, R.B.; Sharpe, A.; Pierce, J.; Roy, S.; Ko, D.; Paladini, C.A.; Beckstead, M.J. Food Restriction Increases Glutamate Receptor-Mediated Burst Firing of Dopamine Neurons. J. Neurosci. 2013, 33, 13861–13872. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Michaelides, M.; Piyis, Y.K.; Wang, G.J.; Volkow, N.D. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 2008, 62, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.S.; Ingram, D.K.; Joseph, J.A. Delayed loss of striatal dopamine receptors during aging of dietarily restricted rats. Brain Res. 1984, 300, 27–32. [Google Scholar] [CrossRef] [PubMed]

| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6–10 Months Old | C/C vs. C/HF | C/C vs. HF/C | C/HF vs. HF/HF | HF/C vs. HF/HF | C/C vs. C/HF | C/C vs. HF/C | C/HF vs. HF/HF | HF/C vs. HF/HF | |
| Body weight | ↓ C/C | - | ↓ C/HF | ↓ HF/C | ↓ C/C | - | - | ↓ HF/C | |
| Motivation | ↑ C/C | - | - | ↑ HF/C | ↑ C/C | ↓ C/C | - | ↑ HF/C | |
| Sucrose preference | S: ↑ C/C M: - | S: - M: - | S: - M: - | S: - M: - | S: ↑ C/C M: - | S: - M: - | S: - M: - | S: ↑ HF/C M: - | |
| 12–16 months old | Body weight | ↓ C/C | - | - | ↓ HF/C | ↓ C/C | - | - | ↓ HF/C |
| Motivation | - | - | - | - | - | - | - | - | |
| Sucrose preference | S: - M: - | S: - M: - | S: - M: - | S: ↑ HF/C M: - | S: ↑ C/C M: ↑ C/C | S: - M: - | S: - M: - | S: ↑ HF/C M: ↑ HF/C | |
| C/C | C/HF | HF/C | HF/HF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Gene or Protein | Males | Females | Males | Females | Males | Females | Males | Females |
| NAc | Drd1a | ↓ | - | - | - | - | - | - | - |
| Drd2 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Crn1 | - | - | - | - | - | - | - | - | |
| Oprm1 | - | - | - | - | - | - | - | - | |
| DAT | - | - | - | - | - | - | - | - | |
| CPu | Drd1a | ↑ | ↑ | - | ↑ | ↑ | ↑ | ↑ | ↑ |
| Drd2 | ↑ | - | - | ↑ | ↑ | - | - | - | |
| Crn1 | ↓ | ↓ | ↓ | - | - | ↓ | - | ↓ | |
| Oprm1 | ↓ | ↓ | ↓ | - | ↓ | - | - | - | |
| DAT | - | - | - | - | - | - | - | - | |
| PFC | Drd1a | - | - | - | - | - | - | - | - |
| Drd2 | - | ↓ | ↓ | - | - | - | - | - | |
| Crn1 | - | ↓ | - | - | - | ↓ | ↓ | ↓ | |
| Oprm1 | - | - | - | - | - | - | - | - | |
| DAT | - | - | - | - | ↓ | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contu, L.; Heath, C.J.; Hawkes, C.A. Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding. Nutrients 2022, 14, 5161. https://doi.org/10.3390/nu14235161
Contu L, Heath CJ, Hawkes CA. Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding. Nutrients. 2022; 14(23):5161. https://doi.org/10.3390/nu14235161
Chicago/Turabian StyleContu, Laura, Christopher J. Heath, and Cheryl A. Hawkes. 2022. "Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding" Nutrients 14, no. 23: 5161. https://doi.org/10.3390/nu14235161
APA StyleContu, L., Heath, C. J., & Hawkes, C. A. (2022). Appetitive Motivation and Associated Neurobiology Change Differentially across the Life Course of Mouse Offspring Exposed to Peri- and Postnatal High Fat Feeding. Nutrients, 14(23), 5161. https://doi.org/10.3390/nu14235161







