Correlations between the Changing Levels of Tissue Plasminogen Activator and Adiposity Following Exercise-Induced Weight Loss
Abstract
1. Introduction
- In people who lose weight through exercise, how strong are the correlations between changes in adiposity and changes in respective TPA and FIBR levels?
- Is there a difference between changes in VAT and SAT in terms of the correlations to TPA or FIBR levels?
- Is there a difference between TPA and FIBR in terms of these correlations, that is, which factor is more sensitive to the changing adiposity?
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Subcutaneous Adipose Tissue
3.2. Visceral Adipose Tissue
3.3. Tissue Plasminogen Activator
3.4. Fibrinogen
3.5. Relationship between Changes in VAT and SAT Amounts and Changes in TPA Levels by Group
3.6. Relationship between Changes in VAT and SAT Amounts and Changes in FIBR Levels by Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Atlas Writing Group; ESC Atlas of Cardiology is a Compendium of Cardiovascular Statistics Compiled by the European Heart Agency, a Department of the European Society of Cardiology; Developed in Collaboration with the National Societies of the European Society of Cardiology Member Countries; Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019 (Executive Summary). Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Dyer, A.; Cai, X.; Garside, D.B.; Ning, H.; Thomas, A.; Greenland, P.; Van Horn, L.; Tracy, R.P.; Lloyd-Jones, D.M. Lifetime risks of cardiovascular disease. N. Engl. J. Med. 2012, 366, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef] [PubMed]
- Michonska, I.; Luszczki, E.; Zielinska, M.; Oleksy, L.; Stolarczyk, A.; Deren, K. Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases-A Narrative Review. Nutrients 2022, 14, 4422. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Temprosa, M.G.; Mather, K.J.; Orchard, T.J.; Kitabchi, A.E.; Watson, K.E.; Diabetes Prevention Program Research, G. Lifestyle and metformin interventions have a durable effect to lower CRP and tPA levels in the diabetes prevention program except in those who develop diabetes. Diabetes Care 2014, 37, 2253–2260. [Google Scholar] [CrossRef]
- Stroke Study Group National Institute of Neurological Disorders. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Loscalzo, J.; Braunwald, E. Tissue plasminogen activator. N. Engl. J. Med. 1988, 319, 925–931. [Google Scholar] [CrossRef]
- Mavri, A.; Stegnar, M.; Krebs, M.; Sentocnik, J.T.; Geiger, M.; Binder, B.R. Impact of adipose tissue on plasma plasminogen activator inhibitor-1 in dieting obese women. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1582–1587. [Google Scholar] [CrossRef][Green Version]
- Binder, B.R.; Christ, G.; Gruber, F.; Grubic, N.; Hufnagl, P.; Krebs, M.; Mihaly, J.; Prager, G.W. Plasminogen activator inhibitor 1: Physiological and pathophysiological roles. Physiology 2002, 17, 56–61. [Google Scholar] [CrossRef]
- Alessi, M.-C.; Poggi, M.; Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Juhan-Vague, I.; Alessi, M.C. Plasminogen activator inhibitor 1 and atherothrombosis. Thromb. Haemost. 1993, 70, 138–143. [Google Scholar] [CrossRef]
- Cook, N.S.; Ubben, D. Fibrinogen as a major risk factor in cardiovascular disease. Trends Pharmacol. Sci. 1990, 11, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Influence of fibrinogen on cardiovascular disease. Drugs 1997, 54, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Papageorgiou, N.; Androulakis, E.; Briasoulis, A.; Antoniades, C.; Stefanadis, C. Fibrinogen and cardiovascular disease: Genetics and biomarkers. Blood Rev. 2011, 25, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Stec, J.J.; Silbershatz, H.; Tofler, G.H.; Matheney, T.H.; Sutherland, P.; Lipinska, I.; Massaro, J.M.; Wilson, P.F.; Muller, J.E.; D’Agostino, R.B., Sr. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation 2000, 102, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Galbreath, M.; Campbell, B.; LaBounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.L.; Levers, K.; Galvan, E.; et al. Effects of Adherence to a Higher Protein Diet on Weight Loss, Markers of Health, and Functional Capacity in Older Women Participating in a Resistance-Based Exercise Program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854–1866. [Google Scholar] [CrossRef]
- Meijer, J.L.; Roderka, M.N.; Chinburg, E.L.; Renier, T.J.; McClure, A.C.; Rothstein, R.I.; Barry, E.L.; Billmeier, S.; Gilbert-Diamond, D. Alterations in Fecal Short-Chain Fatty Acids after Bariatric Surgery: Relationship with Dietary Intake and Weight Loss. Nutrients 2022, 14, 4243. [Google Scholar] [CrossRef]
- Carroll, J.F.; Fulda, K.G.; Chiapa, A.L.; Rodriquez, M.; Phelps, D.R.; Cardarelli, K.M.; Vishwanatha, J.K.; Cardarelli, R. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity 2009, 17, 1420–1427. [Google Scholar] [CrossRef]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F., Jr.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Cigolini, M.; Targher, G.; Bergamo Andreis, I.A.; Tonoli, M.; Agostino, G.; De Sandre, G. Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 368–374. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Williams, K.; Karter, A.; Mayer-Davis, E.; Tracy, R.; Haffner, S. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. 2001, 25, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Ermetici, F.; Cereda, E.; Coman, C.; Locati, M.; Morricone, L.; Corsi, M.M.; Ambrosi, B.J.N. Epicardial fat thickness: Relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Sattar, N.; Rumley, A.; Whincup, P.H.; Lennon, L.; Lowe, G.D. Tissue plasminogen activator, von Willebrand factor, and risk of type 2 diabetes in older men. Diabetes Care 2008, 31, 995–1000. [Google Scholar] [CrossRef][Green Version]
- Eliasson, M.C.; Jansson, J.H.; Lindahl, B.; Stegmayr, B. High levels of tissue plasminogen activator (tPA) antigen precede the development of type 2 diabetes in a longitudinal population study. The Northern Sweden MONICA study. Cardiovasc. Diabetol. 2003, 2, 19. [Google Scholar] [CrossRef][Green Version]
- Ditschuneit, H.H.; Flechtner-Mors, M.; Adler, G. Fibrinogen in obesity before and after weight reduction. Obes. Res. 1995, 3, 43–48. [Google Scholar] [CrossRef]
- Hafez, M.; El-Masry, S.; Musa, N.; Fathy, M.; Hassan, M.; Hassan, N.; El Husseiny, M.; Tareef, M. Relationship between visceral obesity and plasma fibrinogen in obese children. J. Pediatr. Endocrinol. Metab. 2016, 29, 289–296. [Google Scholar] [CrossRef]
- Janand-Delenne, B.; Chagnaud, C.; Raccah, D.; Alessi, M.; Juhan-Vague, I.; Vague, P. Visceral fat as a main determinant of plasminogen activator inhibitor 1 level in women. Int. J. Obes. 1998, 22, 312–317. [Google Scholar] [CrossRef]
- Vague, P.; Juhan-Vague, I.; Chabert, V.; Alessi, M.; Atlan, C.J.M. Fat distribution and plasminogen activator inhibitor activity in nondiabetic obese women. Metabolism 1989, 38, 913–915. [Google Scholar] [CrossRef]
- Goldberg, R.; Temprosa, M.; Otvos, J.; Brunzell, J.; Marcovina, S.; Mather, K.; Arakaki, R.; Watson, K.; Horton, E.; Barrett-Connor, E. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J. Clin. Endocrinol. Metab. 2013, 98, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr. Pract. 2006, 12, 20–24. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. Diabetes Care 2000, 23, 1619. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.T. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
| N | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | (Male) | Variable | Baseline | Year 1 | Delta | Baseline | Year 1 | Delta |
| Lifestyle | 339 (110) | Subcutaneous | 347.1 ± 117.1 | 282.1 ± 108.6 | −60.4 ± 57.6 | 488.0 ± 140.5 | 424.5 ± 138.8 | −58.3 ± 75.4 |
| Visceral | 181.2 ± 78.1 | 137.4 ± 67.5 | −44.1 ± 50.8 | 150.4 ± 54.4 | 123.3 ± 53.8 | −28.7 ± 43.5 | ||
| TPA (ng/mL) | 12.0 ± 4.6 | 9.2 ±3.6 | −3.0 ± 3.9 | 10.3 ± 3.5 | 8.5 ± 3.2 | −1.9 ± 2.9 | ||
| FIBR (µmol/L) | 360.7 ± 63.3 | 359.0 ± 66.3 | −1.6 ± 60.7 | 391.2 ± 81.6 | 385.3 ± 82.2 | −5.5 ± 61.8 | ||
| Metformin | 349 (126) | Subcutaneous | 339.1 ± 128.2 | 334.4 ± 146.6 | −14.0 ± 40.2 | 486.1 ± 145.6 | 445.4 ± 131.3 | −25.1 ± 54.1 |
| Visceral | 174.6 ± 70.0 | 172.6 ± 69.6 | −5.6 ± 40.9 | 148.0 ± 58.6 | 138.9 ± 59.5 | −5.6 ± 26.3 | ||
| TPA (ng/mL) | 11.8 ± 4.6 | 9.9 ± 4.1 | −2.0 ± 3.2 | 10.2 ± 3.5 | 8.4 ± 3.5 | −1.7 ± 2.5 | ||
| FIBR (µmol/L) | 353.9 ± 75.5 | 352.1 ± 77.2 | 0.9 ± 50.1 | 393.8 ± 77.4 | 396.4 ± 82.8 | 2.9 ± 69.3 | ||
| Placebo | 349 (116) | Subcutaneous | 362.3 ± 136.4 | 359.6 ± 128.1 | 0.6 ± 39.7 | 487.4 ± 144.4 | 478.3 ± 142.3 | −4.9 ± 45.1 |
| Visceral | 173.9 ± 67.6 | 172.1 ± 66.3 | −2.1 ± 33.5 | 150.4 ± 61.6 | 144.7 ± 52.6 | 0.4 ± 24.7 | ||
| TPA (ng/mL) | 11.9 ± 3.7 | 11.7 ± 3.5 | −0.6 ± 3.0 | 10.7 ± 3.6 | 10.2 ± 3.4 | −0.5 ± 2.9 | ||
| FIBR (µmol/L) | 353.8 ± 82.4 | 354.9 ± 73.4 | −1.1 ± 63.2 | 400.0 ± 88.4 | 398.6 ± 87.3 | −1.1 ± 67.6 | ||
| Men | Women | ||||
|---|---|---|---|---|---|
| Fat Depot | rs | p-Value | rs | p-Value | |
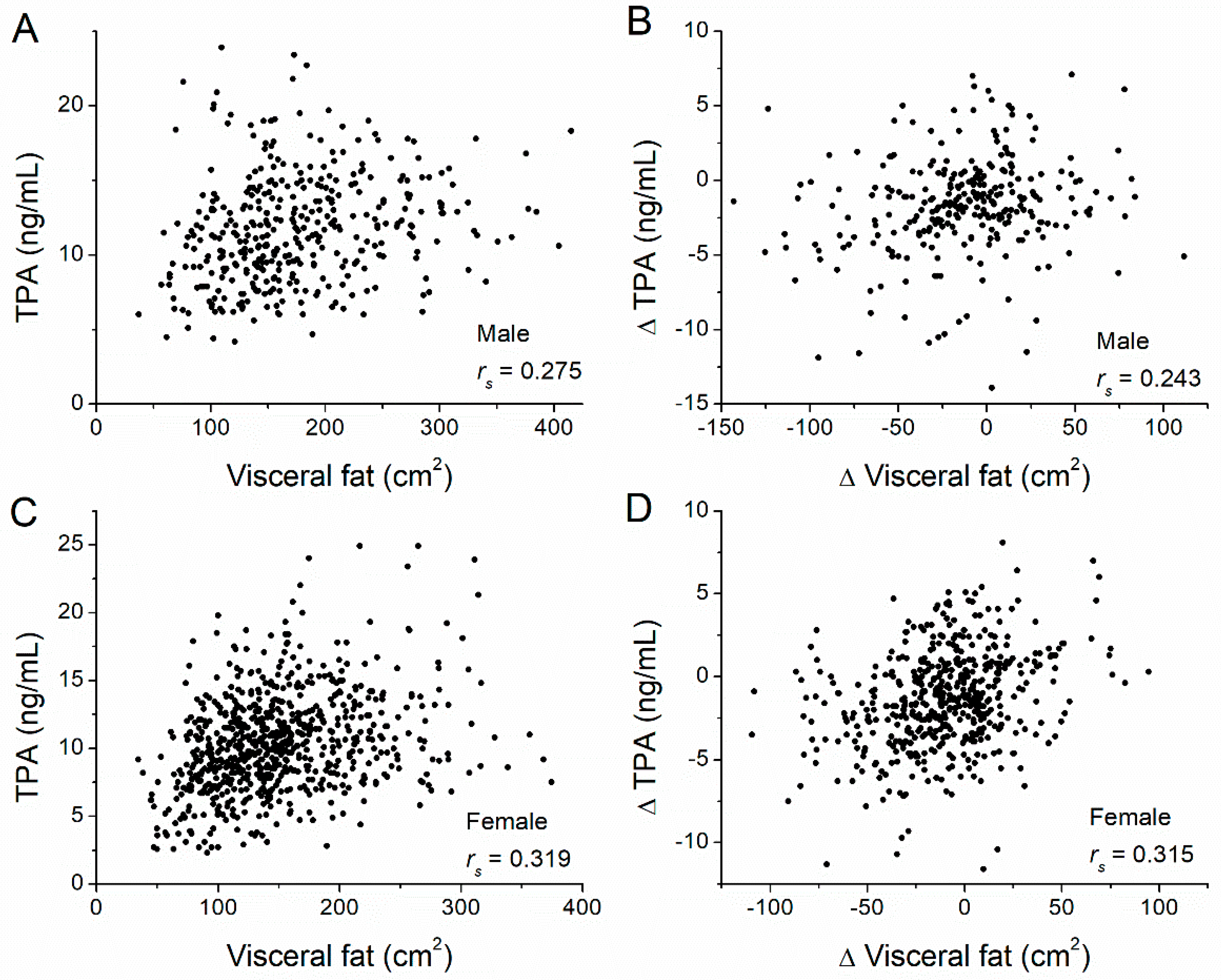
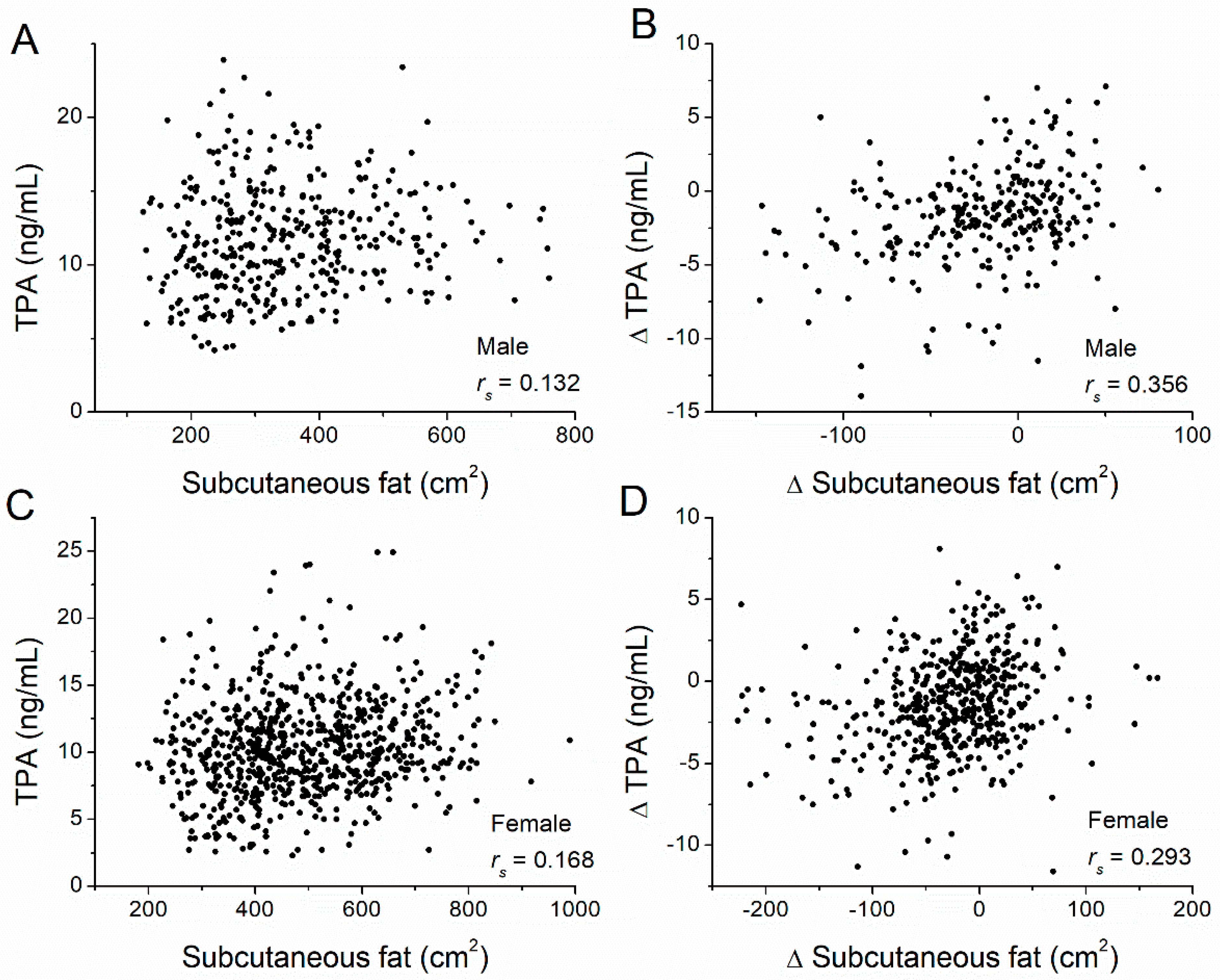
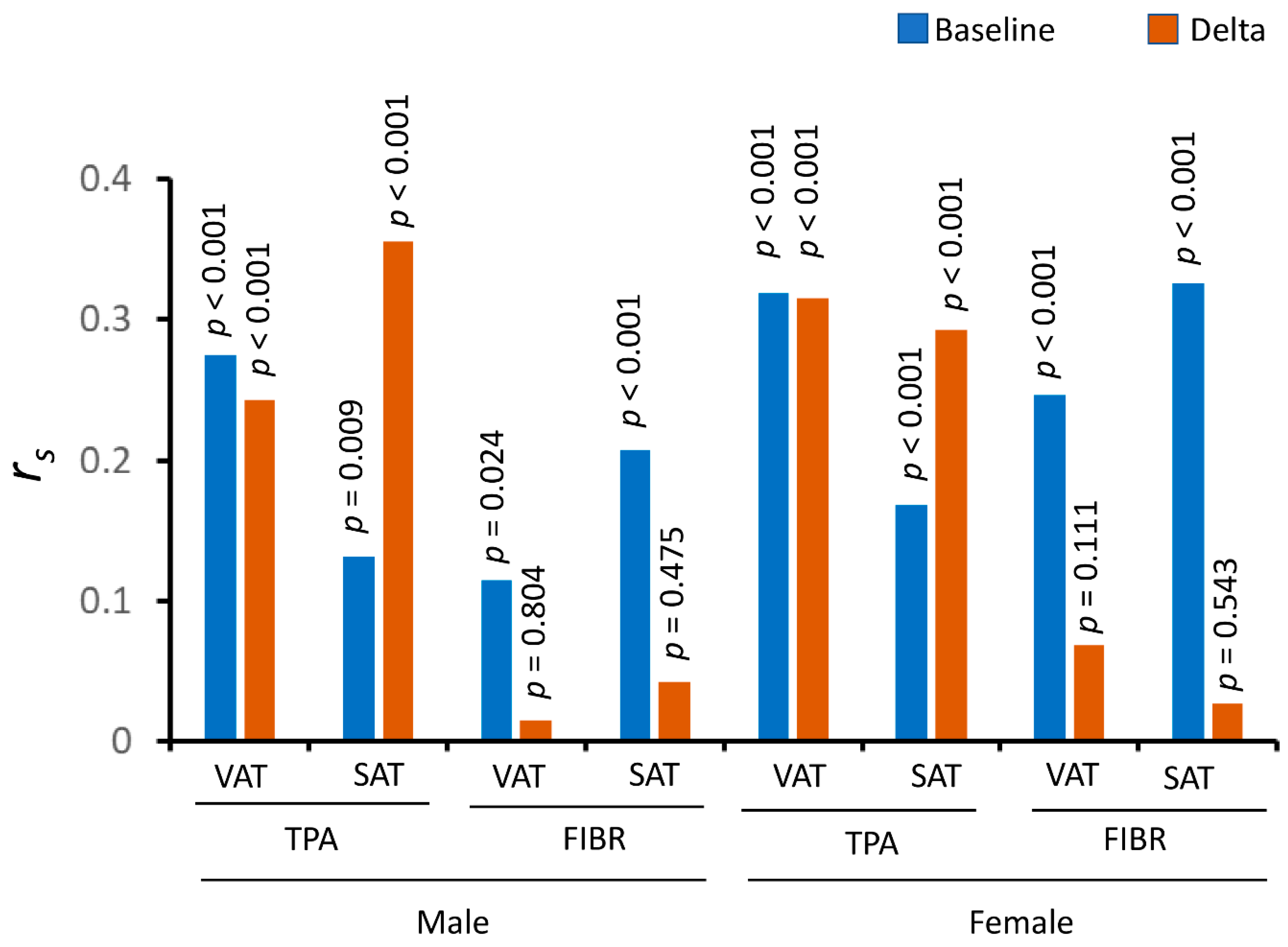
| TPA | Subcutaneous | 0.132 | 0.009 | 0.168 | <0.001 |
| Visceral | 0.275 | <0.001 | 0.319 | <0.001 | |
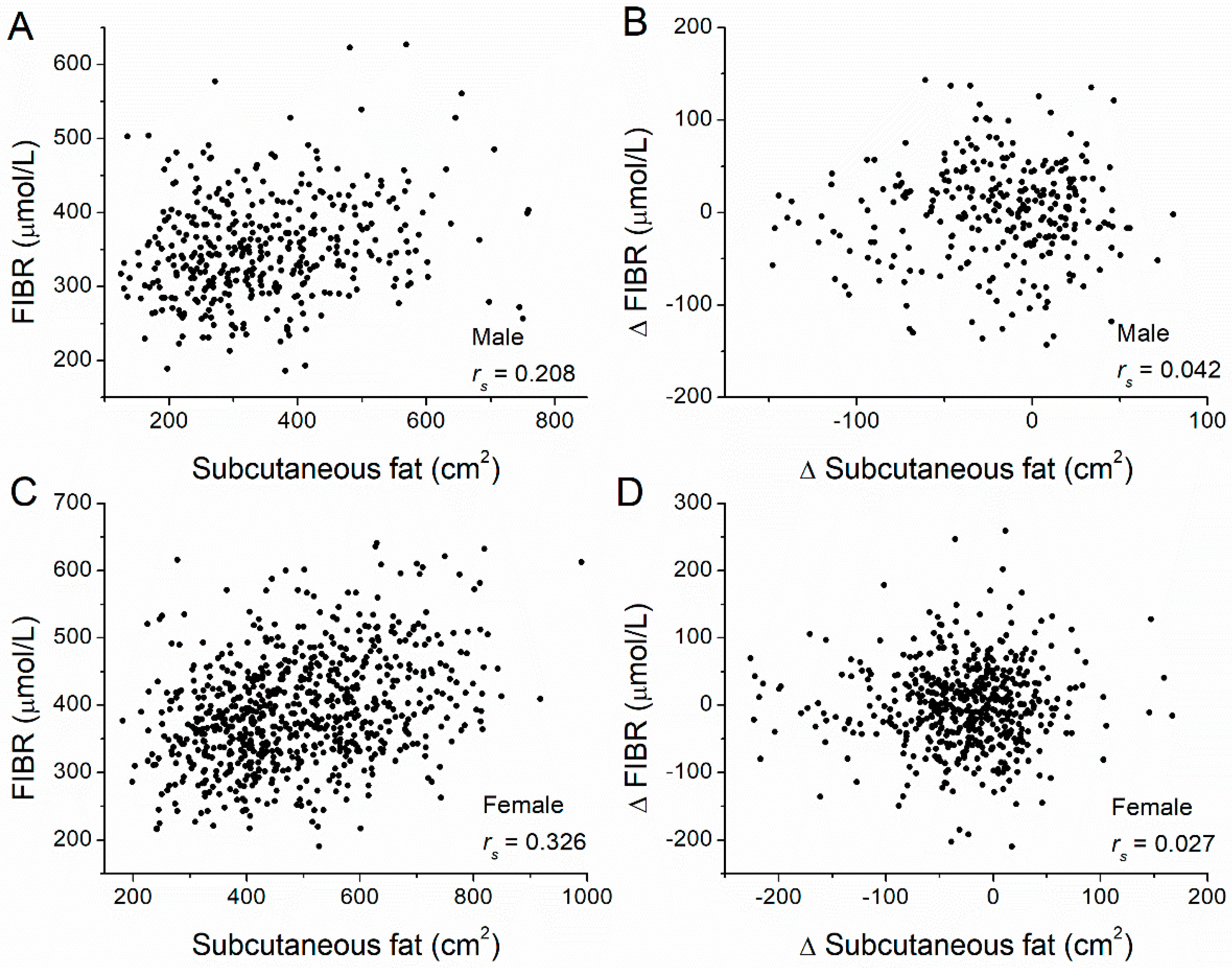
| FIBR | Subcutaneous | 0.208 | <0.001 | 0.326 | <0.001 |
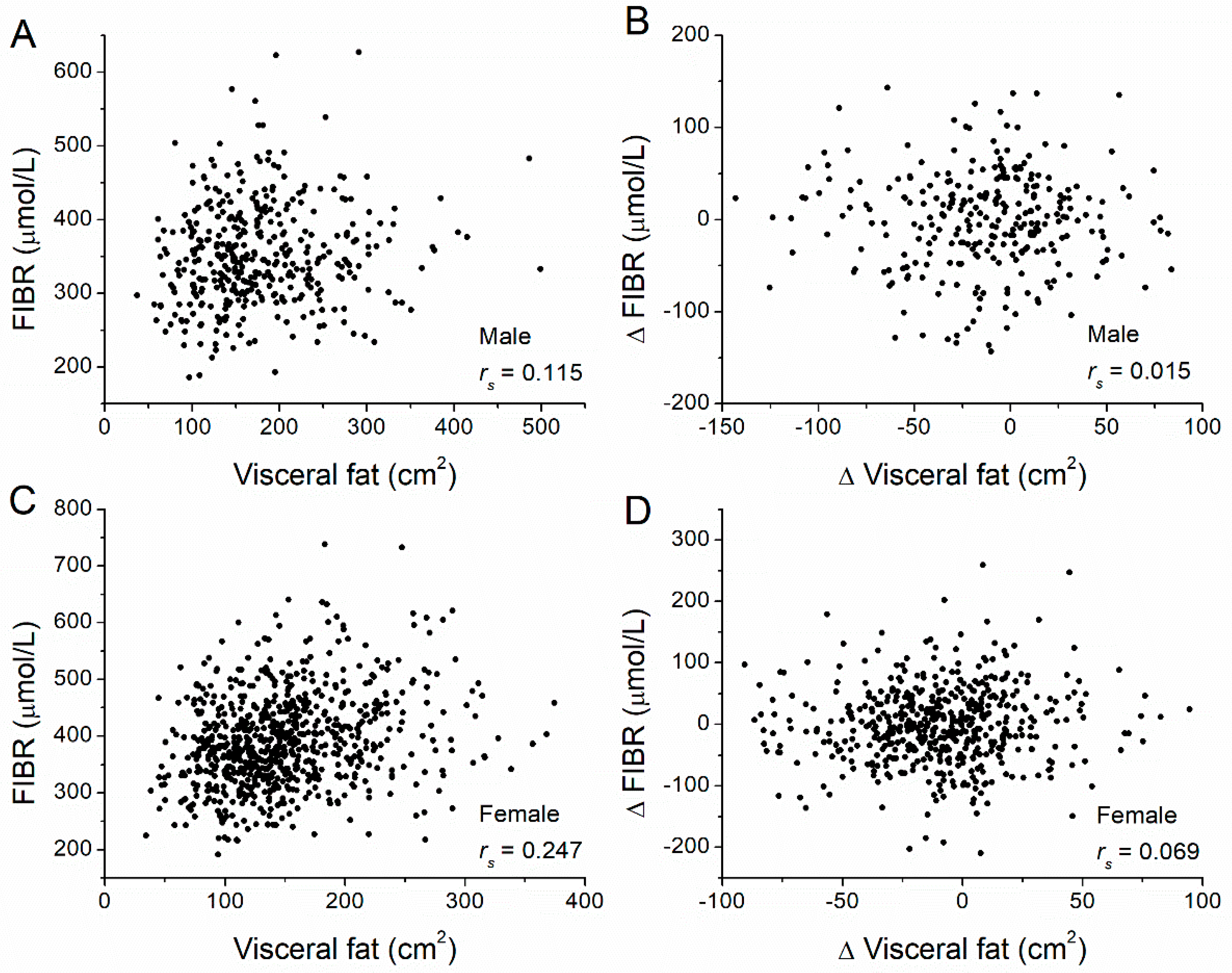
| Visceral | 0.115 | 0.024 | 0.242 | <0.001 | |
| Men | Women | ||||
|---|---|---|---|---|---|
| ΔFat Depot | Markers | rs | p-Value | rs | p-Value |
| Subcutaneous | ΔTPA | 0.356 | <0.001 | 0.293 | <0.001 |
| ΔFIBR | 0.042 | 0.475 | 0.027 | 0.543 | |
| Visceral | ΔTPA | 0.243 | <0.001 | 0.315 | <0.001 |
| ΔFIBR | 0.015 | 0.804 | 0.069 | 0.111 | |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| ΔFat Depot | Marker | rs | p-Value | rs | p-Value | |
| Lifestyle | Subcutaneous | ΔTPA | 0.319 | 0.002 | 0.321 | <0.001 |
| ΔFIBR | 0.228 | 0.031 | 0.124 | 0.103 | ||
| Visceral | ΔTPA | 0.477 | <0.001 | 0.390 | <0.001 | |
| ΔFIBR | 0.207 | 0.050 | 0.030 | 0.692 | ||
| Metformin | Subcutaneous | ΔTPA | 0.178 | 0.070 | 0.317 | <0.001 |
| ΔFIBR | −0.108 | 0.273 | 0.032 | 0.679 | ||
| Visceral | ΔTPA | −0.018 | 0.855 | 0.305 | <0.001 | |
| ΔFIBR | −0.223 | 0.022 | 0.162 | 0.034 | ||
| Placebo | Subcutaneous | ΔTPA | 0.240 | <0.001 | 0.097 | 0.204 |
| ΔFIBR | 0.149 | 0.148 | −0.076 | 0.315 | ||
| Visceral | ΔTPA | 0.155 | 0.134 | 0.078 | 0.306 | |
| ΔFIBR | 0.084 | 0.420 | 0.023 | 0.761 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Salamon, J.; Zhang, R. Correlations between the Changing Levels of Tissue Plasminogen Activator and Adiposity Following Exercise-Induced Weight Loss. Nutrients 2022, 14, 5159. https://doi.org/10.3390/nu14235159
Zhang C, Salamon J, Zhang R. Correlations between the Changing Levels of Tissue Plasminogen Activator and Adiposity Following Exercise-Induced Weight Loss. Nutrients. 2022; 14(23):5159. https://doi.org/10.3390/nu14235159
Chicago/Turabian StyleZhang, Chao, Jonathan Salamon, and Ren Zhang. 2022. "Correlations between the Changing Levels of Tissue Plasminogen Activator and Adiposity Following Exercise-Induced Weight Loss" Nutrients 14, no. 23: 5159. https://doi.org/10.3390/nu14235159
APA StyleZhang, C., Salamon, J., & Zhang, R. (2022). Correlations between the Changing Levels of Tissue Plasminogen Activator and Adiposity Following Exercise-Induced Weight Loss. Nutrients, 14(23), 5159. https://doi.org/10.3390/nu14235159





