The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
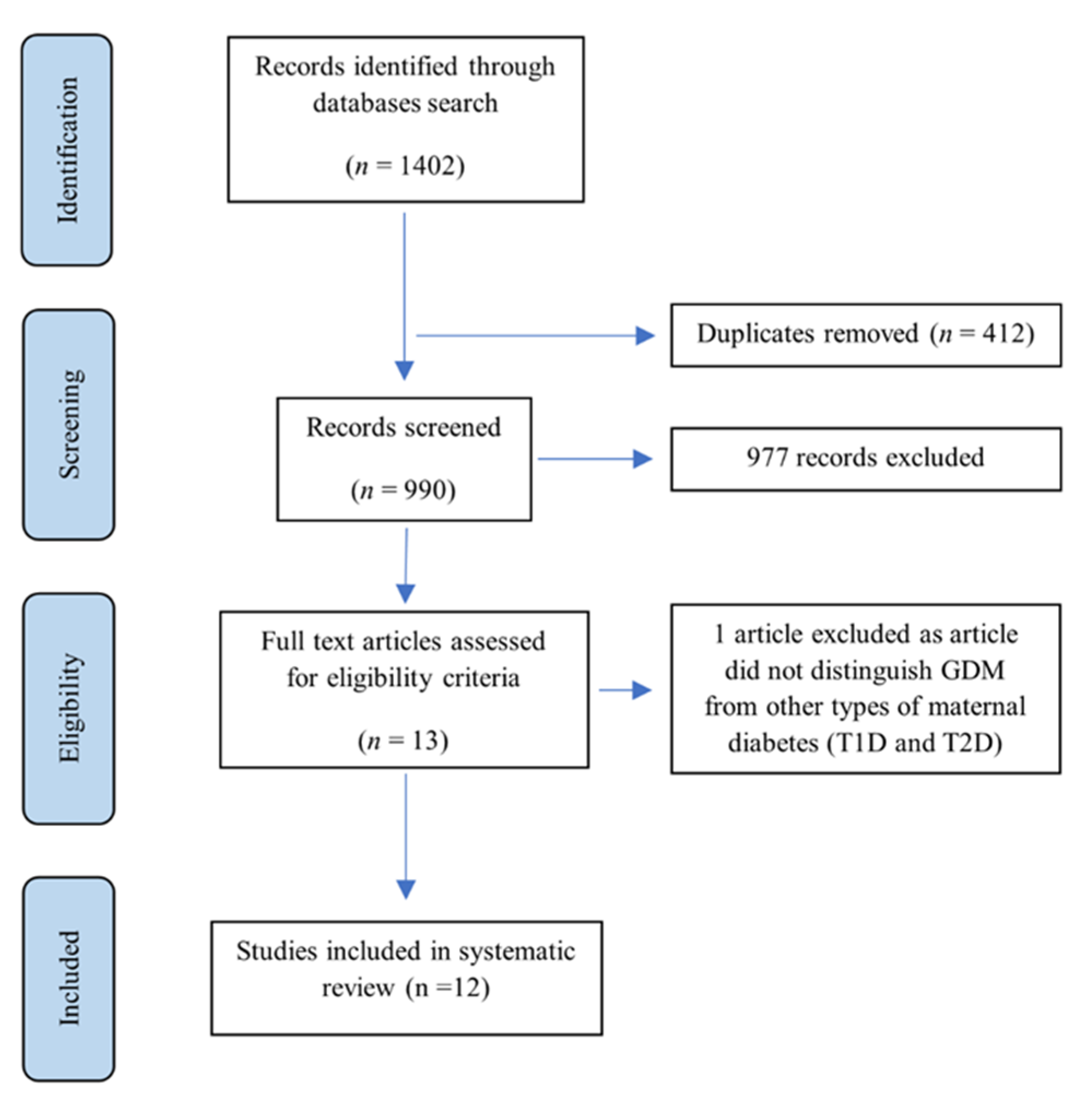
2.4. Selection Process
2.5. Data Extraction
2.6. Quality Assessment
3. Results
3.1. Synthesis
3.2. Description of Studies
3.3. Participant Characteristics
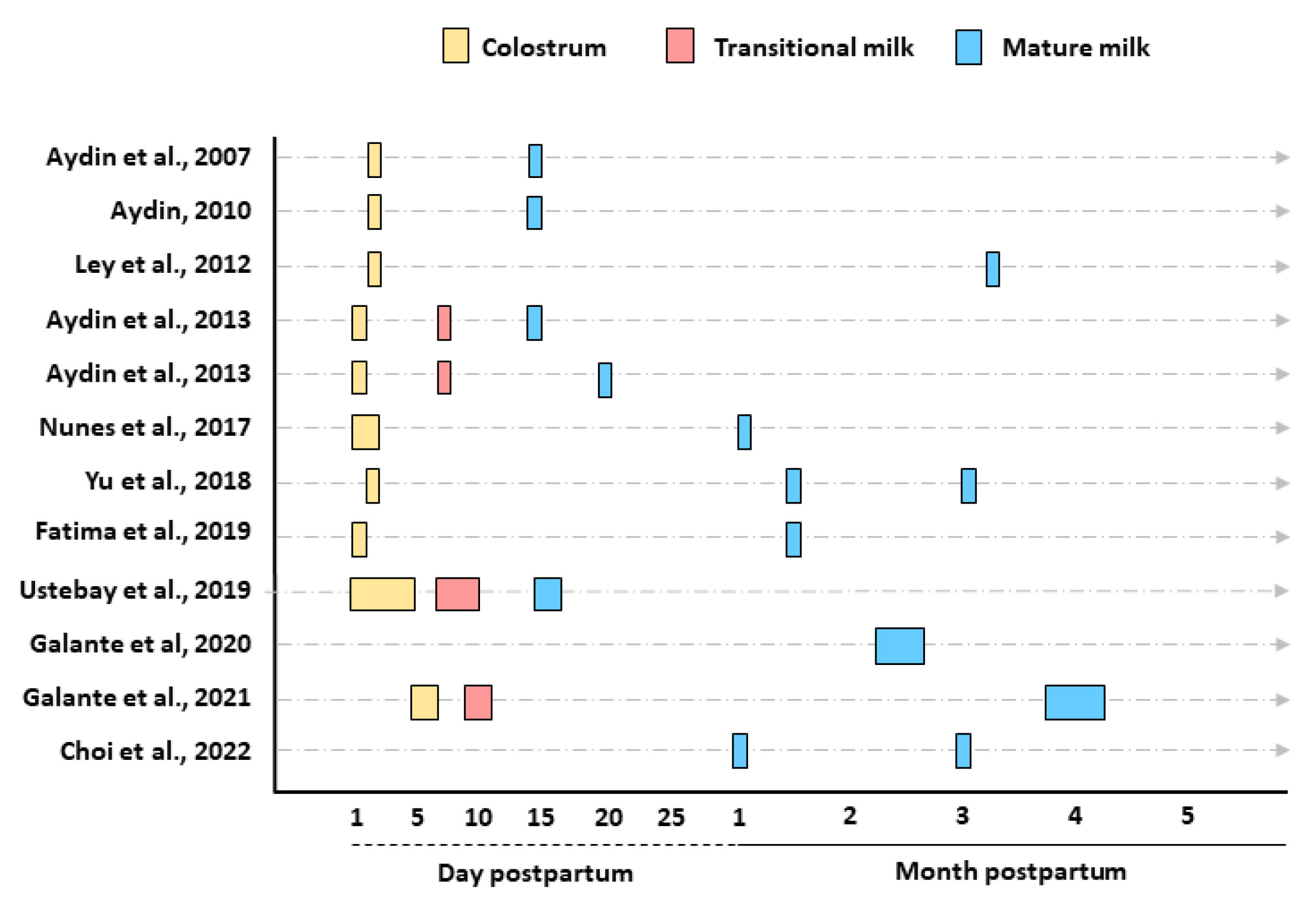
3.4. Stage of Lactation
3.5. Human Milk Sample Collection, Storage, and Preparation for Analysis
3.6. Measurement of Human Milk Metabolic Hormones
3.7. Statistical Analysis
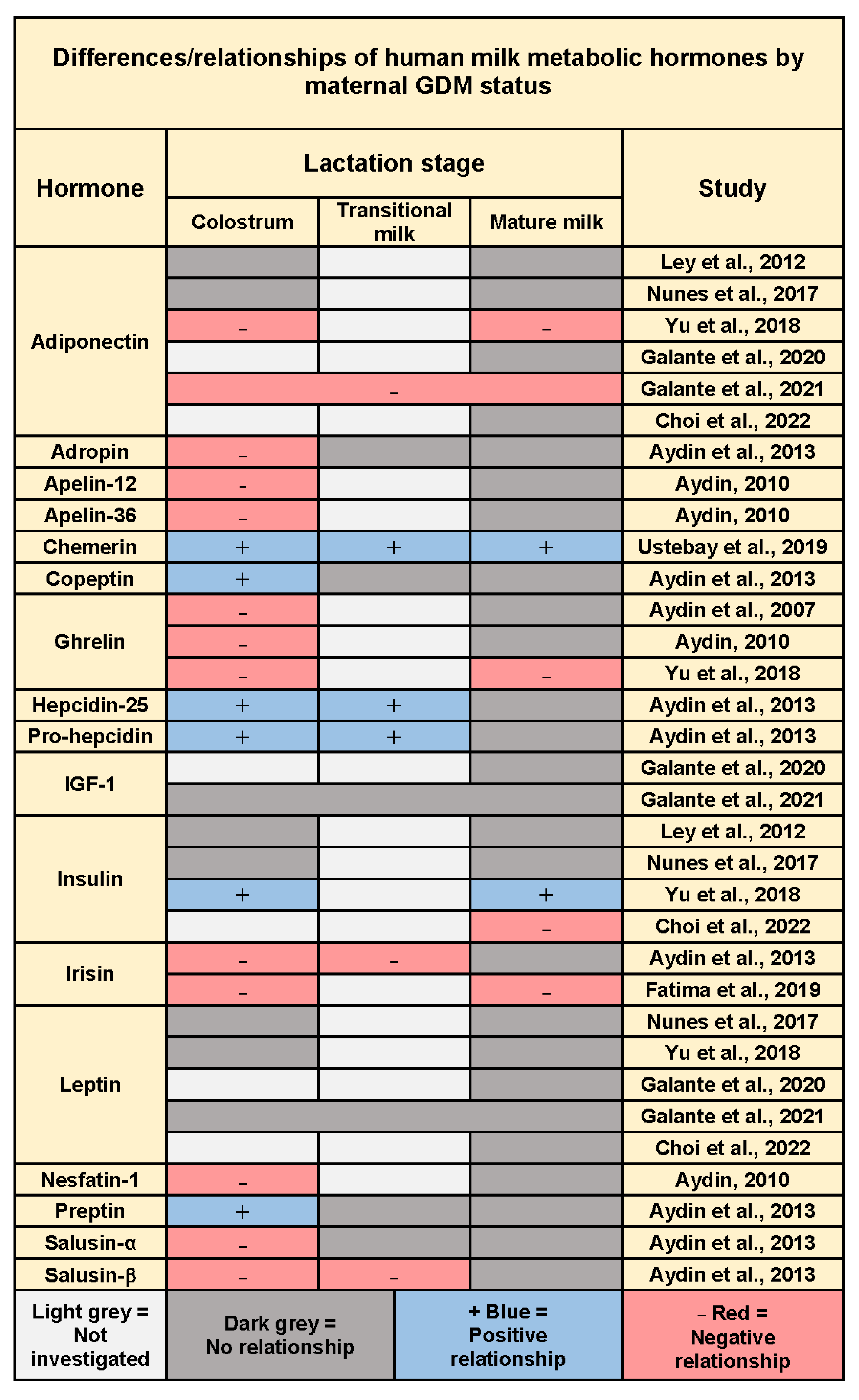
3.8. Gestational Diabetes Mellitus and Human Milk Metabolic Hormones
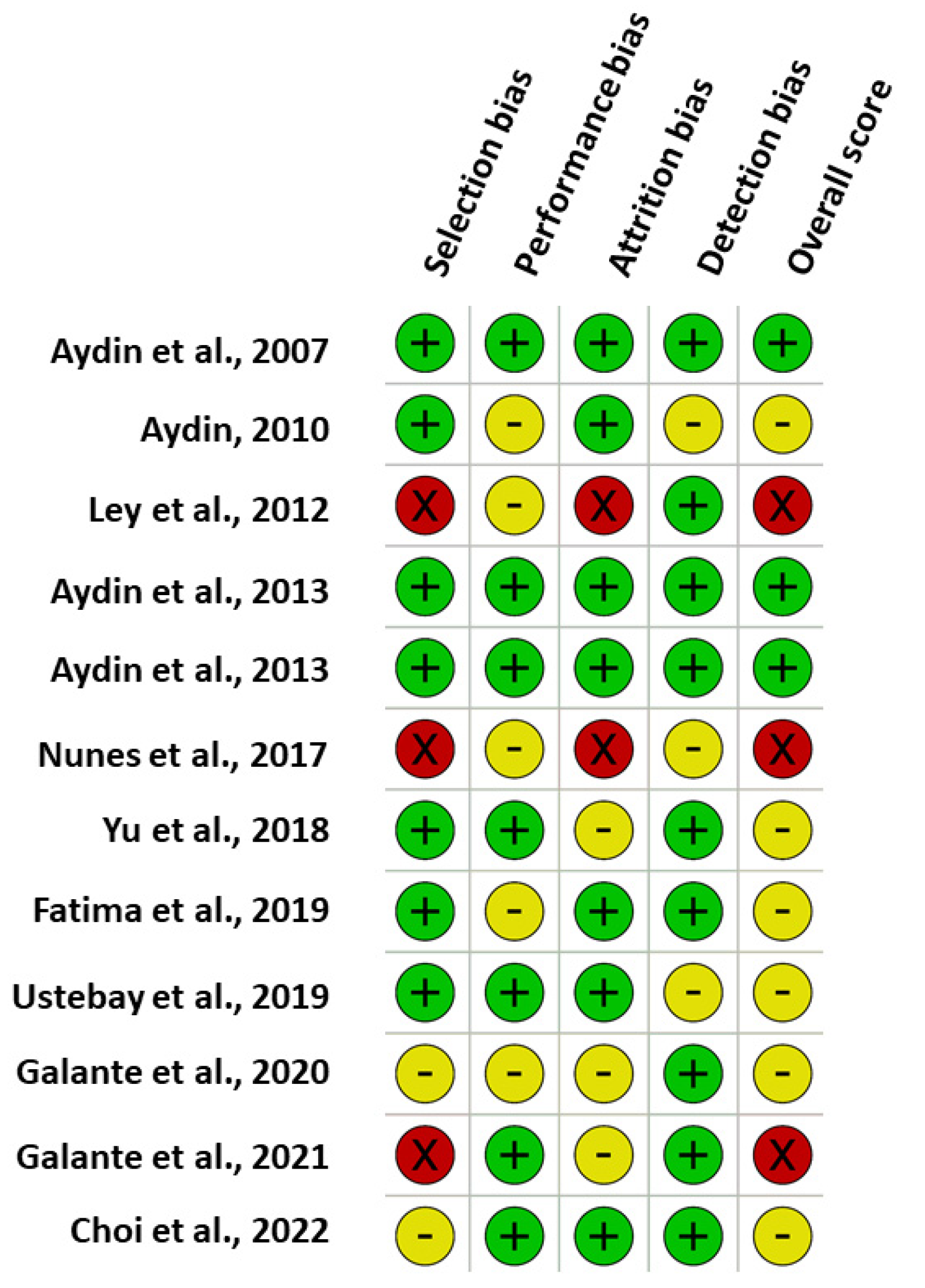
3.9. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Studies | A-Selection Bias | B-Performance Bias | C-Attrition Bias | D-Detection Bias | Overall | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | O | B1 | B2 | B3 | O | C1 | C2 | C3 | O | D1 | D2 | D3 | D4 | D5 | O | ||
| Aydin et al., 2007 [21] | Y | Y | Y | L | Y | NA | NA | L | Y | Y | Y | L | Y | Y | Y | NA | NA | L | L |
| Aydin, 2010 [22] | Y | Y | Y | L | U | NA | NA | U | Y | Y | Y | L | U | Y | U | NA | NA | U | U |
| Ley et al., 2012 [23] | Y | N | N | H | U | NA | NA | U | Y | N | N | H | Y | Y | Y | NA | NA | L | H |
| Aydin et al., 2013 [24] | Y | Y | Y | L | Y | NA | NA | L | Y | Y | Y | L | Y | Y | Y | NA | NA | L | L |
| Aydin et al., 2013 [25] | Y | Y | Y | L | Y | NA | NA | L | Y | Y | Y | L | Y | Y | Y | NA | NA | L | L |
| Nunes et al., 2017 [26] | N | U | Y | H | U | NA | NA | U | Y | N | U | H | Y | Y | U | NA | NA | U | H |
| Yu et al., 2018 [27] | Y | Y | Y | L | Y | NA | NA | L | Y | U | U | U | Y | Y | Y | NA | NA | L | U |
| Fatima et al., 2019 [28] | Y | Y | Y | L | U | NA | NA | U | Y | Y | Y | L | Y | Y | Y | NA | NA | L | U |
| Ustebay et al., 2019 [29] | Y | Y | Y | L | Y | NA | NA | L | Y | Y | Y | L | Y | Y | U | NA | NA | U | U |
| Galante et al., 2020 [30] | Y | Y | U | U | U | NA | NA | U | U | Y | Y | U | Y | Y | Y | NA | NA | L | U |
| Galante et al., 2021 [31] | Y | N | U | H | Y | NA | NA | L | Y | U | U | U | Y | Y | Y | NA | NA | L | H |
| Choi et al., 2022 [32] | Y | Y | U | U | Y | NA | NA | L | Y | Y | U | L | Y | Y | Y | NA | NA | L | U |
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods; World Health Organization: Geneva, Switzerland; The United Nations Children’s Fund (UNICEF): Geneva, Switzerland, 2021; ISBN 978-92-4-001838-9. [Google Scholar]
- Geddes, D.T.; Prescott, S.L. Developmental origins of health and disease: The role of human milk in preventing disease in the 21(st) century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Di Cianni, G.; Miccoli, R.; Volpe, L.; Lencioni, C.; Del Prato, S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab. Res. Rev. 2003, 19, 259–270. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Lewis, C.E.; Lin, Y.; Sorel, M.; Gross, M.; Sidney, S.; Jacobs, D.R., Jr.; Shikany, J.M.; Quesenberry, C.P., Jr. Lactation duration and progression to diabetes in women across the childbearing years: The 30-year CARDIA study. JAMA Intern. Med. 2018, 178, 328–337. [Google Scholar] [CrossRef]
- Ortega-García, J.A.; Kloosterman, N.; Alvarez, L.; Tobarra-Sánchez, E.; Cárceles-Álvarez, A.; Pastor-Valero, R.; López-Hernández, F.A.; Sánchez-Solis, M.; Claudio, L. Full Breastfeeding and Obesity in Children: A Prospective Study from Birth to 6 Years. Child. Obes. 2018, 14, 327–337. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Incidence of Gestational Diabetes in Australia; AIHW: Canberra, Australia, 2019. [Google Scholar]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Gridneva, Z.; Kugananthan, S.; Hepworth, A.R.; Tie, W.J.; Lai, C.T.; Ward, L.C.; Hartmann, P.E.; Geddes, D.T. Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants. Nutrients 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Suárez, P.A.; Rodríguez-Cruz, M.; Nieves-Morales, X. Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. J. Mammary Gland Biol. Neoplasia 2017, 22, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Liguori, S.A.; Fissore, M.F.; Oggero, R. Breast milk hormones and their protective effect on obesity. Int. J. Pediatr. Endocrinol. 2009, 2009, 327505. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Developmental programming of appetite/satiety. Ann. Nutr. Metab. 2014, 64 (Suppl. 1), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Gazzolo, D.; Bertino, E.; Cresi, F.; Coscia, A. Influence of diabetes during pregnancy on human milk composition. Nutrients 2020, 12, 185. [Google Scholar] [CrossRef]
- The EndNote Team. EndNote, EndNote 20; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- National Institute for Health and Care Excellence. The Social Care Guidance Manual: Methodological Checklists for Cohort Studies. Available online: https://www.nice.org.uk/process/pmg10/chapter/appendix-d-methodology-checklist-cohort-studies (accessed on 7 July 2021).
- Aydin, S.; Geckil, H.; Karatas, F.; Donder, E.; Kumru, S.; Kavak, E.C.; Colak, R.; Ozkan, Y.; Sahin, I. Milk and blood ghrelin level in diabetics. Nutrition 2007, 23, 807–811. [Google Scholar] [CrossRef]
- Aydin, S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides 2010, 31, 2236–2240. [Google Scholar] [CrossRef]
- Ley, S.H.; Hanley, A.J.; Sermer, M.; Zinman, B.; O’Connor, D.L. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am. J. Clin. Nutr. 2012, 95, 867–874. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S. Copeptin, adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus. Peptides 2013, 47, 66–70. [Google Scholar] [CrossRef]
- Aydin, S.; Celik, O.; Gurates, B.; Sahin, I.; Ulas, M.; Yilmaz, M.; Kalayci, M.; Kuloglu, T.; Catak, Z.; Aksoy, A.; et al. Concentrations of preptin, salusins and hepcidins in plasma and milk of lactating women with or without gestational diabetes mellitus. Peptides 2013, 49, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.; da Silva, C.H.; Bosa, V.L.; Bernardi, J.R.; Werlang, I.C.R.; Goldani, M.Z. Could a remarkable decrease in leptin and insulin levels from colostrum to mature milk contribute to early growth catch-up of SGA infants? BMC Pregnancy Childbirth 2017, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Rong, S.S.; Sun, X.; Ding, G.; Wan, W.; Zou, L.; Wu, S.; Li, M.; Wang, D. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. Br. J. Nutr. 2018, 120, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Khalid, E.; Ladak, A.A.; Ali, S.A. Colostrum and mature breast milk analysis of serum irisin and sterol regulatory element-binding proteins-1c in gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2019, 32, 2993–2999. [Google Scholar] [CrossRef]
- Ustebay, S.; Baykus, Y.; Deniz, R.; Ugur, K.; Yavuzkir, S.; Yardim, M.; Kalayci, M.; Çaglar, M.; Aydin, S. Chemerin and dermcidin in human milk and their alteration in gestational diabetes. J. Hum. Lact. 2019, 35, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Galante, L.; Lagstrom, H.; Vickers, M.H.; Reynolds, C.M.; Rautava, S.; Milan, A.M.; Cameron-Smith, D.; Pundir, S. Sexually dimorphic associations between maternal factors and human milk hormonal concentrations. Nutrients 2020, 12, 152. [Google Scholar] [CrossRef]
- Galante, L.; Reynolds, C.M.; Milan, A.M.; Alexander, T.; Bloomfield, F.H.; Cameron-Smith, D.; Pundir, S.; Vickers, M.H.; Harding, J.; Meyer, M.; et al. Preterm human milk: Associations between perinatal factors and hormone concentrations throughout lactation. Pediatr. Res. 2021, 89, 1461–1469. [Google Scholar] [CrossRef]
- Choi, Y.; Nagel, E.M.; Kharoud, H.; Johnson, K.E.; Gallagher, T.; Duncan, K.; Kharbanda, E.O.; Fields, D.A.; Gale, C.A.; Jacobs, K.; et al. Gestational diabetes mellitus is associated with differences in human milk hormone and cytokine concentrations in a fully breastfeeding united states cohort. Nutrients 2022, 14, 667. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Sassarini, J.; Kelsey, T.W.; Lindsay, R.S.; Sattar, N.; Nelson, S.M. Accuracy of circulating adiponectin for predicting gestational diabetes: A systematic review and meta-analysis. Diabetologia 2016, 59, 692–699. [Google Scholar] [CrossRef]
- Howlader, M.; Sultana, M.I.; Akter, F.; Hossain, M.M. Adiponectin gene polymorphisms associated with diabetes mellitus: A descriptive review. Heliyon 2021, 7, e07851. [Google Scholar] [CrossRef]
- Gomez-Diaz, R.A.; Gomez-Medina, M.P.; Ramirez-Soriano, E.; Lopez-Robles, L.; Aguilar-Salinas, C.A.; Saucedo, R.; Zarate, A.; Valladares-Salgado, A.; Wacher, N.H. Lower plasma ghrelin levels are found in women with diabetes-complicated pregnancies. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qiao, T.; Xu, F.; Li, Z.; Chen, T.; Su, H.; Chen, G.; Zhang, L.; Xu, D.; Zhang, X. Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: A systematic review and meta-analysis. Cytokine 2020, 126, 154924. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Vilarino-Garcia, T.; Guadix, P.; Duenas, J.L.; Sanchez-Margalet, V. Leptin and nutrition in gestational diabetes. Nutrients 2020, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Hossein-Nezhad, A.; Rahmani, M.; Shafaei, A.R.; Larijani, B. Relationship between leptin concentration and insulin resistance. Horm. Metab. Res. 2007, 39, 903–907. [Google Scholar] [CrossRef]
- Casabiell, X.; Pineiro, V.; Tome, M.A.; Peino, R.; Dieguez, C.; Casanueva, F.F. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metab. 1997, 82, 4270–4273. [Google Scholar] [CrossRef]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between maternal body composition and appetite hormones and macronutrients in human milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef]
- Weyermann, M.; Beermann, C.; Brenner, H.; Rothenbacher, D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin. Chem. 2006, 52, 2095–2102. [Google Scholar] [CrossRef]
- Hassiotou, F.; Savigni, D.; Hartmann, P.; Geddes, D. Mammary cells synthesize appetite hormones that may contribute to breastmilk (38.8). FASEB J. 2014, 28, 38.8. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Gale, C.; Parkinson, J.R.C.; Jeffries, S.; Holmes, E.; Modi, N. Effect of maternal body mass index on hormones in breast milk: A systematic review. PLoS ONE 2014, 9, e115043. [Google Scholar] [CrossRef]
- Kugananthan, S.; Lai, C.T.; Gridneva, Z.; Mark, P.J.; Geddes, D.T.; Kakulas, F. Leptin levels are higher in whole compared to skim human milk, supporting a cellular contribution. Nutrients 2016, 8, 711. [Google Scholar] [CrossRef]
- Mohsen, A.H.; Sallam, S.; Ramzy, M.M.; Hamed, E.K. Investigating the Relationship between Insulin-like Growth Factor-1 (IGF-1) in diabetic mother’s breast milk and the blood serum of their babies. Electron. Physician 2016, 8, 2546–2550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ringholm, L.; Vestgaard, M.; Laugesen, C.S.; Juul, A.; Damm, P.; Mathiesen, E.R. Pregnancy-induced increase in circulating IGF-I is associated with progression of diabetic retinopathy in women with type 1 diabetes. Growth Horm. IGF Res. 2011, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Suwaydi, M.A.; Wlodek, M.E.; Lai, C.T.; Prosser, S.A.; Geddes, D.T.; Perrella, S.L. Delayed secretory activation and low milk production in women with gestational diabetes: A case series. BMC Pregnancy Childbirth 2022, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Fields, D.A.; Pezant, N.P.; Kharoud, H.K.; Gulati, S.; Jacobs, K.; Gale, C.A.; Kharbanda, E.O.; Nagel, E.M.; Demerath, E.W.; et al. Gestational diabetes mellitus is associated with altered abundance of exosomal MicroRNAs in human milk. Clin. Ther. 2022, 44, 172–185.e171. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Rifas-Shiman, S.L.; Zhou, L.; Hu, F.B.; Colditz, G.A.; Gillman, M.W. Breast-feeding and risk for childhood obesity: Does maternal diabetes or obesity status matter? Diabetes Care 2006, 29, 2231–2237. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Hartmann, R.; Pawliczak, J.; Passow, D.; Abou-Dakn, M.; Vetter, K.; Kordonouri, O. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care 2006, 29, 1105–1107. [Google Scholar] [CrossRef]
- Uebel, K.; Pusch, K.; Gedrich, K.; Schneider, K.T.; Hauner, H.; Bader, B.L. Effect of maternal obesity with and without gestational diabetes on offspring subcutaneous and preperitoneal adipose tissue development from birth up to year-1. BMC Pregnancy Childbirth 2014, 14, 138. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Gridneva, Z.; Perrella, S.L.; Wlodek, M.E.; Lai, C.T.; Geddes, D.T. Human milk metabolic hormones: Analytical methods and current understanding. Int. J. Mol. Sci. 2021, 22, 8708. [Google Scholar] [CrossRef]
| Study | Country, Year, Cohort Size (n) | Sample Size/Group | Birth Gestation, Postpartum Glycemic Status a | Lactation Stage (Timing of Sample Collection) | Collection Time, Method, (Storage Temperature) | Hormones Measured | Analytical Method | GDM Outcome Reported | Concentration, Mean ± SD, Median [IQR], Mean Difference (95% CI), and/or β (SEE), p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Aydin et al., [21] | Turkey, 2007 (n = 34) | GDM = 12 T2D = 3 CTL = 14 | Term, no | C (2 d) MM (15 d) | Fasting am Pre-feed, NR (−70 °C) | Ghrelin | RIA, HPLC | GDM: two-fold ↓ C acylated (active) ghrelin No difference in MM | Acyl-ghrelin C (fmol/mL), GDM: 7.75 ± 2.2; non-GDM: 18.99 ± 2.7 (p < 0.05) Acyl-ghrelin MM (fmol/mL), GDM: 16.06 ± 3.2; non-GDM: 16.47 ± 3.3 |
| Aydin, [22] | Turkey 2010 (n = 20) | GDM = 10 CTL = 10 | Term, no | C (2 d) MM (15 d) | Fasting am NR (NR) | Ghrelin Apelin-12 Apelin-36 Nesfatin-1 | EIA: apelin-12, apelin-36 ELISA: ghrelin, nesfatin-1 | GDM: ↓ C ghrelin, apelin-12, apelin-36, nesfatin-1 No difference in MM | Acyl-ghrelin C (pg/mL), GDM: 27.7 ± 2; non-GDM: 39.2 ± 2.0 (p < 0.05) Acyl-ghrelin MM (pg/mL), GDM: 37.7 ± 3.0; non-GDM: 48.2 ± 5.1 Des-acyl ghrelin C (pg/mL), GDM: 338.1 ± 49; non-GDM: 466.1 ± 52 (p < 0.05) Des-acyl ghrelin MM (pg/mL), GDM: 359.1 ± 51.2; non-GDM: 505.1 ± 52 (p < 0.05) Apelin-12 C (ng/mL), GDM: 2.9 ± 0.6; non-GDM: 4.3 ± 1.2 (p < 0.05) Apelin-12 MM (ng/mL), GDM: 3.6 ± 1.2; non-GDM: 5.4 ± 1.8 Apelin-36 C (ng/mL), GDM: 3.2 ± 0.7; non-GDM: 4.9 ± 2.0 (p < 0.05) Apelin-36 MM (ng/mL), GDM: 4.4 ± 1.4; non-GDM: 6.2 ± 1.9 Nesfatin-1 C (ng/mL), GDM: 0.78 ± 0.3; non-GDM: 1.6 ± 0.2 (p < 0.05); Nesfatin-1 MM (ng/mL), GDM: 0.98 ± 0.3; non-GDM: 1.2 ± 0.4 |
| Ley et al., [23] | Canada, 2012 (n = 170) | GDM = 37 CTL = 133 | Term, no | C (median 2 d (1, 3)) MM (median 95 d (91, 102)) | NR C: HE or EBP, MM: complete breast expression from both breasts with EBP (−80 °C) | Adiponectin Insulin | RIA: adiponectin ECLIA: insulin | C, MM adiponectin and insulin not associated with GDM In the analysis restricted to C associations remained non-significant | Concentrations within groups NR Adiponectin C: −0.129 (0.180) (p = 0.47) Adiponectin MM: −0.081 (0.117) (p = 0.49) Insulin C: −0.200 (0.256) (p = 0.44) Insulin MM: 0.102 (0.174) (p = 0.56) |
| Aydin et al., [24] | Turkey, 2013 (n = 44) | GDM = 15 CTL = 15 | Term, no | C (1 d) TM (7 d) MM (15 d) | Fasting am Pre-feed, NR (−40 °C) | Copeptin Irisin Adropin | EIA: copeptin ELISA: irisin, copeptin | GDM: ↑ C copeptin and adropin, ↓ C, TM irisin No difference in MM | For all hormones, results are reported as figures only (p < 0.05) |
| Aydin et al., [25] | Turkey, 2013 (n = 36) | GDM = 12 CTL = 12 | Term, no | C (1 d) TM (7 d) MM (20 d) | Fasting am Pre-feed, NR (−80 °C) | Preptin Salusin-α Salusin-β Pro-hepcidin Hepcidin-25 | ELISA | GDM: ↑ C preptin, ↓ C salusin-α and salusin-β, ↑ C, TM pro-hepcidin and hepcidin No difference in MM | Preptin C (ng/mL), GDM: 14.32 ± 3.06; non-GDM: 9.72 ± 2.26 (p < 0.05) Preptin TM (ng/mL), GDM: 11.72 ± 2.34; non-GDM: 9.02 ± 0.88 Preptin MM (ng/mL), GDM: 10.16 ± 2.19; non-GDM: 11.16 ± 5.70 Salusin-α C (pg/mL), GDM: 187.80 ± 19.01; non-GDM: 261.40 ± 31.35 (p < 0.01) Salusin-α TM (pg/mL), GDM: 211.20 ± 44.61; non-GDM: 242.20 ± 23.97 Salusin-α MM (pg/mL), GDM: 248.80 ± 22.14; non-GDM: 218.60 ± 60.0 Salusin-β C (pg/mL), GDM: 379.0 ± 100.86; non-GDM: 530.20 ± 70.18 (p < 0.05) Salusin-β TM (pg/mL), GDM: 425.0 ± 34.07; non-GDM: 494.40 ± 45.99 (p < 0.05) Salusin-β MM (pg/mL), GDM: 501.0 ± 65.60; non-GDM: 450.0 ± 68.04 Pro-hepcidin C (pg/mL), GDM: 814.0 ± 72.98; non-GDM: 649.60 ± 39.34 (p < 0.01) Pro-hepcidin TM (pg/mL), GDM: 761.40 ± 40.45; non-GDM: 572.0 ± 49.70 (p < 0.01) Pro-hepcidin MM (pg/mL), GDM: 613.60 ± 61.77; non-GDM: 528.80 ± 47.77 Hepcidin-25 C (pg/mL), GDM: 835.80 ± 93.73; non-GDM: 595.0 ± 77.26 (p < 0.01) Hepcidin-25 TM (pg/mL), GDM: 746.20 ± 82.18; non-GDM: 580.60 ± 82.76 (p < 0.05) Hepcidin-25 MM (pg/mL), GDM: 641.20 ± 63.71; non-GDM: 614.0 ± 63.85 |
| Nunes et al., [26] | Brazil, 2017 (n = 69) | GDM = 12 CTL = 21 | Term, no | C (1–2 d) MM (30 d) | NR HE, no control for pre-/post-feed sampling and maternal fasting status (−80 °C) | Adiponectin Insulin Leptin | ELISA | No difference between women with and without GDM | Adiponectin C (ng/mL), GDM: 10.23 [5.63, 22.65]; non-GDM: 8.79 [6.90, 11.35] Adiponectin MM (ng/mL), GDM: 12.43 [6.90, 14.87]; non-GDM: 9.87 [6.33, 11.50] Insulin C µIU/mL, GDM: 49.37 [25.70, 176.54]; non-GDM: 55.04 [11.57, 162.64] Insulin MM, GDM: 22.83 [16.33, 60.43]; non-GDM: 22.03 [13.30, 32.21] Leptin C (ng/mL), GDM: 0.67 [0.45,1.31]; non-GDM: 0.81 [0.42, 1.27] Leptin MM, GDM: 0.46 [0.45, 0.70]; non-GDM: 0.72 [0.49, 0.90] |
| Yu et al., [27] | China, 2018 (n = 96) | GDM = 48 CTL = 48 | Term, yes | C (3 d) MM (42, 90 d) | 3d: 8:00–9:00 Pre-feed 42d, 90d: 14:00–16:00 One breast expression with EBP (−80 °C) | Adiponectin Insulin Leptin Total ghrelin | ELISA | GDM: ↓ adiponectin and total ghrelin, ↑ insulin in C and at d90 No difference in hormone concentrations between women with and without GDM at d42 No difference in leptin concentrations between women with and without GDM | Adiponectin C (log ng/mL), GDM: 21.74 [14.77, 56.10]; non-GDM: 65.81 [29.76, 126.91] (p < 0·001) Adiponectin d42 (log ng/mL), GDM: 11.89 [8.0, 18.37]; non-GDM: 12.22 [9.69, 14.92] (p = 0.89) Adiponectin d90 (log ng/mL), GDM: 11.75 [8.53, 13.91]; non-GDM: 15.31 [11.60, 19.53] (p = 0.009) Insulin C (log µIU/mL), GDM: 22.80 [13.51, 51.25]; non-GDM: 20.41 [7.68, 31.38] (p = 0.047) Insulin d42 (log µIU/mL), GDM: 32.36 [13.06, 58.22]; non-GDM: 28.20 [17.97, 40.05] (p = 0.38) Insulin d90 (log µIU/mL), GDM: 40.63 [22.48, 57.17]; non-GDM: 24.61 [13.40, 31.85] (p = 0.021) Leptin C (log µIU/mL), GDM: 1.28 [0.87, 2.63]; non-GDM: 1.49 [0.56, 3.25] (p = 0.77) Leptin d42 (log µIU/mL), GDM: 0.26 [0.09, 0.47]; non-GDM: 0.21 [0.09, 0.51] (p = 0.69) Leptin d90 (log µIU/mL), GDM: 0.20 [0.12, 0.47]; non-GDM: 0.25 [0.16, 0.45] (p = 0.54) Total ghrelin C (log pg/mL), GDM: 124.43 [89.87, 178.76]; non-GDM: 159.36 [122.62, 234.33] (p = 0·011) Total ghrelin d42 (log pg/mL), GDM: 338.74 [189.98, 432.95]; non-GDM: 337.60 [149.82, 565.77] (p = 0.80) Total ghrelin d90 (log pg/mL), GDM: 104.62 [72.72, 154.71]; non-GDM: 210.91 [147.25, 381.88] (p < 0.001) |
| Fatima et al., [28] | Pakistan, 2019 (n = 66) | GDM = 33 CTL = 33 | NR, no | C (1–3 d) MM (42 d) | 08:00–10:00 2 h after previous breastfeed with manual breast pump (−80 °C) | Irisin | ELISA | GDM: ↓ irisin in C and MM | Irisin C (pg/mL), GDM: 10.36 ± 4.73; non-GDM: 57.08 ± 8.28 (p < 0.001) Irisin MM (pg/mL), GDM: 15.35 ± 0.42; non-GDM: 56.40 ± 9.55 (p < 0.001) |
| Ustebay et al., [29] | Turkey, 2019 (n = 60) | GDM = 26 CTL = 27 | Term, no | C (1–5 d) TM (7–10 d) MM (15–17 d) | Fasting am NR (−80 °C) | Chemerin | ELISA | GDM: ↑ chemerin in C and MM | Results are reported as figure only (p < 0.05) |
| Galante et al., [30] | Finland, 2020 (n = 510) | GDM = 44 CTL = 460 | Term 95.2%; Preterm 4.2%, no | MM (2.6 ± 0.4 mo) | 10:00–12:00 HE full single breast, first few drops of milk discarded (−70 °C) | Adiponectin IGF-1 Leptin | ELISA | No overall difference between women with and without GDM ↓ MM adiponectin in GDM with male infant compared to GDM with female infant or CTL | Adiponectin MM (log 10 ng/mg): −0.012 [−0.099, 0.074] (p = 0.78) IGF-1 MM (log 10 ng/mg): 0.021 [−0.048, 0.091] (p = 0.55) Leptin MM (log 10 ng/mg): −0.018 [−0.093, 0.058] (p = 0.65) Sex-specific differences (p = 0.031) non-significant when correcting for exclusive duration of breastfeeding (p = 0.05) |
| Galante et al., [31] | New Zealand, 2021 (n = 194) | GDM = 36 CTL = 155 | Preterm, no | C (5 ± 2 d) TM (10 ± 2 d) MM (4 ± 0.5 mo) | 10:00–12:00 2–3 h after previous expression or breastfeed, complete right breast expression with EBP (−80 °C) | Adiponectin IGF-1 Leptin | ELISA | GDM: ↓ adiponectin independent of collection time point | Adiponectin (log 10 ng/mg), GDM: 0.199 [0.098, 0.300]; non-GDM: NR (p < 0.001) IGF-1 (log 10 ng/mg), GDM: 0.021 [−0.031, 0.073]; non-GDM: NR (p = 0.42) Leptin (log 10 ng/mg), GDM: −0.048 [−0.078, 0.174]; non-GDM: NR (p = 0.45) |
| Choi et al., [32] | The United States of America, 2021 (n = 189) | GDM = 35 CTL = 154 | Term, no | MM (1 ± 0.2 mo, 3 ± 0.3 mo) | 10:00–12:00 2 h after previous expression or breastfeed, complete right breast expression with EBP (−80 °C) | Adiponectin Insulin Leptin | ELISA | GDM: ↓ MM insulin | Adiponectin mo1 (log ng/mL), GDM: 2.90 ± 0.08; non-GDM: 2.99 ± 0.03; −0.07 (0.10) (p = 0.44) Adiponectin mo3 (log ng/mL), GDM: 2.65 ± 0.08; non-GDM: 2.73 ± 0.06; −0.06 (0.11) (p = 0.61) Insulin mo1 (log µIU/mL), GDM: 2.91 ± 0.14; non-GDM: 3.17 ± 0.06; −0.38 (0.17) (p = 0.03) Insulin mo3 (log µIU/mL), GDM: 2.78 ± 0.14; non-GDM: 3.18 ± 0.06; −0.53 (0.17) (p = 0.003) Leptin mo1 (log pg/mL), GDM: 6.44 ± 0.16; non-GDM: 6.23 ± 0.07; 0.04 (0.19) (p = 0.85) Leptin mo3 (log pg/mL), GDM: 6.19 ± 0.16; non-GDM: 6.03 ± 0.07; −0.01 (0.19) (p = 0.96) |
| Study | Statistical Analyses | Data Expression | Data Transformation and Adjustment for Potential Confounders, Significance Level | Total Cohort Size (Control/GDM Subgroups) | Demographics |
|---|---|---|---|---|---|
| Aydin et al., [21] | Mann–Whitney U test for comparison between groups | Mean ± SD | Correlation coefficients indicate (p < 0.05) | 34 (14/12) | Parity, gestation, and BMI were matched |
| Aydin [22] | Spearman’s correlation analysis for relationship between the groups | Mean ± SD | Correlation coefficients indicate (p < 0.05) | 20 (10/10) | Parity, gestation, and BMI were matched |
| Ley et al., [23] | General linear models for associations of hormones in colostrum and mature milk with prenatal maternal metabolic variables, including GDM status and time from delivery to milk collection | Mean ± SD Median [IQR] β (SEE) | Log transformed concentrations of HM components General linear model analyses with adjustment for maternal age, ethnicity, and time elapsed from birth to milk collection Correlation coefficients indicate (p < 0.05; p < 0.01 for interaction terms) | 170 (133/37) | Pre-pregnancy BMI used to divide the cohort (≥25 vs. ≤25 kg/m2), no significant difference except in HOMA-IR and ISogtt Total of 37 women with GDM 23 ≤ 25 kg/m2 vs. 14 ≥ 25 kg/m2 (no significant difference) |
| Aydin et al., [24] | Mann–Whitney U test for comparison between groups | Mean ± SD | Correlation coefficients indicate (p < 0.05) | 44 (15/15) | BMI higher in lactating women with GDM–no difference in parity and gestation |
| Aydin et al., [25] | Mann–Whitney U test for comparison between groups | Mean ± SD | Correlation coefficients indicate (p < 0.05) | 36 (12/12) | BMI higher in lactating women with GDM–no difference in parity and gestation |
| Nunes et al., [26] | Kruskal–Wallis test with the Games–Howell post-hoc test to assess the difference between the groups | Mean ± SD Mean difference (95% CI) Median [IQR] | 95% confidence intervals were considered and a significance level of 5% (p ≤ 0.05) | 69 (21/12) | Pre-pregnancy and at birth, maternal BMI were significantly higher in GDM compared to CTL |
| Yu et al., [27] | Generalised Estimating Equation (GEE) using longitudinal data to assess the correlation between maternal or obstetrical factors and HM hormone concentrations | Mean ± SD Median [IQR] | Bonferroni correction to control for multiple comparisons Adjustment for maternal age Correlation coefficients indicate (p < 0.05) | 96 (48/48) | BMI significantly higher in GDM group at pre-pregnancy and at day 90 postpartum |
| Fatima et al., [28] | Mann–Whitney U test for comparison between the groups | Mean ± SD | Correlation adjusted for maternal BMI | 66 (33/33) | BMI significantly higher in GDM group |
| Ustebay et al., [29] | t-test and the Mann–Whitney U test for comparisons between the groups | Mean ± SD | Correlation coefficients indicate (p < 0.05) | 53 (27/26) | Age, parity, BMI similar |
| Galante et al., [30] | Multivariate analysis of variance (MANOVA) used to assess the effect of categorical variables on HM composition | Mean difference (95% CI) | Log transformed concentrations of HM components Exclusive breastfeeding used as correcting factor Bonferroni correction to control for multiple comparisons Correlation coefficients indicate (p < 0.05) | 510 (460/44) | 142 women with obesity and overweight status vs. 343 women with normal weight; no details of GDM Some women did not exclusively breastfeed |
| Galante et al., [31] | Mixed-effects modelling used to investigate differences in HM bioactive concentrations over time across the groups defined by participant characteristics, including GDM group | Mean difference (95% CI) | Log transformed concentrations of HM components Bonferroni correction to control for multiple comparisons (p < 0.05) | 169 (155/36) | Preterm cohort, no details of cohorts’ BMI or breastfeeding status at time of sample collection |
| Choi et al., [32] | Mixed-effects modelling to examine the associations of GDM status with HM hormones | Mean ± SD β (SEE) | Log transformed concentrations of HM components Adjustment for multiple covariates (p < 0.05) | 189 (154/35) | Significantly higher BMI in GDM group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwaydi, M.A.; Zhou, X.; Perrella, S.L.; Wlodek, M.E.; Lai, C.T.; Gridneva, Z.; Geddes, D.T. The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review. Nutrients 2022, 14, 3620. https://doi.org/10.3390/nu14173620
Suwaydi MA, Zhou X, Perrella SL, Wlodek ME, Lai CT, Gridneva Z, Geddes DT. The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review. Nutrients. 2022; 14(17):3620. https://doi.org/10.3390/nu14173620
Chicago/Turabian StyleSuwaydi, Majed A., Xiaojie Zhou, Sharon L. Perrella, Mary E. Wlodek, Ching Tat Lai, Zoya Gridneva, and Donna T. Geddes. 2022. "The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review" Nutrients 14, no. 17: 3620. https://doi.org/10.3390/nu14173620
APA StyleSuwaydi, M. A., Zhou, X., Perrella, S. L., Wlodek, M. E., Lai, C. T., Gridneva, Z., & Geddes, D. T. (2022). The Impact of Gestational Diabetes Mellitus on Human Milk Metabolic Hormones: A Systematic Review. Nutrients, 14(17), 3620. https://doi.org/10.3390/nu14173620







