The Power of Suggestion: Subjective Satiety Is Affected by Nutrient and Health-Focused Food Labelling with No Effect on Physiological Gut Hormone Release
Abstract
1. Introduction
2. Materials and Methods
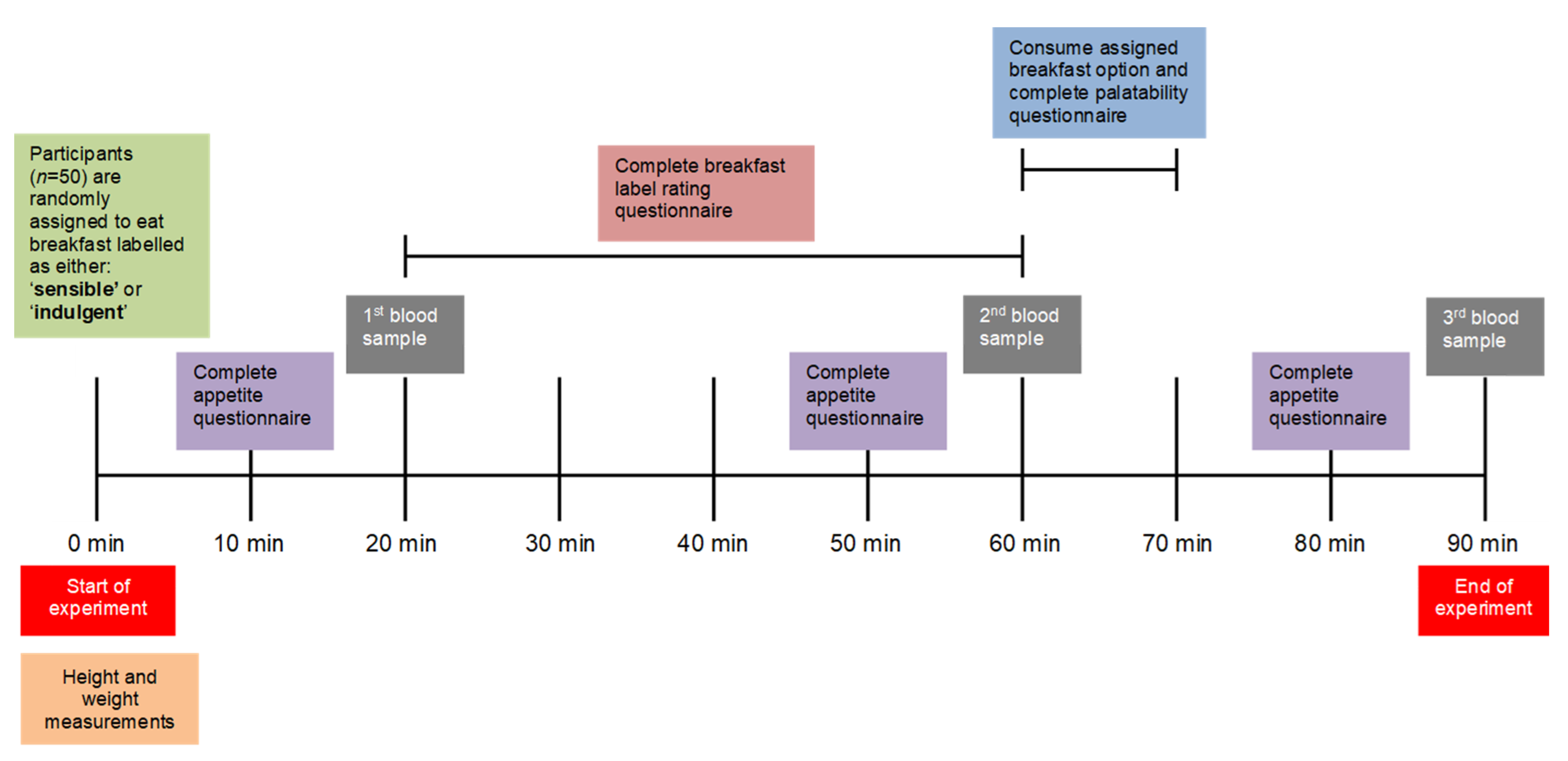
2.1. Study Design
2.2. Participants
2.3. Data Collection and Analysis
2.3.1. Socio-Demographic
2.3.2. Anthropometry
2.3.3. Restrained Eating
2.3.4. Self-Reported Appetite
2.3.5. Breakfast Label Rating
2.3.6. Palatability Rating
2.3.7. Gastrointestinal Hormones
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Rating of Breakfast Labels and Palatability
3.3. Self-Reported Appetite
3.4. Gastrointestinal Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClure, S.M.; Li, J.; Tomlin, D.; Cypert, K.S.; Montague, L.M.; Montague, P. Neural Correlates of Behavioral Preference for Culturally Familiar Drinks. Neuron 2004, 44, 379–387. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Nachtigal, D.J.; Flammer, L.J.; de Araujo, I.E.; Small, D.M. Verbal descriptors influence hypothalamic response to low-calorie drinks. Mol. Metab. 2013, 2, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Turnwald, B.P.; Crum, A.J. Smart food policy for healthy food labeling: Leading with taste, not healthiness, to shift consumption and enjoyment of healthy foods. Prev. Med. 2018, 119, 7–13. [Google Scholar] [CrossRef]
- Raghunathan, R.; Naylor, R.W.; Hoyer, W.D. The Unhealthy = Tasty Intuition and Its Effects on Taste Inferences, Enjoyment, and Choice of Food Products. J. Mark. 2006, 70, 170–184. [Google Scholar] [CrossRef]
- Department of Health Social Services and Public Safety. A Fitter Future for All. Framework for preventing and addressing overweight and obesity in Northern Ireland 2012–2022. 2015. Available online: https://www.health-ni.gov.uk/publications/obesity-prevention-framework-and-reports (accessed on 26 July 2022).
- WHO Regional Office for Europe. The Challenge of Obesity in the WHO European Region and the Strategies for Response; WHO: Copenhagen, Denmark, 2007. [Google Scholar] [CrossRef]
- Miller, L.M.S.; Cassady, D.L. We are what we eat: Healthy eating trends around the world. Appetite 2015, 92, 207–216. [Google Scholar] [CrossRef]
- Benson, T.; Lavelle, F.; Bucher, T.; McCloat, A.; Mooney, E.; Egan, B.; Collins, C.E.; Dean, M. The Impact of Nutrition and Health Claims on Consumer Perceptions and Portion Size Selection: Results from a Nationally Representative Survey. Nutrients 2018, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Fay, S.; Hinton, E.C.; Rogers, P.J.; Brunstrom, J.M. Product labelling can confer sustained increases in expected and actual satiety. Appetite 2011, 57, 557. [Google Scholar] [CrossRef]
- Talati, Z.; Pettigrew, S.; Kelly, B.; Ball, K.; Neal, B.; Dixon, H.; Shilton, T.; Miller, C. Can front-of-pack labels influence portion size judgements for unhealthy foods? Public Health Nutr. 2018, 21, 2776–2781. [Google Scholar] [CrossRef]
- Shide, D.J.; Rolls, B.J. Information About the Fat Content of Preloads Influences Energy Intake in Healthy Women. J. Am. Diet. Assoc. 1995, 95, 993–998. [Google Scholar] [CrossRef]
- Chambers, L.; Ells, H.; Yeomans, M.R. Can the satiating power of a high energy beverage be improved by manipulating sensory characteristics and label information? Food Qual. Preference 2013, 28, 271–278. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Lartamo, S.; Procter, E.L.; Lee, M.D.; Gray, R.W. The actual, but not labelled, fat content of a soup pre-load alters short-term appetite in healthy men. Physiol. Behav. 2001, 73, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, G.P.; Pourshahidi, L.K.; Wallace, J.M.W.; A Kerr, M.; A McCaffrey, T.; E Livingstone, M.B. Perceived ‘healthiness’ of foods can influence consumers’ estimations of energy density and appropriate portion size. Int. J. Obes. 2013, 38, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B.; Chandon, P. Can “Low-Fat” Nutrition Labels Lead to Obesity? J. Mark. Res. 2006, 43, 605–617. [Google Scholar] [CrossRef]
- Kaur, A.; Scarborough, P.; Rayner, M. A systematic review, and meta-analyses, of the impact of health-related claims on dietary choices. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 93. [Google Scholar] [CrossRef]
- Cassady, B.; Considine, R.V.; Mattes, R.D. Beverage consumption, appetite, and energy intake: What did you expect? Am. J. Clin. Nutr. 2012, 95, 587–593. [Google Scholar] [CrossRef]
- Crum, A.J.; Corbin, W.R.; Brownell, K.D.; Salovey, P. Mind over milkshakes: Mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011, 30, 424–429. [Google Scholar] [CrossRef]
- Office of National Statistics. The National Statistics Socio-Economic Classification (NSSEC). 2021. Available online: https://www.ons.gov.uk/methodology/classificationsandstandards/otherclassifications/thenationalstatisticssocioeconomicclassificationnssecrebasedonsoc2010 (accessed on 10 November 2022).
- Schur, E.A.; Cummings, D.E.; Callahan, H.S.; Foster-Schubert, K.E. Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiol. Behav. 2008, 93, 706–712. [Google Scholar] [CrossRef]
- Polivy, J.; Herman, C.P. Restrained Eating and Food Cues: Recent Findings and Conclusions. Curr. Obes. Rep. 2017, 6, 79–85. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assess-ment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.; Ferri, G.-L.; Bacarese-Hamilton, A.; Fuessl, H.; Polak, J.; Bloom, S. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, B.; Ghatei, M.; Williams, G.; Bloom, S. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 330, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Hills, M.; Armitage, P. The two-period cross-over clinical trial. Br. J. Clin. Pharmacol. 1979, 8, 7–20. [Google Scholar] [CrossRef]
- Iles, I.A.; Nan, X.; Verrill, L. Nutrient Content Claims: How They Impact Perceived Healthfulness of Fortified Snack Foods and the Moderating Effects of Nutrition Facts Labels. Health Commun. 2017, 33, 1308–1316. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Nayga, R.M.; Capps, O. Food Label Use, Self-Selectivity, and Diet Quality. J. Consum. Aff. 2001, 35, 346–363. [Google Scholar] [CrossRef]
- Gadah, N.S.; Brunstrom, J.M.; Rogers, P.J. Cross-over studies underestimate energy compensation: The example of sucrose-versus sucralose-containing drinks. Appetite 2016, 107, 398–405. [Google Scholar] [CrossRef]
- Frecka, J.M.; Mattes, R.D. Possible entrainment of ghrelin to habitual meal patterns in humans. Am. J. Physiol. Liver Physiol. 2008, 294, G699–G707. [Google Scholar] [CrossRef]
- Vadiveloo, M.; Morwitz, V.; Chandon, P. The interplay of health claims and taste importance on food consumption and self-reported satiety. Appetite 2013, 71, 349–356. [Google Scholar] [CrossRef]
- Myhre, R.; Kratz, M.; Goldberg, J.; Polivy, J.; Melhorn, S.; Buchwald, D.; Cummings, D.E.; Schur, E.A. A twin study of differences in the response of plasma ghrelin to a milkshake preload in restrained eaters. Physiol. Behav. 2014, 129, 50–56. [Google Scholar] [CrossRef][Green Version]


| Kcal | Fat (g) | Carbohydrate (g) | Protein (g) | |
|---|---|---|---|---|
| Natural yoghurt (150g) | 123 | 6.8 | 8.4 | 7.7 |
| Granola (50g) | 209 | 5.6 | 32.8 | 5.1 |
| Compote (30g) | 48 | 0.3 | 10.5 | 0.2 |
| Total (230g) | 380 | 12.7 | 51.7 | 13 |
| Sample Characteristics | ||
|---|---|---|
| Total sample n (%) | 50 (100) | |
| Sex n (%) | Female | 33 (66) |
| Age (years) mean (SD) | 30.1 (10.4) | |
| Geographical location n (%) | Urban | 31 (62) |
| Suburban | 13 (26) | |
| Rural | 6 (12) | |
| a Occupational classification n (%) | Higher managerial, admin & professional | 11 (22) |
| Intermediate occupations | 8 (16) | |
| Routine & manual occupations | 0 (0) | |
| Student (undergraduate/postgraduate) | 31 (62) | |
| Education level (years) mean (SD) | 18.4 (3.1) | |
| BMI (kg/m2) mean (SD) | 24.6 (3.6) | |
| BMI categories n (%) | Normal weight (18.5–24.9 kg/m2) | 31 (62) |
| Overweight (25–29.9 kg/m2) | 14 (28) | |
| Obese (>30 kg/m2) | 5 (10) | |
| How often read nutrition information on food labels n (%) | Often/always | 29 (60.4) |
| Occasionally/rarely | 18 (37.5) | |
| Never | 1 (2.1) | |
| How often read health claims on food labels n (%) | Often/always | 21 (43.8) |
| Occasionally/rarely | 18 (45.8) | |
| Never | 5 (10.4) |
| Indulgent Breakfast | Sensible Breakfast | |||||
|---|---|---|---|---|---|---|
| Visual Analogue Scales | Sample Size | Mean (SD) | Sample Size | Mean (SD) | Mean Difference a (95% CI) | p |
| Breakfast package rating | ||||||
| Appeal | 48 | 87.12 (10.5) | 48 | 69.7 (19.3) | 17.3 (11.6, 23.0) | <0.001 |
| Perceived healthiness | 48 | 45.8 (21.1) | 48 | 70.5 (13.6) | −24.8 (−31.6, −17.9) | <0.001 |
| Palatability rating | ||||||
| Breakfast (food) appearance | 48 | 84.3 (12.4) | 48 | 79.1 (13.8) | 5.0 (0.7, 9.3) | 0.024 |
| Smell of the breakfast | 48 | 77.1 (16.2) | 48 | 75.8 (13.7) | 1.5 (−2.4, 5.3) | 0.455 |
| Taste of the Breakfast | 48 | 80.0 (13.0) | 48 | 78.3 (13.2) | 1.65 (−2.8, 6.1) | 0.462 |
| Overall palatability | 48 | 79.4 (16.1) | 48 | 80.2 (13.8) | −0.7 (−6.0, 4.5) | 0.777 |
| Enjoyed the breakfast | 47 | 80.2 (16.1) | 47 | 77.3 (15.6) | 2.8 (−3.0, 8.6) | 0.336 |
| Healthy feeling while eating breakfast | 47 | 58.9 (19.8) | 47 | 72.2 (13.3) | −13.2 (−18.8, −7.6) | <0.001 |
| Indulgent Breakfast | Sensible Breakfast | |||||
|---|---|---|---|---|---|---|
| Time Point | Sample Size | Mean Change (SD) | Mean Change (SD) | Mean Difference (95% CI) | p | |
| Hunger | BL | 48 | 63.80 (19.31) * | 62.72 (76.05) * | - | - |
| BL-ANT | 48 | 15.29 (14.03 | 13. 33 (17.86) | 1.73 (−4.40, 7.86) | 0.572 | |
| ANT-POST | 48 | −55.28 (22.48) | −50.09 (22.61) | −5.06 (−11.4, 1.27) | 0.114 | |
| BL-POST | 48 | −39.99 (23.55) | −36.76 (22.43) | −3.33 (−9.48, 2.83) | 0.282 | |
| Satisfied | BL | 48 | 33.56 (22.17) * | 34.29 (21.43) * | - | - |
| BL-ANT | 48 | −7.33 (15.08) | −8.14 (15.75) | 0.99 (−4.87, 6.85) | 0.736 | |
| ANT-POST | 48 | 51.66 (25.09) | 48.32 (24.78) | 3.40 (−2.59, 9.39) | 0.259 | |
| BL-POST | 48 | 44.32 (27.45) | 40.19 (25.14) | 4.39 (−2.99, 11.8) | 0.237 | |
| Fullness | BL | 48 | 21.96 (16.32) * | 22.24 (19.67) * | - | - |
| BL-ANT | 48 | −5.73 (9.70) | −2.94 (14.56) | −2.89 (−5.90, 0.12) | 0.059 | |
| ANT-POST | 48 | 57.18 (21.66) | 49.96 (22.90) | 7.19 (0.73, 13.6) | 0.030 | |
| BL-POST | 48 | 51.45 (21.19) | 47.03 (21.45) | 4.30 (−2.04, 10.6) | 0.179 | |
| a Quantity | BL | 48 | 62.98 (16.49) * | 62.49 (15.62) | - | - |
| BL-ANT | 48 | 10.70 (12.10) | 9.95 (11.23) | 0.63 (−4.35, 5.60) | 0.801 | |
| ANT-POST | 48 | −46.21 (19.03) | −45.18 (19.59) | −1.05 (−5.70 3.60) | 0.653 | |
| BL-POST | 48 | −35.51 (17.31) | −35.23 (19.31) | −0.42 (−5.96, 5.11) | 0.879 | |
| b Desire | BL | 48 | 67.98 (21.66) * | 65.21 (21.16) * | - | - |
| strength | BL-ANT | 48 | 10.53 (14.21) | 12.32 (14.58) | −1.94 (−6.01, 2.12) | 0.340 |
| ANT-POST | 48 | −52.45 (23.42) | −47.53 (21.19) | −4.82 (−11.9, 2.24) | 0.176 | |
| BL-POST | 48 | −41.92 (26.82) | −35.21 (24.79) | −6.77 (−13.8, 0.24) | 0.058 | |
| Indulgent Breakfast | Sensible Breakfast | |||||
|---|---|---|---|---|---|---|
| Gut Hormone | Time Point | Sample Size | Mean Change (SD) | Mean Change (SD) | Mean Difference (95% CI) | p |
| Acylated | BL | 32.46 (41.86) * | 32.59 (44.83) * | - | - | |
| ghrelin | BL-ANT | 38 | 3.04 (13.10) | 4.66 (15.65) | −0.86 (−5.02, 3.31) | 0.680 |
| pmol/L | ANT-POST | 38 | −8.38 (10.88) | −10.36 (15.8) | 1.68 (−3.96, 7.32) | 0.549 |
| BL-POST | 38 | −5.35 (14.58) | −5.70 (11.21) | 0.83 (−5.17, 6.83) | 0.781 | |
| PYY pmol/L | BL | 36.66 (31.45) * | 34.00 (33.21) * | - | - | |
| BL-ANT | 40 | −2.15 (8.99) | −2.13 (8.96) | 0.01 (−4.29, 4.32) | 0.995 | |
| ANT-POST | 40 | 6.23 (10.78) | 6.74 (10.49) | −0.65 (−5.09, 3.79) | 0.768 | |
| BL-POST | 40 | 4.01 (11.93) | 4.74 (8.35) | −0.64 (−5.79, 4.51) | 0.803 | |
| GLP-1 pmol/L | BL | 40.35 (10.83) * | 40.46 (11.89) * | - | - | |
| BL-ANT | 44 | 0.10 (5.96) | 0.17 (5.59) | −0.14 (−2.68, 2.41) | 0.913 | |
| ANT-POST | 44 | 14.34 (11.67) | 14.14 (11.25) | 0.41 (−3.93, 4.76) | 0.848 | |
| BL-POST | 44 | 14.44 (11.46) | 14.31 (11.29) | 0.28 (−4.33, 4.88) | 0.904 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, S.; O’Hara, H.; Reveendran, D.; Cardwell, C.; Murphy, K.G.; Benson, T.; Dean, M.; Woodside, J.V. The Power of Suggestion: Subjective Satiety Is Affected by Nutrient and Health-Focused Food Labelling with No Effect on Physiological Gut Hormone Release. Nutrients 2022, 14, 5100. https://doi.org/10.3390/nu14235100
Watson S, O’Hara H, Reveendran D, Cardwell C, Murphy KG, Benson T, Dean M, Woodside JV. The Power of Suggestion: Subjective Satiety Is Affected by Nutrient and Health-Focused Food Labelling with No Effect on Physiological Gut Hormone Release. Nutrients. 2022; 14(23):5100. https://doi.org/10.3390/nu14235100
Chicago/Turabian StyleWatson, Sinead, Hannah O’Hara, Dharsshini Reveendran, Christopher Cardwell, Kevin G. Murphy, Tony Benson, Moira Dean, and Jayne V. Woodside. 2022. "The Power of Suggestion: Subjective Satiety Is Affected by Nutrient and Health-Focused Food Labelling with No Effect on Physiological Gut Hormone Release" Nutrients 14, no. 23: 5100. https://doi.org/10.3390/nu14235100
APA StyleWatson, S., O’Hara, H., Reveendran, D., Cardwell, C., Murphy, K. G., Benson, T., Dean, M., & Woodside, J. V. (2022). The Power of Suggestion: Subjective Satiety Is Affected by Nutrient and Health-Focused Food Labelling with No Effect on Physiological Gut Hormone Release. Nutrients, 14(23), 5100. https://doi.org/10.3390/nu14235100







