Nutraceuticals and the Network of Obesity Modulators
Abstract
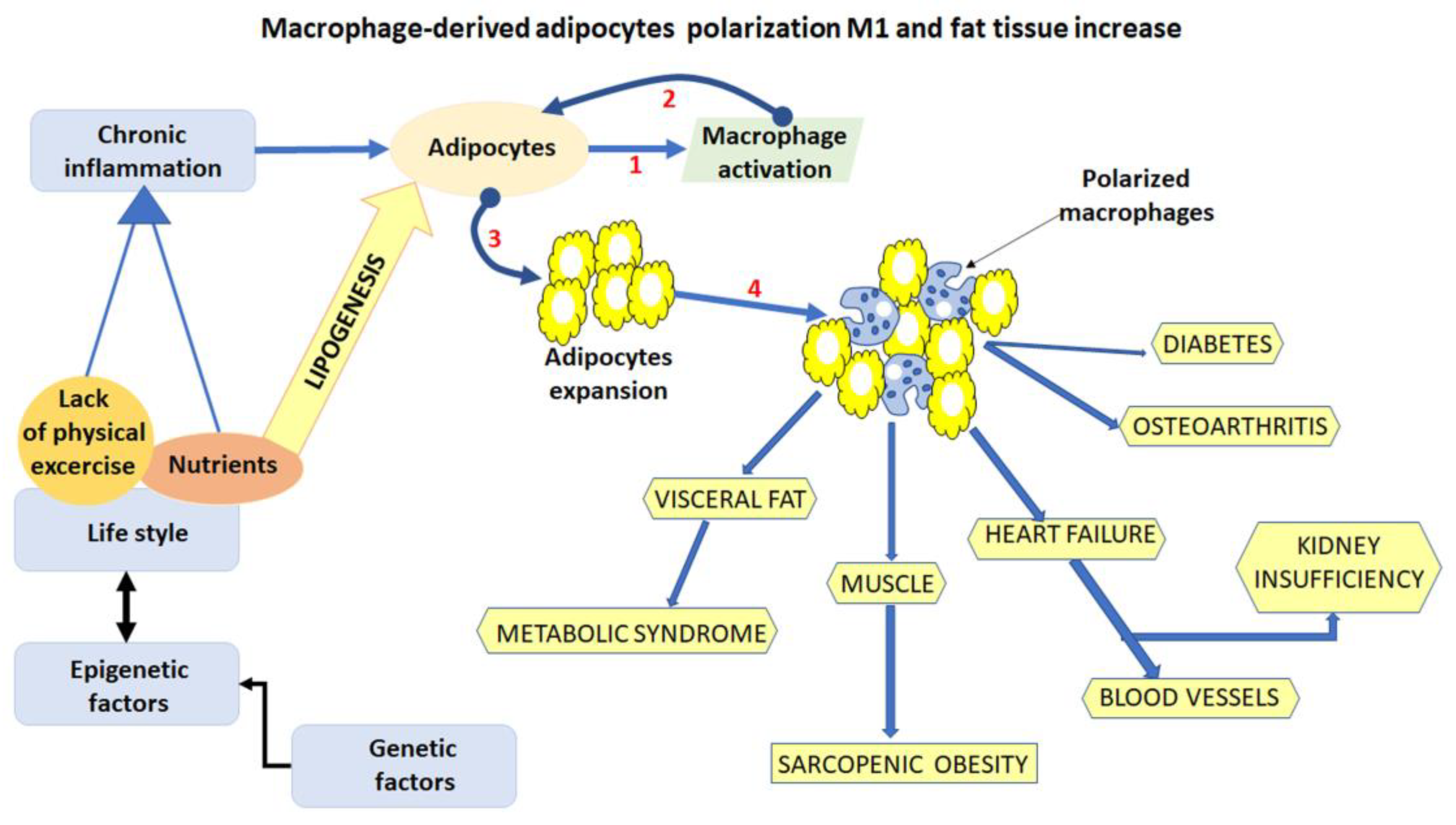
1. Introduction
2. Results
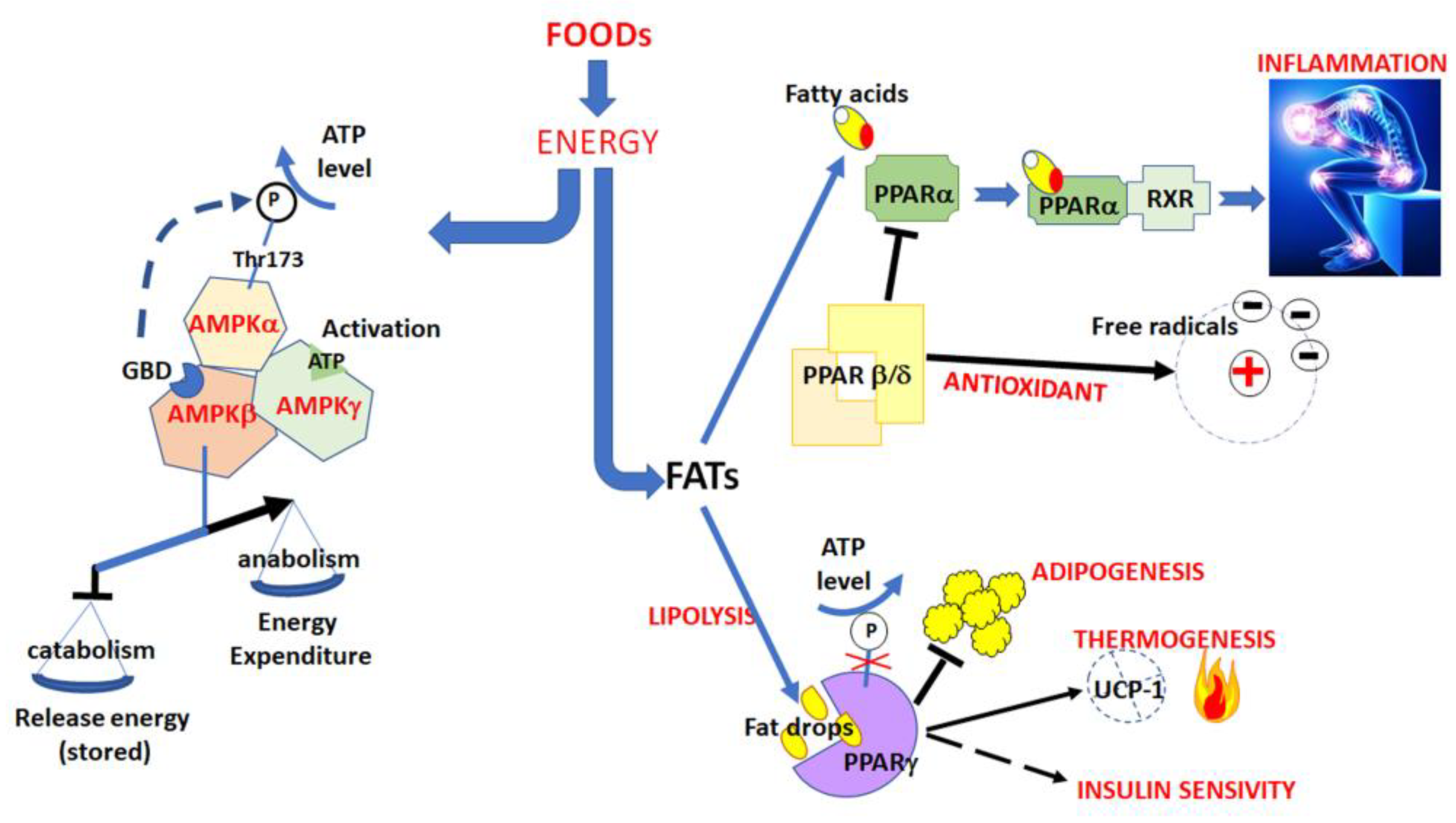
2.1. The Activation of AMPK Pathway
2.2. The Inhibition of the PPAR Pathway
2.3. Reducing Inflammation
2.3.1. Activation of Inflammosome
2.3.2. TNF-α Pathway
2.3.3. MAPK Signalling
2.3.4. Production Control and Scavenging of Free Radicals
2.4. Regulation of Lipase Activity
2.5. Effectors on Neuromodulators
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Kyle, U.U.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Thirumurugan, K. Autophagy: A molecular switch to regulate adipogenesis and lipolysis. Mol. Cell. Biochem. 2022, 477, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, M.S.; Pae, M.; Yamamoto, Y.; Eberlé, D.; Shimada, T.; Kamei, N.; Park, H.S.; Sasorith, S.; Woo, J.R.; et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016, 23, 685–698. [Google Scholar] [CrossRef]
- Czech, M. Macrophages dispose of catecholamines in adipose tissue. Nat. Med. 2017, 23, 1255–1257. [Google Scholar] [CrossRef]
- Gomes, A.; Leite, F.; Ribeiro, L. Adipocytes and macrophages secretomes coregulate catecholamine-synthesizing enzymes. Int. J. Med. Sci. 2021, 18, 582–592. [Google Scholar] [CrossRef]
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Cheminform. 2020, 12, 20. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, G.M.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Ràfols, M.E. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Yucel, N.; Arany, Z. Fat, Obesity, and the Endothelium. Curr. Opin. Physiol. 2019, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Chylikova, J.; Dvorackova, J.; Tauber, Z.; Kamarad, V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2018, 162, 79–82. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The New Role of AMP-Activated Protein Kinase in Regulating Fat Metabolism and Energy Expenditure in Adipose Tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.D.S.; Teixeira, L.; Gambero, A.; Ribeiro, M.L. Guarana (Paullinia cupana) stimulates mitochondrial biogenesis in mice fed high-fat diet. Nutrients 2018, 10, 165. [Google Scholar] [CrossRef]
- Yavari, A.; Stocker, C.J.; Ghaffari, S.; Wargent, E.T.; Steeples, V.; Czibik, G.; Pinter, K.; Bellahcene, M.; Woods, A.; Martínez de Morentin, P.B.; et al. Chronic Activation of γ2 AMPK Induces Obesity and Reduces β Cell Function. Cell Metab. 2016, 23, 821–836. [Google Scholar] [CrossRef]
- Kim, C.O.; Kim, Y.N.; Lee, D.C. Effects of Gelidium elegans on Weight and Fat Mass Reduction and Obesity Biomarkers in Overweight or Obese Adults: A Randomized Double-Blinded Study. Nutrients 2019, 11, 1513. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.J.; Koh, E.J.; Lee, B.Y. Gelidium elegans regulates the AMPK-PRDM16-UCP-1 pathway and has a synergistic effect with orlistat on obesity-associated features in mice fed a high-fat diet. Nutrients 2017, 9, 342. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Ferreira, E.D.F.; de Paula, M.N.; Klein, T.; de Mello, J.C.P. Paullinia cupana: A multipurpose plant—A review. Rev. Bras. Farmacogn. 2019, 29, 77–110. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef]
- Lee, G.-H.; Peng, C.; Jeong, S.-Y.; Park, S.-A.; Lee, H.-Y.; Hoang, T.-H.; Kim, J.; Chae, H.-J. Ginger extract controls mTOR-SREBP1-ER stress-mitochondria dysfunction through AMPK activation in obesity model. J. Funct. Food 2021, 87, 104628. [Google Scholar] [CrossRef]
- Aminifard, T.; Razavi, B.M.; Hosseinzadeh, H. The effects of ginseng on the metabolic syndrome: An updated review. Food Sci. Nutr. 2021, 9, 5293–5311. [Google Scholar] [CrossRef]
- Lee, K.; Seo, Y.J.; Song, J.H.; Chei, S.; Lee, B.Y. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J. Ginseng Res. 2019, 43, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Rodriguez Lanzi, C.; Perdicaro, D.J.; Gambarte Tudela, J.; Muscia, V.; Fontana, A.R.; Oteiza, P.I.; Vazquez Prieto, M.A. Grape pomace extract supplementation activates FNDC5/irisin in muscle and promotes white adipose browning in rats fed a high-fat diet. Food Funct. 2020, 11, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Buhlmann, E.; Horváth, C.; Houriet, J.; Kiehlmann, E.; Radtke, J.; Marcourt, L.; Wolfender, J.-L.; Wolfrum, C.; Schröder, S. Puerariae lobatae root extracts and the regulation of brown fat activity. Phytomedicine 2019, 64, 153075. [Google Scholar] [CrossRef]
- Jung, H.W.; Kang, A.N.; Kang, S.Y.; Park, Y.K.; Song, M.Y. The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Gong, G.; Han, G.; He, H.; Dong, T.T.X.; Tsim, K.W.K.; Zheng, Y. An Ancient Chinese Herbal Decoction Containing Angelicae sinensis Radix, Astragali Radix, Jujuba Fructus, and Zingiberis Rhizoma Recens Stimulates the Browning Conversion of White Adipocyte in Cultured 3T3-L1 Cells. Evid. -Based Complementary Altern. Med. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger Extract Ameliorates Obesity and Inflammation via Regulating MicroRNA-21/132 Expression and AMPK Activation in White Adipose Tissue. Nutrients 2018, 10, 1567. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef]
- Yang, L.L.; Xiao, N.; Liu, J.; Liu, K.; Liu, B.; Li, P.; Qi, L.W. Differential regulation of baicalin and scutellarin on AMPK and Akt in promoting adipose cell glucose disposal. Biochim. Biophys. Acta (BBA) -Mol. Basis Dis. 2017, 1863, 598–606. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, K.H.; Jung, H.K.; Sim, M.O.; Kim, T.M.; Woo, K.W.; An, B.K.; Cho, J.H.; Cho, H.W. Anti-inflammatory effects of 6′-O-acetyl mangiferin from Iris rossii Baker via NF-κb signal blocking in lipopolysaccharide-stimulated RAW 264.7 cells. Chemico-Biol. Interact. 2016, 257, 54–60. [Google Scholar] [CrossRef]
- Sim, M.O.; Lee, H.J.; Jeong, D.E.; Jang, J.H.; Jung, H.K.; Cho, H.W. 6′-O-acetyl mangiferin from Iris rossii Baker inhibits lipid accumulation partly via AMPK activation in adipogenesis. Chem. Biol. Interact. 2019, 311, 108755. [Google Scholar] [CrossRef]
- Enes, B.N.; de Paula, L.; Moreira, D.; Lopes Toledo, R.C.; Aguiar Moraes, E.; de Castro Moreira, M.E.; Miranda, H.H.; Noratto, H.G.; Mertens-Talcott, S.U.; Talcott, S.; et al. Effect of different fractions of chia (Salvia hispanica L.) on glucose metabolism, in vivo and in vitro. J. Funct. Foods 2020, 71, 104026. [Google Scholar] [CrossRef]
- Pandurangan, S.B.; Al-Maiman, S.A.; Al-Harbi, L.N.; Alshatwi, A.A. Beneficial Fatty Acid Ratio of Salvia hispanica L. (Chia Seed) Potentially Inhibits Adipocyte Hypertrophy, and Decreases Adipokines Expression and Inflammation in Macrophage. Foods 2020, 9, 368. [Google Scholar] [CrossRef]
- Willows, R.; Navaratnam, N.; Lima, A.; Read, J.; Carling, D. Effect of different γ-subunit isoforms on the regulation of AMPK. Biochem. J. 2017, 474, 1741–1754. [Google Scholar] [CrossRef]
- Wu, W.; Feng, J.; Jiang, D.; Zhou, X.; Jiang, Q.; Cai, M.; Wang, X.; Shan, T.; Wang, Y. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine. Sci. Rep. 2017, 7, 41606. [Google Scholar] [CrossRef]
- Yao, B.; Christian, K.M.; He, C.; Jin, P.; Ming, G.L.; Song, H. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016, 17, 537–549. [Google Scholar] [CrossRef]
- Antonio, J.; Knafo, S.; Kapoor, R.; Tartar, J.L. A fat mass and obesity-associated gene polymorphism influences fat mass in exercise-trained individuals. J. Int. Soc. Sports Nutr. 2018, 15, 40. [Google Scholar] [CrossRef]
- Zhong, T.; Duan, X.Y.; Zhang, H.; Li, L.; Zhang, H.P.; Niu, L. Angelica sinensis Suppresses Body Weight Gain and Alters Expression of the FTO Gene in High-Fat-Diet Induced Obese Mice. BioMed Res. Int. 2017, 2017, 6280972. [Google Scholar] [CrossRef]
- Ma, P.; Sun, C.; Li, W.; Deng, W.; Adu-Frimpong, M.; Yu, J.; Xu, X. Extraction and structural analysis of Angelica sinensis polysaccharide with low molecular weight and its lipid-lowering effect on nonalcoholic fatty liver disease. Food Sci. Nutr. 2020, 8, 3212–3224. [Google Scholar] [CrossRef]
- Asuquo, E.A.; Nwodo, O.F.C.; Assumpta, A.C.; Orizu, U.N.; Oziamara, O.N.; Solomon, O.A. FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation. Open Life Sci. 2022, 17, 641–658. [Google Scholar] [CrossRef]
- Yarmohammadi, F.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effect of eggplant (Solanum melongena) on the metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2021, 24, 420–427. [Google Scholar]
- Fanale, D.; Amodeo, V.; Caruso, S. The Interplay between Metabolism, PPAR Signaling Pathway, and Cancer. PPAR Res. 2017, 2017, 1830626. [Google Scholar] [CrossRef]
- Wafer, R.; Tandon, P.; Minchin, J.E.N. The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) in Adipogenesis: Applying Knowledge from the Fish Aquaculture Industry to Biomedical Research. Front. Endocrinol. 2017, 8, 102. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Phua, W.W.T.; Wong, M.X.Y.; Liao, Z.; Tan, N.S. An aPPARent Functional Consequence in Skeletal Muscle Physiology via Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2018, 19, 1425. [Google Scholar] [CrossRef]
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: A patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Dias, M.M.G.; Batista, F.A.H.; Tittanegro, T.H.; de Oliveira, A.G.; Le Maire, A.; Torres, F.R.; Filho, H.V.R.; Silveira, L.R.; Figueira, A.C.M. PPARγ S273 Phosphorylation Modifies the Dynamics of Coregulator Proteins Recruitment. Front. Endocrinol. 2020, 11, 561256. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, K.; Sharma, R.; Purohit, R.; Padwad, Y. Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting PPARγ/Cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 2019, 383, 111480. [Google Scholar] [CrossRef]
- Bu, Y.; Okunishi, K.; Yogosawa, S.; Mizuno, K.; Irudayam, M.J.; Brown, C.W.; Izumi, T. Insulin Regulates Lipolysis and Fat Mass by Upregulating Growth/Differentiation Factor 3 in Adipose Tissue Macrophages. Diabetes 2018, 67, 1761–1772. [Google Scholar] [CrossRef]
- Esakkimuthu, S.; Nagulkumar, S.; Darvin, S.S.; Buvanesvaragurunathan, K.; Sathya, T.N.; Navaneethakrishnan, K.R.; Kumaravel, T.S.; Murugan, S.S.; Shirota, O.; Balakrishna, K.; et al. Antihyperlipidemic effect of iridoid glycoside deacetylasperulosidic acid isolated from the seeds of Spermacoce hispida L. -A traditional antiobesity herb. J. Ethnopharmacol. 2019, 245, 112–170. [Google Scholar] [CrossRef]
- Baiyisaiti, A.; Wang, Y.; Zhang, X.; Chen, W.; Qi, R. Rosa rugosa flavonoids exhibited PPARα agonist-like effects on genetic severe hypertriglyceridemia of mice. J. Ethnopharmacol. 2019, 240, 111952. [Google Scholar] [CrossRef]
- Choi, D.H.; Han, J.H.; Hong, M.; Lee, S.Y.; Lee, S.U.; Kwon, T.H. Antioxidant and lipid-reducing effects of Rosa rugosa root extract in 3T3-L1 cell. Food Sci. Biotechnol. 2021, 31, 121–129. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.Y.; Guo, Y.; Chu, C.; Zheng, G.D.; Liu, E.H.; Li, F. Enrichment of polymethoxyflavones from Citrus reticulata ‘Chachi’ peels and their hypolipidemic effect. J. Chromatogr. B 2019, 1124, 226–232. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, C.T.; Pan, M.H. Immature Citrus reticulata Extract Promotes Browning of Beige Adipocytes in High-Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 2018, 66, 9697–9703. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Safi, S.; Mazaheri, M.; Salehi-Abargouei, A.; Abdollahi, N.; Nazari, M.; Fallahzadeh, H.; Nadjarzadeh, A. Effects of oral Nigella sativa oil on the expression levels and serum concentrations of adiponectin, PPAR-γ, and TNF-α in overweight and obese women: A study protocol for a crossover-designed, double-blind, placebo-controlled randomized clinical trial. Trials 2019, 20, 512. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: A randomized double-blind, placebo-controlled clinical trial. J. Clin. Lipidol. 2016, 10, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Hoo, R.L.; Wong, J.Y.; Qiao, C.; Xu, A.; Xu, H.; Lam, K.S. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr. Metab. 2010, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Zhao, S.; Mao, L.; Yang, Y.; Sun, W.; Lin, X.; Liu, S.; Li, K.; Sun, Y.; Li, P.; et al. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPARγ activity. Br. J. Pharmacol. 2018, 175, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef]
- Savova, M.S.; Vasileva, L.V.; Mladenova, S.G.; Amirova, K.M.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed. Pharmacother. 2021, 141, 111934. [Google Scholar] [CrossRef]
- Halib, H.; Ismail, A.; Mohd Yusof, B.N.; Osakabe, N.; Mat Daud, Z.A. Effects of Cocoa Polyphenols and Dark Chocolate on Obese Adults: A Scoping Review. Nutrients 2020, 12, 3695. [Google Scholar] [CrossRef]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.H.; Cypess, A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, X.; Yan, Z.; Wang, X.; Gun, S. Isoimperatorin enhances 3T3-L1 preadipocyte differentiation by regulating PPARγ and C/EBPα through the Akt signaling pathway. Exp. Ther. Med. 2019, 18, 2160–2166. [Google Scholar] [CrossRef]
- Han, H.S.; Jeon, H.; Kang, S.C. Phellopterin isolated from Angelica dahurica reduces blood glucose level in diabetic mice. Heliyon 2018, 4, e00577. [Google Scholar] [CrossRef]
- Liang, Y.C.; Yang, M.T.; Lin, C.J.; Chang, C.L.; Yang, W.C. Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. Sci. Rep. 2016, 6, 24285. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.H.; Ali, M.Y.; Min, B.S.; Kim, G.D.; Jung, H.A. Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-α and PPAR-γ. Fitoterapia 2014, 98, 199–208. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Tian, W.; Lu, Y.; Xu, Y.; Wang, F.; Qin, W.; Ma, X.; Puno, P.T.; Xiong, W. Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS. Molecules 2019, 24, 158. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Zhou, J.; Yin, Y.; Zhang, T.; Chen, D. PPARγ provides anti-inflammatory and protective effects in intrahepatic cholestasis of pregnancy through NF-κB pathway. Biochem. Biophys. Res. Commun. 2018, 504, 834–842. [Google Scholar] [CrossRef]
- Patil, V.S.; Khatib, N.A. Triterpene saponins from Barringtonia acutangula (L.) Gaertn as a potent inhibitor of 11β-HSD1 for type 2 diabetes mellitus, obesity, and metabolic syndrome. Clin. Phytosci. 2020, 6, 61. [Google Scholar] [CrossRef]
- Lee, D.; Shin, Y.; Jang, J.; Park, Y.; Ahn, J.; Jeong, S.; Shin, S.S.; Yoon, M. The herbal extract ALS-L1023 from Melissa officinalis alleviates visceral obesity and insulin resistance in obese female C57BL/6J mice. J. Ethnopharmacol. 2020, 253, 112646. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, J.; Peak, J.; Ki, M.; Lee, S.; Choe, G.; Jung, J.; Jung, H.; Jeon, S.; Park, T.-S.; et al. Effect of Melissa officinalis L. leaf extract on lipid accumulation by modulating specific adipogenic gene transcription factors in 3T3-L1 adipocytes. J. App. Biol. Chem. 2020, 63, 169–174. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, D.; Ahn, J.; Lee, M.; Shin, S.S.; Yoon, M. The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and β-cell loss in Otsuka Long-Evans Tokushima fatty rats. J. Ethnopharmacol. 2021, 264, 113360. [Google Scholar] [CrossRef]
- Hussain, F.; Hafeez, J.; Khalifa, A.S.; Naeem, M.; Ali, T.; Eed, E.M. In vitro and in vivo study of inhibitory potentials of α-glucosidase and acetylcholinesterase and biochemical profiling of M. charantia in alloxan-induced diabetic rat models. Am. J. Transl. Res. 2022, 14, 3824–3839. [Google Scholar]
- Helal, M.; Ali, M.A.; Nadrin, A.H.; Awad, Y.I.; Younis, N.K.; Alasyed, B.M.; Jamal, M.; Eid, D.H.; Soliman, H.A.; Eissa, S.A.; et al. Association between IRS-1, PPAR-γ and LEP genes polymorphisms and growth traits in rabbits. Anim. Biotechnol. 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2008, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.J.; Noh, J.W.; Lee, B.C. Mechanisms and Effect of Coptidis rhizoma on Obesity-Induced Inflammation: In Silico and In Vivo Approaches. Int. J. Mol. Sci. 2021, 22, 8075. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3a. Biosci. Rep. 2019, 39, bsr20192059. [Google Scholar] [CrossRef]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.I. Pharmacological properties of Morus nigra L. (Black Mulberry) as a promising nutraceutical resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef]
- Vafaeipour, Z.; Razavi, B.M.; Hosseinzadeh, H. Effects of turmeric (Curcuma longa) and its constituent (curcumin) on the metabolic syndrome: An updated review. J. Integr. Med. 2022, 20, 193–203. [Google Scholar] [CrossRef]
- Liu, E.; Tsuboi, H.; Ikegami, S.; Kamiyama, T.; Asami, Y.; Ye, L.; Oda, M.; Ji, Z.S. Effects of Nelumbo nucifera Leaf Extract on Obesity. Plant Foods Hum. Nutr. 2021, 76, 377–384. [Google Scholar] [CrossRef]
- Shandiz, H.T.; Razavi, B.M.; Hosseinzadeh, H. Review of Garcinia mangostana and its Xanthones in Metabolic Syndrome and Related Complications. Phytother. Res. 2017, 8, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Eltokhy, A.K.; Khattab, H.A.; Rabah, H.M. The impact of cichorium intybus L. On GDF-15 level in obese diabetic albino mice as compared with metformin effect. J. Diabetes Metab. Disord. 2021, 20, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, E.; Lee, S.; Kwon, Y.; Kang, K.S.; Kim, C.E.; Kim, D. System-level investigation of anti-obesity effects and the potential pathways of Cordyceps militaris in ovariectomized rats. BMC Complement. Med. Ther. 2022, 22, 132. [Google Scholar] [CrossRef]
- de Sousa, A.R.; Castro Moreira, M.E.; Grancieri, M.; Lopes Toledo, R.C.; de Oliveira Araújo, F.; Cuquetto Mantovani, H.; Vieira Queiroz, V.A.; Duarte Martino, H.S. Extruded sorghum (Sorghum bicolor L.) improves gut microbiota, reduces inflammation, and oxidative stress in obese rats fed a high-fat diet. J. Funct. Foods 2019, 58, 282–291. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, B.; Li, H.; Ren, W.; Zhang, Q.; Liu, Y.; Zhao, J. Comprehensive analysis of MAPK cascade genes in sorghum (Sorghum bicolor L.) reveals SbMPK14 as a potential target for drought sensitivity regulation. Genomics 2022, 114, 110311. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Shapira, N. The metabolic concept of meal sequence vs. Satiety: Glycemic and oxidative responses with reference to inflammation risk, protective principles and mediterranean diet. Nutrients 2019, 11, 2373. [Google Scholar] [CrossRef]
- Mu, R.F.; Niu, Y.F.; Wang, Q.; Zhou, H.M.; Hu, J.; Qin, W.Y.; Xiong, W.Y. Eriocalyxin B Inhibits Adipogenesis in 3T3-L1 Adipocytes by Cell Cycle Arrest. Nat. Prod. Bioprospect. 2020, 10, 131–140. [Google Scholar] [CrossRef]
- Kil, Y.S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharm. Res. 2017, 40, 655–675. [Google Scholar] [CrossRef]
- Ohta, M.; Fujinami, A.; Oishi, K.; Kobayashi, N.; Ohnishi, K.; Ohkura, N. Ashitaba (Angelica keiskei) Exudate Prevents Increases in Plasminogen Activator Inhibitor-1 Induced by Obesity in Tsumura Suzuki Obese Diabetic Mice. J. Diet Suppl. 2019, 16, 331–344. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Md Noor, S. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Llorente, M.; Unamuno, X.; Gómez-Ambrosi, J.; Frühbeck, G. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: Role of tenascin C. Int. J. Obes. 2018, 42, 1458–1470. [Google Scholar] [CrossRef]
- Ohnogi, H.; Hayami, S.; Kudo, Y.; Deguchi, S.; Mizutani, S.; Enoki, T.; Tanimura, Y.; Aoi, W.; Naito, Y.; Kato, I.; et al. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci. Biotechnol. Biochem. 2012, 76, 928–932. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Hill, B.G. Regulation of obesity and insulin resistance by nitric oxide. Free Rad. Biol. Med. 2014, 73, 383–399. [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 118, 2216–2223. [Google Scholar] [CrossRef]
- Willer, J.; Zidorn, C.; Juan-Vicedo, J. Ethnopharmacology, phytochemistry, and bioactivities of Hieracium L. and Pilosella Hill (Cichorieae, Asteraceae) species. J. Ethnopharmacol. 2021, 281, 114465. [Google Scholar] [CrossRef]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a Source of Biologically Active Compounds Supporting the Therapy of Co-Existing Diseases in Metabolic Syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef]
- Liu, P.K.; Weng, Z.M.; Ge, G.B.; Li, H.L.; Ding, L.L.; Dai, Z.R.; Hou, X.D.; Leng, Y.H.; Yu, Y.; Hou, J. Biflavones from Ginkgo biloba as novel pancreatic lipase inhibitors: Inhibition potentials and mechanism. Int. J. Biol. Macromol. 2018, 118 Pt B, 2216–2223. [Google Scholar] [CrossRef]
- Hirata, B.K.; Banin, R.M.; Dornellas, A.P.; de Andrade, I.S.; Zemdegs, J.C.; Caperuto, L.C.; Oyama, L.M.; Ribeiro, E.B.; Telles, M.M. Ginkgo biloba extract improves insulin signaling and attenuates inflammation in retroperitoneal adipose tissue depot of obese rats. Mediat. Inflamm. 2015, 2015, 419106. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Profile of Phenolic Compounds of Prunus armeniaca L. Leaf Extract Determined by LC-ESI-QTOF-MS/MS and Their Antioxidant, Anti-Diabetic, Anti-Cholinesterase, and Anti-Inflammatory Potency. Antioxidants 2021, 10, 1869. [Google Scholar] [CrossRef]
- Suruga, K.; Tomita, T.; Kadokura, K.; Arai, T. Rhus verniciflua leaf extract suppresses obesity in high-fat diet-induced obese mice. Food Nutr. Res. 2019, 63, 3601. [Google Scholar] [CrossRef]
- Hoang, T.H.; Yoon, Y.; Park, S.A.; Lee, H.Y.; Peng, C.; Kim, J.H.; Lee, G.H.; Chae, H.J. IBF-R, a botanical extract of Rhus verniciflua controls obesity in which AMPK-SIRT1 axis and ROS regulatory mechanism are involved in mice. J. Funct. Foods 2021, 87, 104804. [Google Scholar] [CrossRef]
- Cheong, S.A.; Kim, R.; Park, Y.K.; Baek, S.Y.; Yeo, S.-H.; Lee, C.H. Anti-Obesity Effect of Fermented Detoxified Rhus verniciflua Vinegar Supplementation in Diet-Induced Obese Rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1771–1778. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, J.; Kim, M.-J. Alpha glucosidase inhibitory effect, anti-microbial activity and UPLC analysis of Rhus verniciflua under various extract conditions. J. Med. Plants Res. 2011, 5, 778–783. [Google Scholar]
- Lee, S.O.; Kim, S.J.; Kim, J.S.; Ji, H.; Lee, E.O.; Lee, H.J. Comparison of the main components and bioactivity of Rhus verniciflua Stokes extracts by different detoxification processing methods. BMC Complement. Altern. Med. 2018, 18, 242. [Google Scholar] [CrossRef]
- Caron, A.; Richard, D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann. N. Y. Acad. Sci. 2017, 1391, 35–53. [Google Scholar] [CrossRef]
- Fan, L.; Peng, Y.; Wu, D.; Hu, J.; Shi, X.; Yang, G.; Li, X. Dietary supplementation of Morus nigra L. leaves decrease fat mass partially through elevating leptin-stimulated lipolysis in pig model. J. Ethnopharmacol. 2020, 249, 112416. [Google Scholar] [CrossRef]
- Gyengesi, E.; Paxinos, G.B.; Andrews, Z. Oxidative Stress in the Hypothalamus: The Importance of Calcium Signaling and Mitochondrial ROS in Body Weight Regulation. Curr. Neuropharmacol. 2015, 10, 344–353. [Google Scholar] [CrossRef]
- Phung, H.M.; Jang, D.; Trinh, T.A.; Lee, D.; Nguyen, Q.N.; Kim, C.-E.; Kang, K.S. Regulation of appetite-related neuropeptides by Panax ginseng: A novel approach for obesity treatment. J. Ginseng Res. 2022, 46, 609–619. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Luo, P.; Chen, Y.; Xi, Q.; Wu, H.; Zhao, W.; Shu, G.; Wang, S.; Gao, P.; et al. Oral supplementation with ginseng polysaccharide promotes food intake in mice. Brain Behav. 2019, 9, e01340. [Google Scholar] [CrossRef]
- Hamerski, L.; Somner, G.V.; Tamaio, N. Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plants Res. 2013, 7, 2221–2229. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Dias, T.R.; Alves, M.G.; Oliveira, P.F.; Monteiro, M.P.; Silva, B.M. Anti-obesity potential of natural methylxanthines. J. Funct. Foods 2018, 43, 84–94. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Gelidium elegans [20,21] | Unknown | AMPK-PRDM16 | Anti-obesity |
| Iris rossii Baker [36,37] | Inhibits pre-adipocytes differentiation | AMPK | Decreases lipid accumulation |
| Panax ginseng [25,26] | Increases AMPK phosphorylation | AMPK PRDM16 PGC1α | Improves insulin sensitivity and glucose metabolism |
| Paullinia cupana [22,18] | Stimulates thermogenesis | AMPK PPARγ coactivator 1-alpha (PGC1-α) UCP-1 | Anti-hyperlipidemic |
| Pueraria montana var lobata [29,30] | Regulation of brown fat activity | PPAR pathway UCP1 | Stimulates thermogenesis |
| Salvia hispanica L. [38,39] | Increases protein kinase B 1 (AKT1)[pS473] | AMPK mRNA HSP, PGC-1α Forkhead box protein 1 (FOXO1) | Improves glucose and insulin tolerance |
| Scutellaria baicalensis [34,35] | Hemostasis of glucose and lipid metabolisms | (WNT)/β-catenin pathway AMPK PPARγ | Anti-hyperlipidemic |
| Zingiber officinalis [32,33] | Upregulating β-oxidation | AMPK | Controls fat accumulation |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Angelicae sinensis [44,45] | Controls adipocytes expansion | FTO gene AMPK | Suppresses body weight gain |
| Solanum melongena or aethiopicum [46,47] | Controls the adipocytes in hypothalamus | FTO gene AMPK | Decreases feed consumption |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Astragali membranaceus var. mongholicus [64,65] | Increases thermogenesis | PPARγ | Antidiabetic/anti-inflammatory |
| Citrus reticulata [60,61] | Increases hepatic fatty acid oxidation | PPARα | Anti-hyperlipidemic activity |
| Nigella sativa [62,63] | Reduces adipogenesis | PPARγ TNF-α Adipokines | Decreases appetite Reduces body weight |
| Rosa rugosa [58,59] | AMPK pathway activation | PPARα agonist | Control of dyslipidemia |
| Spermacoce hispida [57] | Decreases lipid accumulation | PPARα | Anti-hyperlipidemic activity |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Angelica dahuricae [70,71] | Inhibits adipogenesis | C/EBPβ signaling | Controls adipose tissue |
| Bidens pilosa [72] | Decreases the adipogenesis and lipid accumulation | C/EBPs PPARγ Egr2 | Decreases fat content |
| Theobroma cacao [68,69] | Stimulates thermogenesis | C/EBPα | Controls body weight |
| Coptis chinensis [73] | Inhibits adipocyte differentiation | C/EBPα PPARγ | Reduces obesity |
| Isodon adenantha [74] | Inhibits adipogenesis decreasing the ROS amount | C/EBPβ signaling | Controls adipose tissue |
| Ziziphus jujuba [67] | Affects adipogenic differentiation | C/EBP PPARγ PI3K/AKT | Anti-adipogenic |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Barringtonia acutangular [76] | Controls metabolism of lipids | 11β-HSD1 | Reduces hyperlipidemia |
| Melissa officinalis [77,78] | Increases fatty acid β-oxidation and decreases lipogenesis | FAS SREBP-1 CPT-1 | Reduces visceral adiposity |
| Momordica charantia [81] | Insulin signalling | IRS-1 | Reduces weight gain |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Coptidis rhizoma [84,85] | Decreases adipose tissue macrophages | NLRP3 | Reduces obesity |
| Morus alba [86,87] | Adipocyte differentiation | NLRP3 PPARγ and C/EBPα | Reduces dyslipidemia |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Curcuma longa [89] | Anti-inflammatory | TNF-α | Reduces obesity |
| Garcinia mangostana [91] | Anti-inflammatory | TNF-α IL-6 | Reduces body weight |
| Nelumbo nucifera [90] | Inhibition of cAMP pathway | TNF-α, leptin, insulin | Prevents triglyceride accumulation and promote lipolysis |
| Cichorium intybus [92] | GDF-15 signalling pathways | PI3K/AKT | Weight reduction |
| Source | Proposed Mechanism | Target | Effect |
|---|---|---|---|
| Cordyceps militaris [94] | Decreases population of negativebacillus | MAPK signaling pathway; PI3K–Akt signaling pathway | Reduce body weight, fat accumulation. Stimulate lipolysis |
| Sorghum bicolor [95,96] | Inhibits preadipocyte differentiation | MAPK signaling production of ROS | Reduce intracellular lipid accumulation |
| Source | Proposed Mechanism | Target | Effect |
|---|---|---|---|
| Angelica keiskei [100,101] | Anti-inflammatory | TNF-α | Reduce gains in body weight |
| Isodon eriocalyx [99] | Anti-inflammatory | NF-κB | Inhibit adipogenesis |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Ginkgo biloba [109,110] | Adipocyte hypertrophy | FAS Perilipin 1 | Reduce the adipocyte volume |
| Hieracium sp. [107] | Decrease lypolysis | Pancreatic lipase | Decrease fat accumulation |
| Morus nigra [87,117] | Leptin-stimulated lipolysis | Hormone-sensitive lipase FAS | Decrease fat mass |
| Prunus armeniaca [111] | Inhibit adipogenesis | pancreatic lipase | Prevention of obesity |
| Rhus verniciflua [112,113,114,115,116] | Inhibit nonesterified fatty acid and glycerol absorption | SREBP1 alpha-glucosidase | Reduce body weight gain |
| Taraxacum officinale [108] | Increase plasma superoxide radical scavenging | Alkaline phosphatase | Decrease in lipid peroxidation |
| Source | Proposed Mechanism | Target | Effects |
|---|---|---|---|
| Panax ginseng [120,121] | Regulates appetite-related neuropeptides | Pro-opiomelanocortin, cholecystokinin, and cocaine- and agouti-related protein, neuropeptide Y | Decreases adipogenesis |
| Paullinia cupana [122] | Inhibits adipogenesis | Ghrelin | Decreases fat mass |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammendola, S.; Scotto d’Abusco, A. Nutraceuticals and the Network of Obesity Modulators. Nutrients 2022, 14, 5099. https://doi.org/10.3390/nu14235099
Ammendola S, Scotto d’Abusco A. Nutraceuticals and the Network of Obesity Modulators. Nutrients. 2022; 14(23):5099. https://doi.org/10.3390/nu14235099
Chicago/Turabian StyleAmmendola, Sergio, and Anna Scotto d’Abusco. 2022. "Nutraceuticals and the Network of Obesity Modulators" Nutrients 14, no. 23: 5099. https://doi.org/10.3390/nu14235099
APA StyleAmmendola, S., & Scotto d’Abusco, A. (2022). Nutraceuticals and the Network of Obesity Modulators. Nutrients, 14(23), 5099. https://doi.org/10.3390/nu14235099






