Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies
Abstract
1. Introduction
2. Methods
Search Strategy and Study Eligibility Criteria
3. NNS and Outcomes
4. Data Extraction and Assessment of Study Quality for Individual Studies
5. Statistical Analyses
6. Evaluation of Publication Bias and Quality of a Whole Body of Evidence
7. Results
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Symonds, M.E.; Sebert, S.P.; Hyatt, M.A.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef]
- Jen, V.; Erler, N.S.; Tielemans, M.J.; Braun, K.V.; Jaddoe, V.W.; Franco, O.H.; Voortman, T. Mothers’ intake of sugar-containing beverages during pregnancy and body composition of their children during childhood: The Generation R Study. Am. J. Clin. Nutr. 2017, 105, 834–841. [Google Scholar] [CrossRef]
- Casas, R.; Barquero, S.C.; Estruch, R. Impact of Sugary Food Consumption on Pregnancy: A Review. Nutrients 2020, 12, 3574. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Tofigh, A.M.; Jahangiri, L.; Nikniaz, Z.; Nikniaz, L. Sugar-sweetened beverages intake and the risk of obesity in children: An updated systematic review and dose-response meta-analysis. Pediatr. Obes. 2022, 17, e12914. [Google Scholar] [CrossRef]
- Azad, M.B.; Sharma, A.K.; de Souza, R.J.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association between Artificially Sweetened Beverage Consumption during Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016, 170, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Aris, I.M.; Rifas-Shiman, S.L.; Goran, M.I.; Oken, E. Associations of maternal non-nutritive sweetener intake during pregnancy with offspring body mass index and body fat from birth to adolescence. Int. J. Obes. 2022, 46, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Rifas-Shiman, S.L.; Fernandez-Barres, S.; Kleinman, K.; Taveras, E.M.; Oken, E. Beverage Intake during Pregnancy and Childhood Adiposity. Pediatrics 2017, 140, e20170031. [Google Scholar] [CrossRef]
- Institute of Medicine. Nutrition Standards for Foods in Schools: Leading the Way Toward Healthier Youth; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Azad, M.B.; Archibald, A.; Tomczyk, M.M.; Head, A.; Cheung, K.G.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: Evidence from humans, mice, and cells. Int. J. Obes. 2020, 44, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Rawal, S.; Hinkle, S.N.; Yeung, E.H.; Chavarro, J.E.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Salavati, N.; Vinke, P.C.; Lewis, F.; Bakker, M.K.; Erwich, J.J.H.M.; van der Beek, E.M. Offspring birth weight is associated with specific preconception maternal food group intake: Data from a linked population-based birth cohort. Nutrients 2020, 12, 3172. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Figueroa, J.; Rother, K.I.; Goran, M.I.; Welsh, J.A. Trends in Low-Calorie Sweetener Consumption among Pregnant Women in the United States. Curr. Dev. Nutr. 2019, 3, nzz004. [Google Scholar] [CrossRef]
- Olivier-Van Stichelen, S.; Rother, K.I.; Hanover, J.A. Maternal Exposure to Non-nutritive Sweeteners Impacts Progeny’s Metabolism and Microbiome. Front. Microbiol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Archibald, A.J.; Dolinsky, V.W.; Azad, M.B. Early-Life Exposure to Non-Nutritive Sweeteners and the Developmental Origins of Childhood Obesity: Global Evidence from Human and Rodent Studies. Nutrients 2018, 10, 194. [Google Scholar] [CrossRef]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Palatnik, A.; Moosreiner, A.; Olivier-Van Stichelen, S. Consumption of non-nutritive sweeteners during pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 211–218. [Google Scholar] [CrossRef]
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.; Cory Slechta, D.; et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Saleh, S.M.; Andres, B.; Inglis, A.; Al-Rabiah, R.; Al-Mohanna, F.A. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS ONE 2012, 7, e31570. [Google Scholar] [CrossRef] [PubMed]
- Kozyrskyj, A.L.; Kalu, R.; Koleva, P.T.; Bridgman, S.L. Fetal programming of overweight through the microbiome: Boys are disproportionately affected. J. Dev. Orig. Health Dis. 2016, 7, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Prete, A.; Citro, F.; Bertolotto, A.; Aragona, M.; de Gennaro, G.; Del Prato, S.; Bianchi, C. Use of non-nutritive-sweetened soft drink and risk of gestational diabetes. Diabetes Res. Clin. Pract. 2021, 178, 108943. [Google Scholar] [CrossRef]
- Ayoob, K.T. Consumption of non-nutritive sweeteners during pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, W.M.; Simões, R.S.; Buzzini, R.F.; Nunes, V.M.; Glina, F. Adverse effects of the consumption of artificial sweeteners—Systematic review. Rev. Assoc. Med. Bras. 2016, 62, 120–122. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Morahan, H.L.; Leenaars, C.H.C.; Boakes, R.A.; Rooney, K.B. Metabolic and behavioural effects of prenatal exposure to non-nutritive sweeteners: A systematic review and meta-analysis of rodent models. Physiol. Behav. 2020, 213, 112696. [Google Scholar] [CrossRef]
- Cai, C.; Sivak, A.; Davenport, M.H. Effects of prenatal artificial sweeteners consumption on birth outcomes: A systematic review and meta-analysis. Public Health Nutr. 2021, 24, 5024–5033. [Google Scholar] [CrossRef]
- Maslova, E.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS ONE 2013, 8, e57261. [Google Scholar] [CrossRef]
- Nakai, Y.; Shinga-Ishihara, C.; Kaji, M.; Moriya, K.; Murakami-Yamanaka, K.; Takimura, M. Xylitol gum and maternal transmission of mutans streptococci. J. Dent. Res. 2010, 89, 56–60. [Google Scholar] [CrossRef] [PubMed]
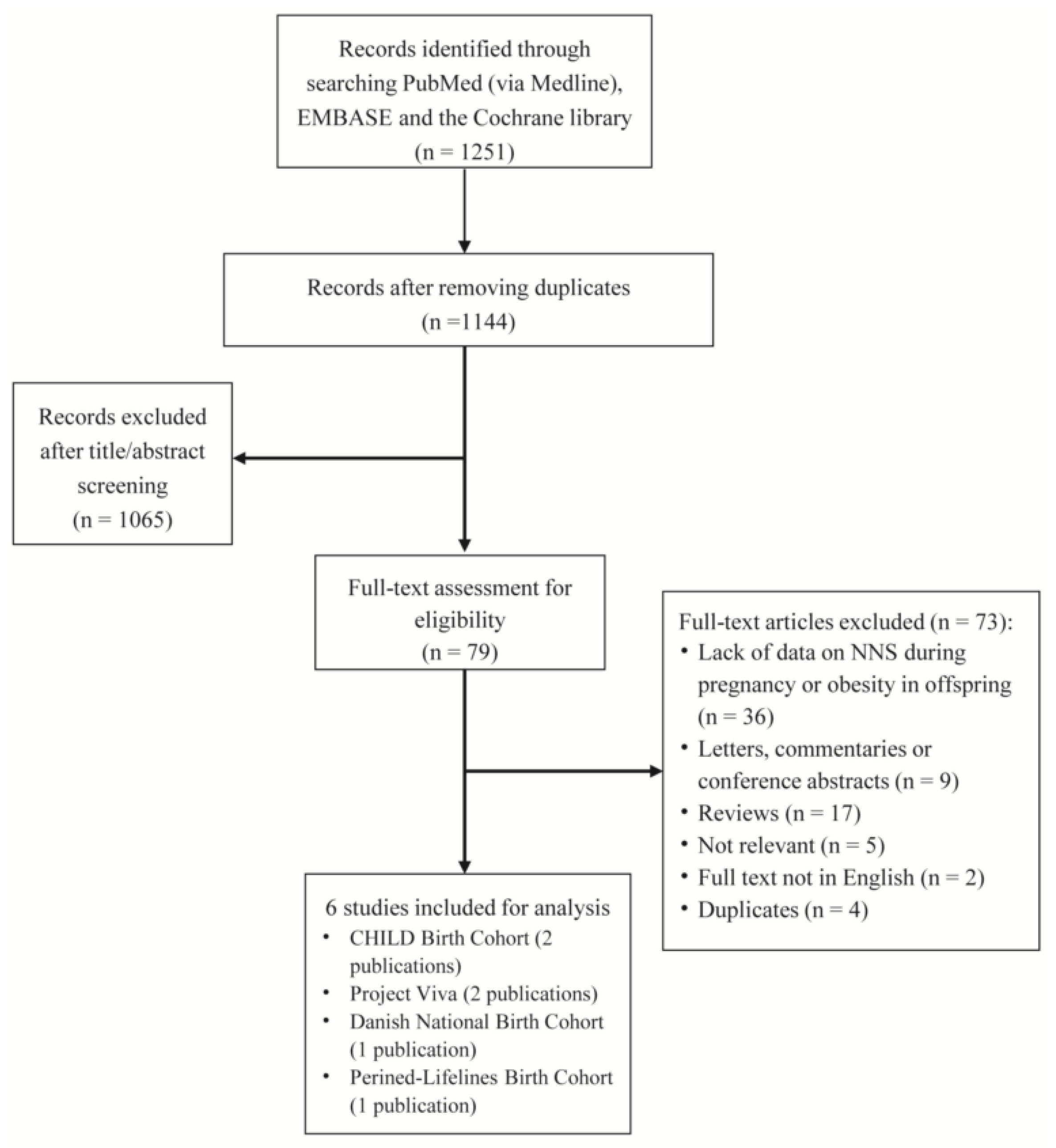

| First Author, Publication Year | Country/Study Name | No. of Mothers/Offspring | Age of Mothers (in Years) | BMI of Mothers (in kg/m2) | Energy Intake of Mothers | Percentage of Mothers Smoking | Percentage of Gestational Diabetes | Girl Percentage of Offspring | Breastfeeding Duration for Offspring | Type of NNS | Measurement of NNS Intake | Outcomes Reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azad, 2016 | Canada/CHILD Birth Cohort | 2413/2413 | 32.5 (SD: 4.6) | 24.8 (SD: 5.4) | 2007 kcal/day | 7.9% | 4.4% | NA | 8.8 months | Artificial | FFQ | Age- and sex-specific BMI-for-age z scores; offspring being overweight |
| Zhu, 2017 | Denmark/Danish National Birth Cohort | 918/918 | 31.3 | 27.6 | 2391 kcal/day | 16.1% | 100% | 44% | NA | Artificial | FFQ | Age- and sex-specific BMI z-scores; offspring being overweight or obese; large-for-gestational age (LGA) |
| Salavati, 2020 | The Netherlands/Perined-Lifelines Birth Cohort | 1698/1698 | 29 (27–32) | 23.8 (21.7–26.6) | 1813 kcal/day | NA | NA | NA | NA | Artificial | FFQ | Birth weight |
| Plows, 2022 | USA/Project Viva | 1683/1683 | 32.2 (SD: 5.0) | 24.6 (SD: 5.2) | NA | 11% | 5% | 49% | NA | Artificial | FFQ | Age- and sex-specific BMI z-scores; sum of skinfolds; fat mass index |
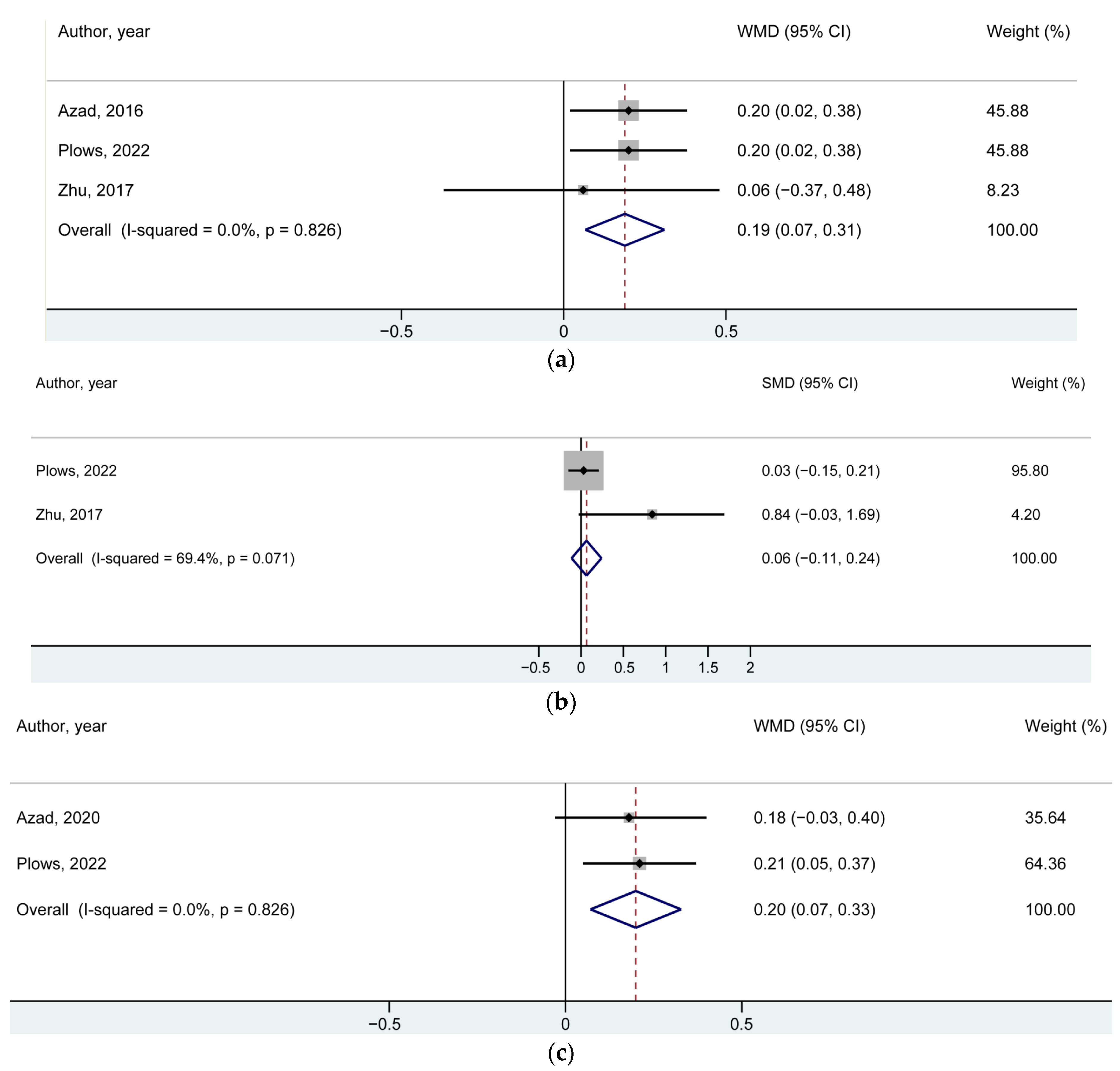
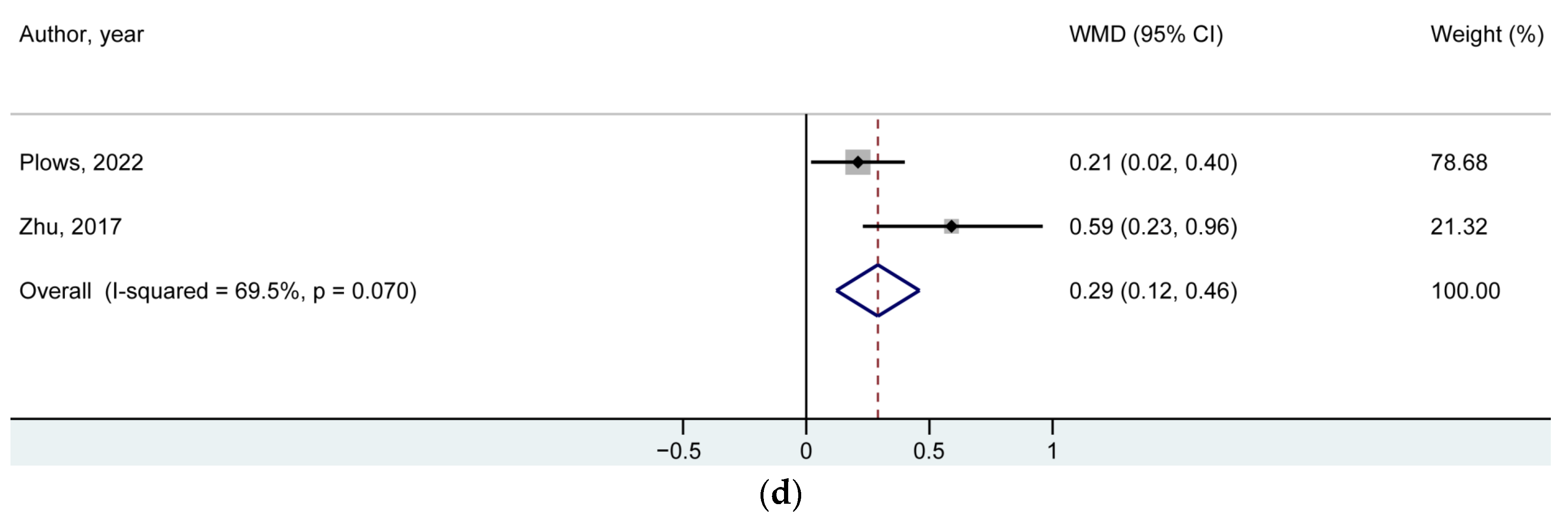
| BMI z-Score | Number of Studies/Offspring | Reference Number for the Included Studies | WMD (95% CI) | p-Value |
|---|---|---|---|---|
| At 1 year of age | 3/5014 | [6,7,14] | 0.19 (0.07, 0.31) | 0.002 |
| At birth | 2/2601 | [7,14] | 0.06 (−0.11, 0.24) * | 0.48 |
| At early childhood | 2/3981 | [7,13] | 0.20 (0.07, 0.33) | 0.002 |
| At mid-childhood | 2/2601 | [7,14] | 0.29 (0.12, 0.46) | 0.001 |
| Study Design Factor | Issue Descriptions |
|---|---|
| Population |
|
| Exposure |
|
| Control |
|
| Outcome |
|
| Time |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wang, R.; Zhang, C.; Li, L.; Zhang, J.; Sun, G. Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies. Nutrients 2022, 14, 5098. https://doi.org/10.3390/nu14235098
Li G, Wang R, Zhang C, Li L, Zhang J, Sun G. Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies. Nutrients. 2022; 14(23):5098. https://doi.org/10.3390/nu14235098
Chicago/Turabian StyleLi, Guowei, Ruoting Wang, Changfa Zhang, Likang Li, Jingyi Zhang, and Guiju Sun. 2022. "Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies" Nutrients 14, no. 23: 5098. https://doi.org/10.3390/nu14235098
APA StyleLi, G., Wang, R., Zhang, C., Li, L., Zhang, J., & Sun, G. (2022). Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies. Nutrients, 14(23), 5098. https://doi.org/10.3390/nu14235098






