Plasma FGF21 Levels Are Not Associated with Weight Loss or Improvements in Metabolic Health Markers upon 12 Weeks of Energy Restriction: Secondary Analysis of an RCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Dietary Intervention
2.3. General Health Measures
2.4. Plasma FGF21
2.5. Intra-Hepatic Lipid Content and Abdominal Fat Distribution
2.6. Clinical Chemistry
2.7. Habitual Dietary Intake and Sweet-Taste Preference
2.8. Statistical Analyses
3. Results
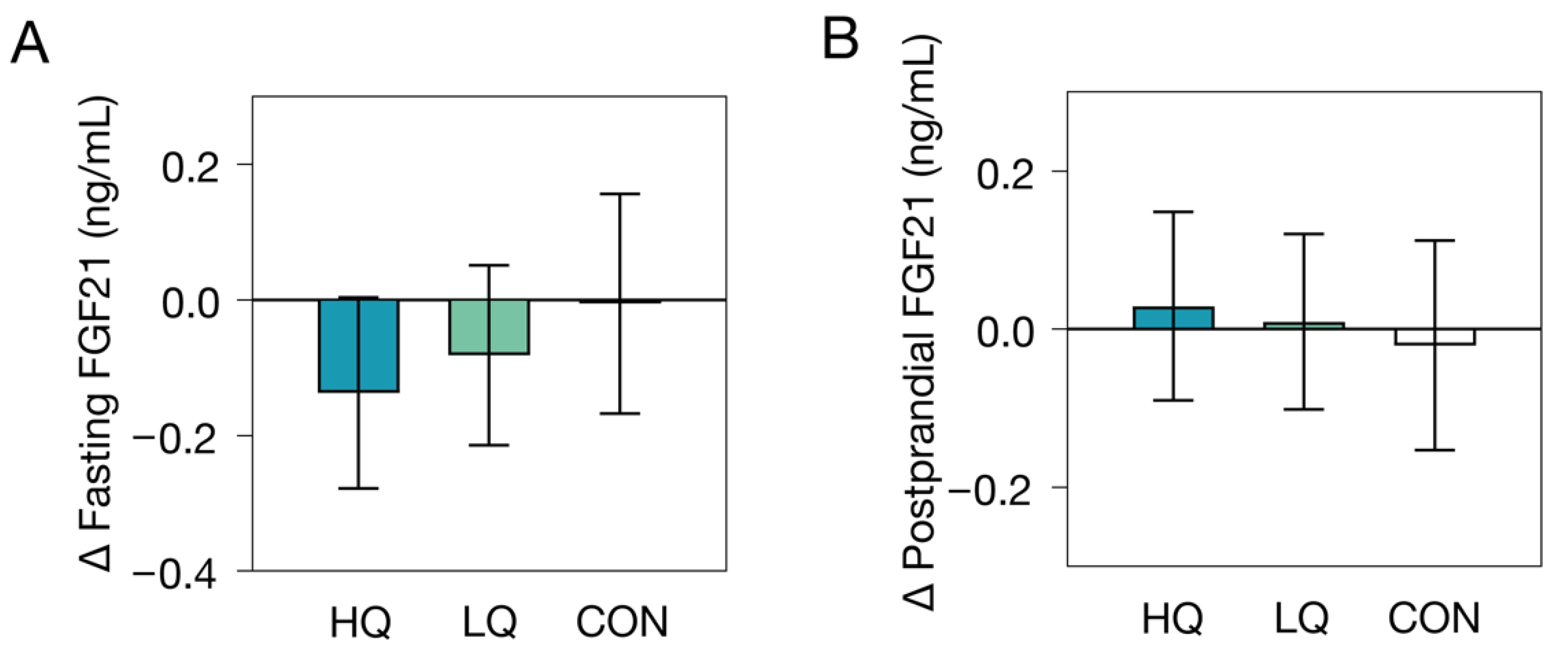
3.1. Effects of Dietary Interventions on Plasma FGF21
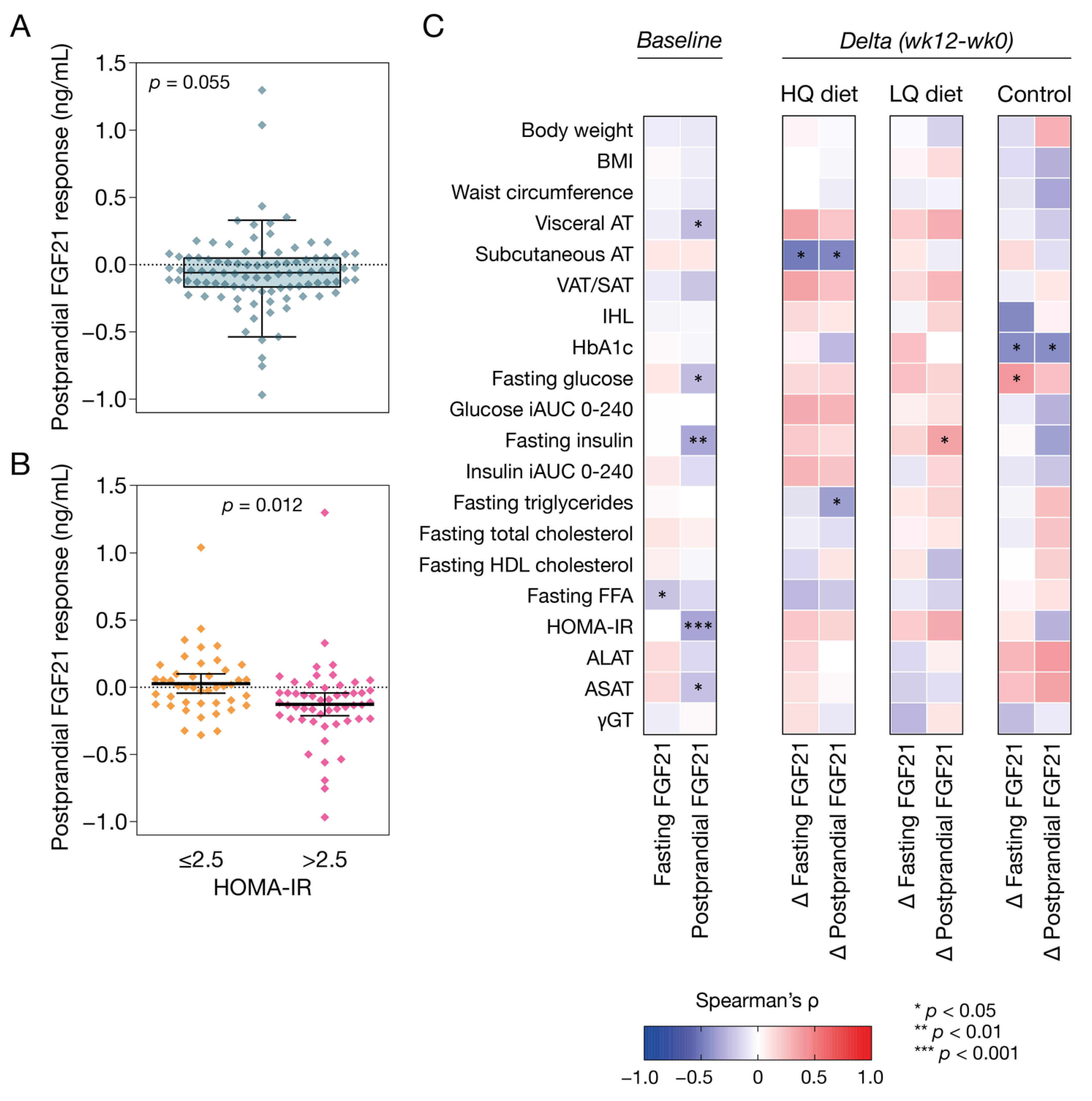
3.2. Postprandial Plasma FGF21 Response at Baseline
3.3. Plasma FGF21 and Correlations with Markers of Metabolic Health
3.4. Plasma FGF21 and Habitual (Macro)Nutrient Intake and Sweet-Taste Preference
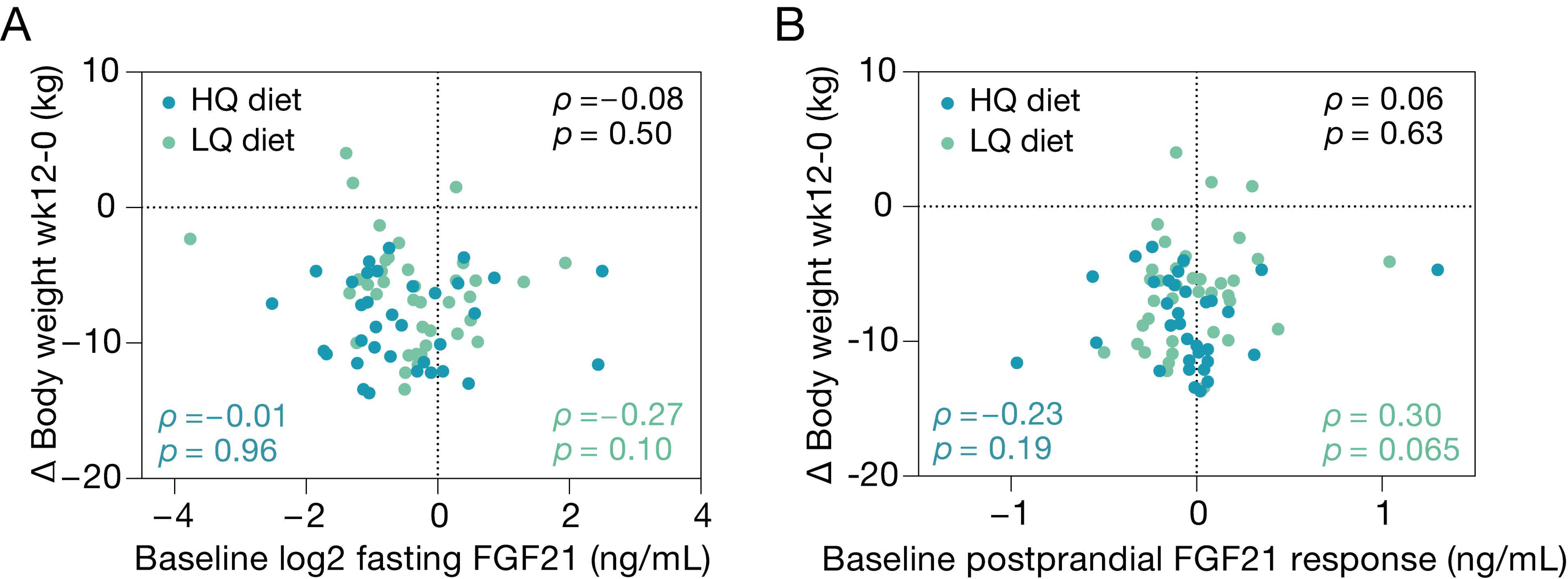
3.5. Baseline Plasma FGF21 and Weight Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Ebling, F.J.P.; Samms, R.J.; Tsintzas, K. Going Back to the Biology of FGF21: New Insights. Trends Endocrinol. Metab. 2019, 30, 491–504. [Google Scholar] [CrossRef]
- Chavez, A.O.; Molina-Carrion, M.; Abdul-Ghani, M.A.; Folli, F.; Defronzo, R.A.; Tripathy, D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009, 32, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela-Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez-Chantar, M.L.; Maratos-Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef]
- Kralisch, S.; Tönjes, A.; Krause, K.; Richter, J.; Lossner, U.; Kovacs, P.; Ebert, T.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Fibroblast growth factor-21 serum concentrations are associated with metabolic and hepatic markers in humans. J. Endocrinol. 2013, 216, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, T.; Schwarz, F.; Fischer-Rosinsky, A.; Pfeiffer, A.F.; Möhlig, M.; Mai, K.; Spranger, J. Fibroblast growth factor 21 predicts the metabolic syndrome and type 2 diabetes in Caucasians. Diabetes Care 2013, 36, 145–149. [Google Scholar] [CrossRef]
- Ong, K.L.; McClelland, R.L.; Allison, M.A.; Kokkinos, J.; Wu, B.J.; Barter, P.J.; Rye, K.A. Association of elevated circulating fibroblast growth factor 21 levels with prevalent and incident metabolic syndrome: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2019, 281, 200–206. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Gomez-Arbelaez, D.; Zulet, M.A.; Carreira, M.C.; Sajoux, I.; de Luis, D.; Castro, A.I.; Baltar, J.; Baamonde, I.; Sueiro, A.; et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: A marker of metabolic stress? Int. J. Obes. 2017, 41, 1570–1578. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef]
- Melhem, S.; Steven, S.; Taylor, R.; Al-Mrabeh, A. Effect of Weight Loss by Low-Calorie Diet on Cardiovascular Health in Type 2 Diabetes: An Interventional Cohort Study. Nutrients 2021, 13, 1465. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef]
- Lips, M.A.; de Groot, G.H.; Berends, F.J.; Wiezer, R.; van Wagensveld, B.A.; Swank, D.J.; Luijten, A.; van Dijk, K.W.; Pijl, H.; Jansen, P.L.M.; et al. Calorie restriction and Roux-en-Y gastric bypass have opposing effects on circulating FGF21 in morbidly obese subjects. Clin. Endocrinol. 2014, 81, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Effects of Weight Loss on FGF-21 in Human Subjects: An Exploratory Study. Int. J. Environ. Res. Public Health 2019, 16, 4877. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Schwarz, F.; Bobbert, T.; Andres, J.; Assmann, A.; Pfeiffer, A.F.; Spranger, J. Relation between fibroblast growth factor-21, adiposity, metabolism, and weight reduction. Metabolism 2011, 60, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Telgenkamp, I.; Kusters, Y.; Schalkwijk, C.G.; Houben, A.; Kooi, M.E.; Lindeboom, L.; Bons, J.A.P.; Schaper, N.C.; Joris, P.J.; Plat, J.; et al. Contribution of Liver Fat to Weight Loss-Induced Changes in Serum Hepatokines: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2019, 104, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Tok, Ö.; Kişioğlu, S.V.; Ersöz, H.; Kahveci, B.; Göktaş, Z. Effects of increased physical activity and/or weight loss diet on serum myokine and adipokine levels in overweight adults with impaired glucose metabolism. J. Diabetes Complicat. 2021, 35, 107892. [Google Scholar] [CrossRef]
- Xu, C.; Markova, M.; Seebeck, N.; Loft, A.; Hornemann, S.; Gantert, T.; Kabisch, S.; Herz, K.; Loske, J.; Ost, M.; et al. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and FGF21 levels. Liver Int. 2020, 40, 2982–2997. [Google Scholar] [CrossRef]
- Asghari, S.; Rezaei, M.; Rafraf, M.; Taghizadeh, M.; Asghari-Jafarabadi, M.; Ebadi, M. Effects of Calorie Restricted Diet on Oxidative/Antioxidative Status Biomarkers and Serum Fibroblast Growth Factor 21 Levels in Nonalcoholic Fatty Liver Disease Patients: A Randomized, Controlled Clinical Trial. Nutrients 2022, 14, 2509. [Google Scholar] [CrossRef]
- Heinitz, S.; Hollstein, T.; Ando, T.; Walter, M.; Basolo, A.; Krakoff, J.; Votruba, S.B.; Piaggi, P. Early adaptive thermogenesis is a determinant of weight loss after six weeks of caloric restriction in overweight subjects. Metabolism 2020, 110, 154303. [Google Scholar] [CrossRef]
- Figarska, S.M.; Rigdon, J.; Ganna, A.; Elmståhl, S.; Lind, L.; Gardner, C.D.; Ingelsson, E. Proteomic profiles before and during weight loss: Results from randomized trial of dietary intervention. Sci. Rep. 2020, 10, 7913. [Google Scholar] [CrossRef]
- Heinitz, S.; Piaggi, P.; Yang, S.; Bonfiglio, S.; Steel, J.; Krakoff, J.; Votruba, S.B. Response of skeletal muscle UCP2-expression during metabolic adaptation to caloric restriction. Int. J. Obes. 2018, 42, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Vinales, K.L.; Begaye, B.; Bogardus, C.; Walter, M.; Krakoff, J.; Piaggi, P. FGF21 Is a Hormonal Mediator of the Human “Thrifty” Metabolic Phenotype. Diabetes 2019, 68, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.Y.; Workalemahu, T.; Paynter, N.P.; Rose, L.M.; Giulianini, F.; Tanaka, T.; Ngwa, J.S.; Qi, Q.; Curhan, G.C.; Rimm, E.B.; et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 2013, 22, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Beaumont, R.N.; Jones, S.E.; Yaghootkar, H.; Tuke, M.A.; Ruth, K.S.; Casanova, F.; West, B.; Locke, J.; Sharp, S.; et al. A Common Allele in FGF21 Associated with Sugar Intake Is Associated with Body Shape, Lower Total Body-Fat Percentage, and Higher Blood Pressure. Cell Rep. 2018, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Søberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017, 25, 1045–1053.e6. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ngwa, J.S.; van Rooij, F.J.; Zillikens, M.C.; Wojczynski, M.K.; Frazier-Wood, A.C.; Houston, D.K.; Kanoni, S.; Lemaitre, R.N.; Luan, J.; et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 2013, 97, 1395–1402. [Google Scholar] [CrossRef]
- Basolo, A.; Hollstein, T.; Shah, M.H.; Walter, M.; Krakoff, J.; Votruba, S.B.; Piaggi, P. Higher fasting plasma FGF21 concentration is associated with lower ad libitum soda consumption in humans. Am. J. Clin. Nutr. 2021, 114, 1518–1522. [Google Scholar] [CrossRef]
- von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef]
- Søberg, S.; Andersen, E.S.; Dalsgaard, N.B.; Jarlhelt, I.; Hansen, N.L.; Hoffmann, N.; Vilsbøll, T.; Chenchar, A.; Jensen, M.; Grevengoed, T.J.; et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol. Metab. 2018, 11, 96–103. [Google Scholar] [CrossRef]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Schutte, S.; Esser, D.; Siebelink, E.; Michielsen, C.J.R.; Daanje, M.; Matualatupauw, J.C.; Boshuizen, H.C.; Mensink, M.; Afman, L.A.; Wageningen Belly Fat Study team. Diverging metabolic effects of 2 energy-restricted diets differing in nutrient quality: A 12-week randomized controlled trial in subjects with abdominal obesity. Am. J. Clin. Nutr. 2022, 116, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-Gonzalez, A.; Rodriguez-Alvarez, M.X.; Gude, F.; Cadarso-Suarez, C.; García, F.; De Francisco, A. Insulin resistance index (HOMA-IR) levels in a general adult population: Curves percentile by gender and age. The EPIRCE study. Diabetes Res. Clin. Pract. 2011, 94, 146–155. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. 2005, 288, E462–E468. [Google Scholar] [CrossRef]
- Positano, V.; Gastaldelli, A.; Sironi, A.m.; Santarelli, M.F.; Lombardi, M.; Landini, L. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J. Magn. Reson. Imaging 2004, 20, 684–689. [Google Scholar] [CrossRef]
- Trijsburg, L.; de Vries, J.H.; Boshuizen, H.C.; Hulshof, P.J.; Hollman, P.C.; van’t Veer, P.; Geelen, A. Comparison of duplicate portion and 24 h recall as reference methods for validating a FFQ using urinary markers as the estimate of true intake. Br. J. Nutr. 2015, 114, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; de Vries, J.H.; Meijboom, S.; Beekman, M.; de Craen, A.J.; Slagboom, P.E.; Feskens, E.J. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef]
- de Bruijn, S.E.M.; de Vries, Y.C.; de Graaf, C.; Boesveldt, S.; Jager, G. The reliability and validity of the Macronutrient and Taste Preference Ranking Task: A new method to measure food preferences. Food Qual. Prefer. 2017, 57, 32–40. [Google Scholar] [CrossRef]
- Mraz, M.; Bartlova, M.; Lacinova, Z.; Michalsky, D.; Kasalicky, M.; Haluzikova, D.; Matoulek, M.; Dostalova, I.; Humenanska, V.; Haluzik, M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009, 71, 369–375. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Roever, L.; Alizadeh, S. Surgery-Induced Weight Loss and Changes in Hormonally Active Fibroblast Growth Factors: A Systematic Review and Meta-Analysis. Obes. Surg. 2020, 30, 4046–4060. [Google Scholar] [CrossRef]
- Herpich, C.; Haß, U.; Kochlik, B.; Franz, K.; Laeger, T.; Klaus, S.; Bosy-Westphal, A.; Norman, K. Postprandial dynamics and response of fibroblast growth factor 21 in older adults. Clin. Nutr. 2021, 40, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Leszczak, S.; Ober, I.; Karrasch, T.; Schäffler, A. Short-term and divergent regulation of FGF-19 and FGF-21 during oral lipid tolerance test but not oral glucose tolerance test. Exp. Clin. Endocrinol. Diabetes. 2015, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, N.; Taskinen, M.R.; Stennabb, S.; Lundbom, N.; Hakkarainen, A.; Vaaralahti, K.; Raivio, T. Decrease in circulating fibroblast growth factor 21 after an oral fat load is related to postprandial triglyceride-rich lipoproteins and liver fat. Eur. J. Endocrinol. 2012, 166, 487–492. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Xia, F.; Lam, K.S.; Wang, Y.; Bao, Y.; Zhang, J.; Gu, Y.; Zhou, P.; Lu, J.; Jia, W.; et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin. Chem. 2011, 57, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Konishi, M.; Murata, Y.; Itoh, N. Time-imposed daily restricted feeding induces rhythmic expression of Fgf21 in white adipose tissue of mice. Biochem. Biophys. Res. Commun. 2011, 412, 396–400. [Google Scholar] [CrossRef]
- Dushay, J.R.; Toschi, E.; Mitten, E.K.; Fisher, F.M.; Herman, M.A.; Maratos-Flier, E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 2015, 4, 51–57. [Google Scholar] [CrossRef]
- ter Horst, K.W.; Gilijamse, P.W.; Demirkiran, A.; van Wagensveld, B.A.; Ackermans, M.T.; Verheij, J.; Romijn, J.A.; Nieuwdorp, M.; Maratos-Flier, E.; Herman, M.A.; et al. The FGF21 response to fructose predicts metabolic health and persists after bariatric surgery in obese humans. Mol. Metab. 2017, 6, 1493–1502. [Google Scholar] [CrossRef]
- Vienberg, S.G.; Jacobsen, S.H.; Worm, D.; Hvolris, L.E.; Naver, L.; Almdal, T.; Hansen, D.L.; Wulff, B.S.; Clausen, T.R.; Madsbad, S.; et al. Increased glucose-stimulated FGF21 response to oral glucose in obese nondiabetic subjects after Roux-en-Y gastric bypass. Clin. Endocrinol. 2017, 86, 156–159. [Google Scholar] [CrossRef]
- Iroz, A.; Montagner, A.; Benhamed, F.; Levavasseur, F.; Polizzi, A.; Anthony, E.; Régnier, M.; Fouché, E.; Lukowicz, C.; Cauzac, M.; et al. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017, 21, 403–416. [Google Scholar] [CrossRef]
- Coppage, A.L.; Heard, K.R.; DiMare, M.T.; Liu, Y.; Wu, W.; Lai, J.H.; Bachovchin, W.W. Human FGF-21 Is a Substrate of Fibroblast Activation Protein. PLoS ONE 2016, 11, e0151269. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise Increases Serum Fibroblast Growth Factor 21 (FGF21) Levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef] [PubMed]

| High-Nutrient- Quality Diet (n = 34) | Low-Nutrient- Quality Diet (n = 38) | Control Group (n = 26) | |
|---|---|---|---|
| Women, n (%) | 18 (52.9%) | 21 (55.3%) | 13 (50.0%) |
| Age, years | 62 (53,65) | 64 (54, 65) | 61 (56, 66) |
| BMI, kg/m2 | 31.1 (28.7, 33.8) | 30.8 (28.9, 33.4) | 30.3 (28.1, 32.6) |
| Intra-hepatic lipid content, % | 3.1 (1.4, 10.2) | 4.9 (2.6, 9.7) | 3.7 (2.1, 8.4) |
| NAFLD, n (%) | 9 (26.5%) | 14 (36.8%) | 7 (26.9%) |
| HbA1c, mmol/mol | 37 ± 3 | 36 ± 2 | 35 ± 3 |
| HOMA-IR | 2.7 (2.0, 4.9) | 2.8 (1.6, 5.0) | 2.6 (1.9, 4.4) |
| HOMA-IR > 2.5, n (%) | 19 (55.9%) | 21 (55.3%) | 14 (53.8%) |
| Plasma glucose, mmol/L | 5.7 ± 0.5 | 5.6 ± 0.7 | 5.7 ± 0.4 |
| Plasma insulin, mU/L | 10.9 (8.2, 17.5) | 11.6 (7.0, 18.6) | 10.9 (7.9, 17.0) |
| Plasma triglycerides, mmol/L | 1.5 (1.1, 1.9) | 1.7 (1.3, 2.3) | 1.7 (1.4, 2.1) |
| Serum total cholesterol, mmol/L | 5.5 ± 0.8 | 5.8 ± 1.0 | 5.5 ± 1.0 |
| Serum HDL cholesterol, mmol/L | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 |
| Plasma free fatty acids, mmol/L | 0.5 (0.4, 0.6) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) |
| Systolic blood pressure, mmHg | 131 ± 15 | 126 ± 19 | 126 ± 14 |
| Diastolic blood pressure, mmHg | 76 ± 9 | 72 ± 8 | 75 ± 9 |
| Alanine aminotransferase, U/L | 24 (19, 33) | 24 (20, 33) | 25 (20, 33) |
| Aspartate aminotransferase, U/L | 22 (19, 26) | 22 (19, 25) | 24 (20, 32) |
| Gamma-glutamyl transferase, U/L | 23 (18, 33) | 26 (18, 36) | 23 (17, 32) |
| Baseline a | Change after 12 Wks b | p-Value c | |
|---|---|---|---|
| Fasting FGF21 (ng/mL) | |||
| Control group (n = 26) | 0.95 ± 1.86 | −0.01 (−0.17, 0.16) | 0.48 |
| Low-Nutrient-Quality Diet (n = 38) | 0.77 ± 1.89 | −0.08 (−0.21, 0.05) | |
| High-Nutrient-Quality Diet (n = 34) | 0.71 ± 1.92 | −0.14 (−0.28, 0.004) | |
| Postprandial FGF21 (ng/mL) | |||
| Control group (n = 26) | 0.82 ± 2.00 | 0.00 (−0.15, 0.15) | 0.57 |
| Low-Nutrient-Quality Diet (n = 37) | 0.71 ± 1.93 | −0.09 (−0.21, 0.04) | |
| High-Nutrient-Quality Diet (n = 32) | 0.64 ± 1.88 | −0.10 (−0.24, 0.04) | |
| Postprandial FGF21 response (ng/mL) | |||
| Control group (n = 26) | −0.12 ± 0.22 | −0.02 (−0.15, 0.11) | 0.86 |
| Low-Nutrient-Quality Diet (n = 37) | −0.02 ± 0.27 | 0.01 (−0.10, 0.12) | |
| High-Nutrient-Quality Diet (n = 32) | −0.05 ± 0.34 | 0.03 (−0.09, 0.15) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gijbels, A.; Schutte, S.; Esser, D.; Michielsen, C.C.J.R.; Siebelink, E.; Mars, M.; Mensink, M.; Afman, L.A. Plasma FGF21 Levels Are Not Associated with Weight Loss or Improvements in Metabolic Health Markers upon 12 Weeks of Energy Restriction: Secondary Analysis of an RCT. Nutrients 2022, 14, 5061. https://doi.org/10.3390/nu14235061
Gijbels A, Schutte S, Esser D, Michielsen CCJR, Siebelink E, Mars M, Mensink M, Afman LA. Plasma FGF21 Levels Are Not Associated with Weight Loss or Improvements in Metabolic Health Markers upon 12 Weeks of Energy Restriction: Secondary Analysis of an RCT. Nutrients. 2022; 14(23):5061. https://doi.org/10.3390/nu14235061
Chicago/Turabian StyleGijbels, Anouk, Sophie Schutte, Diederik Esser, Charlotte C. J. R. Michielsen, Els Siebelink, Monica Mars, Marco Mensink, and Lydia A. Afman. 2022. "Plasma FGF21 Levels Are Not Associated with Weight Loss or Improvements in Metabolic Health Markers upon 12 Weeks of Energy Restriction: Secondary Analysis of an RCT" Nutrients 14, no. 23: 5061. https://doi.org/10.3390/nu14235061
APA StyleGijbels, A., Schutte, S., Esser, D., Michielsen, C. C. J. R., Siebelink, E., Mars, M., Mensink, M., & Afman, L. A. (2022). Plasma FGF21 Levels Are Not Associated with Weight Loss or Improvements in Metabolic Health Markers upon 12 Weeks of Energy Restriction: Secondary Analysis of an RCT. Nutrients, 14(23), 5061. https://doi.org/10.3390/nu14235061






