Periconceptional Maternal Protein Intake from Animal and Plant Sources and the Impact on Early and Late Prenatal Growth and Birthweight: The Rotterdam Periconceptional Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Embryonic Growth and Gestational Age
2.2.2. Fetal Growth and Birthweight
2.2.3. Food Frequency Questionnaire
2.2.4. Maternal Characteristics
2.3. Statistical Analysis
* +1 g/day in the first trimester, +9 g/day in the second trimester, and
+28 g/day in the third trimester of pregnancy
3. Results
3.1. Baseline Characteristics
3.2. Protein Intake
3.3. Embryonic Growth
3.4. Fetal Growth and Birthweight
4. Discussion
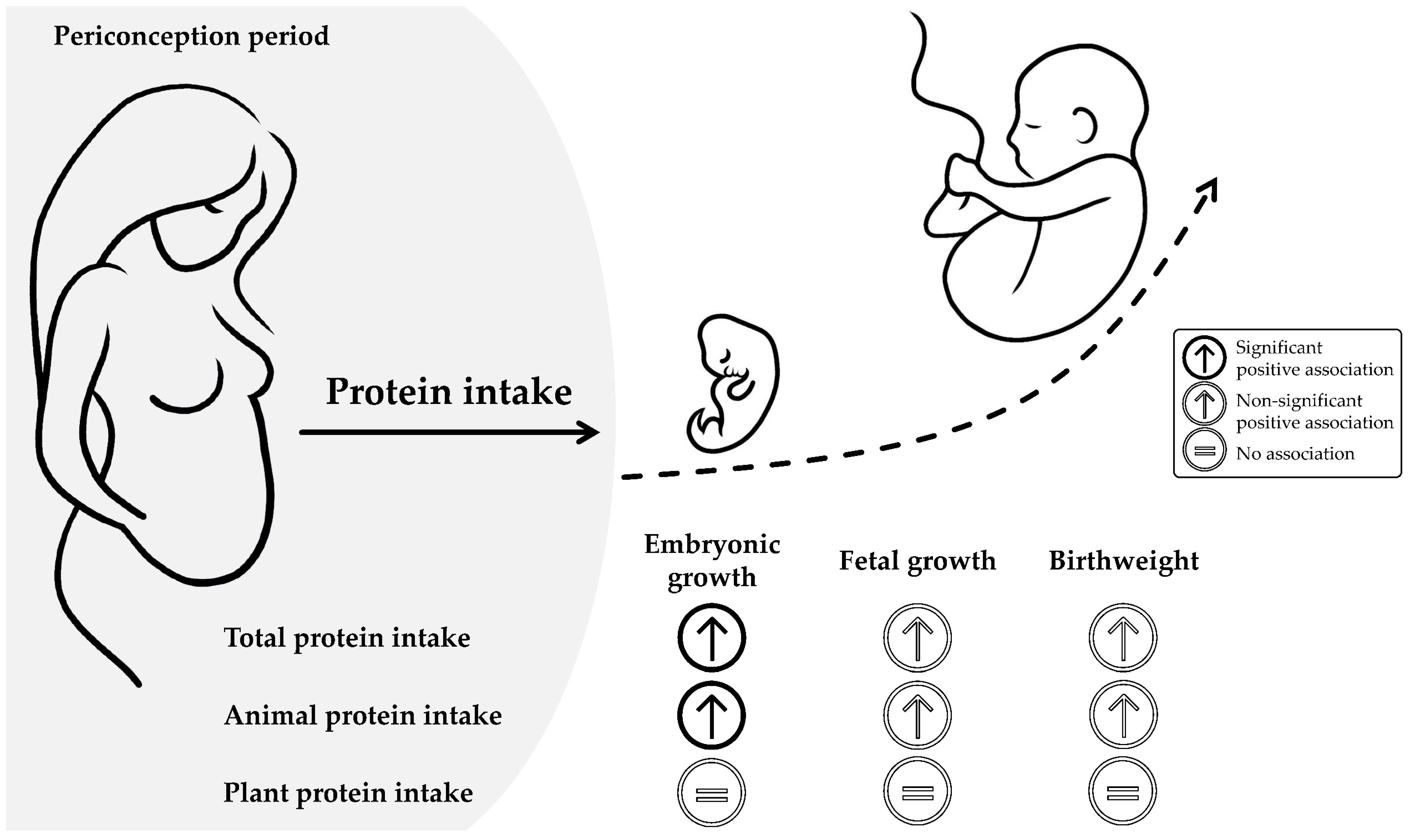
4.1. Main Findings
4.2. Interpretation of Findings and Comparison with Previous Studies
4.2.1. Maternal Protein Intake
4.2.2. Maternal Animal Protein Intake
4.2.3. Maternal Plant Protein Intake
4.3. Strengths and Limitations
4.4. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence: WHO European Office for the Prevention and Control of Noncommunicable Diseases; Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Jenkins, A.L.; Augustin, L.S.; Ludwig, D.S.; Barnard, N.D.; Anderson, J.W. Type 2 diabetes and the vegetarian diet. Am. J. Clin. Nutr. 2003, 78, 610S–616S. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef]
- Rousian, M.; Schoenmakers, S.; Eggink, A.J.; Gootjes, D.V.; Koning, A.H.; Koster, M.P.; Mulders, A.G.; Baart, E.B.; Reiss, I.K.; Laven, J.S.; et al. Cohort profile update: The Rotterdam periconceptional cohort and embryonic and fetal measurements using 3D ultrasound and virtual reality techniques. Int. J. Epidemiol. 2021, 50, 1426–1427. [Google Scholar] [CrossRef]
- Cucó, G.; Arija, V.; Iranzo, R.; Vilà, J.; Prieto, M.T.; Fernández-Ballart, J. Association of maternal protein intake before conception and throughout pregnancy with birth weight. Acta Obstet. Gynecol. Scand. 2006, 85, 413–421. [Google Scholar] [CrossRef]
- Moore, V.M.; Davies, M.J.; Willson, K.J.; Worsley, A.; Robinson, J.S. Dietary composition of pregnant women is related to size of the baby at birth. J. Nutr. 2004, 134, 1820–1826. [Google Scholar] [CrossRef]
- Salavati, N.; Bakker, M.K.; Lewis, F.; Vinke, P.C.; Mubarik, F.; Erwich, J.H.M.; van der Beek, E.M. Associations between preconception macronutrient intake and birth weight across strata of maternal BMI. PLoS ONE 2020, 15, e0243200. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Q.; Tian, X.; Zhang, B.; Zeng, L.; Yan, H.; Dang, S.; Li, Y.H. Dietary protein intake during pregnancy and birth weight among Chinese pregnant women with low intake of protein. Nutr. Metab. 2022, 19, 43. [Google Scholar] [CrossRef]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 120, 1432–1440. [Google Scholar] [CrossRef]
- Van Uitert, E.M.; Exalto, N.; Burton, G.J.; Willemsen, S.P.; Koning, A.H.; Eilers, P.H.; Laven, J.S.; Steegers, E.A.; Steegers-Theunissen, R.P. Human embryonic growth trajectories and associations with fetal growth and birthweight. Hum. Reprod. 2013, 28, 1753–1761. [Google Scholar] [CrossRef]
- Jaddoe, V.W.; de Jonge, L.L.; Hofman, A.; Franco, O.H.; Steegers, E.A.; Gaillard, R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ 2014, 348, g14. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Steegers, E.A.; Eilers, P.H.; Raat, H.; Hofman, A.; Jaddoe, V.W. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 2010, 303, 527–534. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Verheijden-Paulissen, J.J.; van Uitert, E.M.; Wildhagen, M.F.; Exalto, N.; Koning, A.H.; Eggink, A.J.; Duvekot, J.J.; Laven, J.S.; Tibboel, D.; et al. Cohort profile: The Rotterdam periconceptional cohort (Predict study). Int. J. Epidemiol. 2016, 45, 374–381. [Google Scholar] [CrossRef]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Mumford, S.L.; Sjaarda, L.A.; Connell, M.T.; Plowden, T.C.; Andriessen, V.C.; Perkins, N.J.; Hill, M.J.; Silver, R.M.; Schisterman, E.F. Prediction of pregnancy loss by early first trimester ultrasound characteristics. Am. J. Obstet. Gynecol. 2020, 223, 242.e1–242.e22. [Google Scholar] [CrossRef]
- Baken, L.; van Heesch, P.N.; Wildschut, H.I.; Koning, A.H.; van der Spek, P.J.; Steegers, E.A.; Exalto, N. First-trimester crown-rump length and embryonic volume of aneuploid fetuses measured in virtual reality. Ultrasound Obstet. Gynecol. 2013, 41, 521–525. [Google Scholar] [CrossRef]
- Rousian, M.; Koning, A.H.; van Oppenraaij, R.H.; Hop, W.C.; Verwoerd-Dikkeboom, C.M.; van der Spek, P.J.; Exalto, N.; Steegers, E.A. An innovative virtual reality technique for automated human embryonic volume measurements. Hum. Reprod. 2010, 25, 2210–2216. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Gaillard, R.; de Ridder, M.A.; Verburg, B.O.; Witteman, J.C.; Mackenbach, J.P.; Moll, H.A.; Hofman, A.; Steegers, E.A.; Jaddoe, V.W. Individually customised fetal weight charts derived from ultrasound measurements: The Generation R Study. Eur. J. Epidemiol. 2011, 26, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Hoftiezer, L.; Hukkelhoven, C.W.; Hogeveen, M.; Straatman, H.M.; van Lingen, R.A. Defining small-for-gestational-age: Prescriptive versus descriptive birthweight standards. Eur. J. Pediatr. 2016, 175, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Fayet, F.; Flood, V.; Petocz, P.; Samman, S. Relative and biomarker-based validity of a food frequency questionnaire that measures the intakes of vitamin B(12), folate, iron, and zinc in young women. Nutr. Res. 2011, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Verkleij-Hagoort, A.C.; de Vries, J.H.; Stegers, M.P.; Lindemans, J.; Ursem, N.T.; Steegers-Theunissen, R.P. Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: The method of triads. Eur. J. Clin. Nutr. 2007, 61, 610–615. [Google Scholar] [CrossRef]
- NEVO-Online Versie 2021/7.0. Available online: https://www.rivm.nl/nederlands-voedingsstoffenbestand (accessed on 3 January 2022).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Smit, A.J.P.; Hojeij, B.; Rousian, M.; Schoenmakers, S.; Willemsen, S.P.; Steegers-Theunissen, R.P.M.; van Rossem, L. A high periconceptional maternal ultra-processed food consumption impairs embryonic growth: The Rotterdam periconceptional cohort. Clin. Nutr. 2022, 41, 1667–1675. [Google Scholar] [CrossRef]
- Schneider, S.L. The international standard classification of education 2011. In Class and Stratification Analysis; Emerald Group Publishing Limited: Bingley, UK, 2013. [Google Scholar]
- Alders, M. Classification of the population with a foreign background in The Netherlands. In Proceedings of the Measure and Mismeasure of Populations. The Statistical Use of Ethnic and Racial Categories in Multicultural Societies, Paris, France, 17–18 December 2001; p. 18. [Google Scholar]
- EFSA Panel on Dietetic Products, N.a.A.N. Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Bukowski, R.; Smith, G.C.; Malone, F.D.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Hankins, G.D.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; et al. Human sexual size dimorphism in early pregnancy. Am. J. Epidemiol. 2007, 165, 1216–1218. [Google Scholar] [CrossRef]
- Shah, P.S. Parity and low birth weight and preterm birth: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 862–875. [Google Scholar] [CrossRef]
- Van Uitert, E.M.; van der Elst-Otte, N.; Wilbers, J.J.; Exalto, N.; Willemsen, S.P.; Eilers, P.H.; Koning, A.H.; Steegers, E.A.; Steegers-Theunissen, R.P. Periconception maternal characteristics and embryonic growth trajectories: The Rotterdam Predict study. Hum. Reprod. 2013, 28, 3188–3196. [Google Scholar] [CrossRef]
- Van Dijk, M.R.; Borggreven, N.V.; Willemsen, S.P.; Koning, A.H.J.; Steegers-Theunissen, R.P.M.; Koster, M.P.H. Maternal Lifestyle Impairs Embryonic Growth: The Rotterdam Periconception Cohort. Reprod. Sci. 2018, 25, 916–922. [Google Scholar] [CrossRef]
- Van Duijn, L.; Rousian, M.; Laven, J.S.E.; Steegers-Theunissen, R.P.M. Periconceptional maternal body mass index and the impact on post-implantation (sex-specific) embryonic growth and morphological development. Int. J. Obes. 2021, 45, 2369–2376. [Google Scholar] [CrossRef]
- Bottomley, C.; Daemen, A.; Mukri, F.; Papageorghiou, A.T.; Kirk, E.; Pexsters, A.; De Moor, B.; Timmerman, D.; Bourne, T. Assessing first trimester growth: The influence of ethnic background and maternal age. Hum. Reprod. 2009, 24, 284–290. [Google Scholar] [CrossRef]
- Eindhoven, S.C.; van Uitert, E.M.; Laven, J.S.; Willemsen, S.P.; Koning, A.H.; Eilers, P.H.; Exalto, N.; Steegers, E.A.; Steegers-Theunissen, R.P. The influence of IVF/ICSI treatment on human embryonic growth trajectories. Hum. Reprod. 2014, 29, 2628–2636. [Google Scholar] [CrossRef]
- Jackson, R.A.; Gibson, K.A.; Wu, Y.W.; Croughan, M.S. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet. Gynecol. 2004, 103, 551–563. [Google Scholar] [CrossRef]
- Mackerras, D. Energy adjustment: The concepts underlying the debate. J. Clin. Epidemiol. 1996, 49, 957–962. [Google Scholar] [CrossRef]
- Mendez, M.A.; Popkin, B.M.; Buckland, G.; Schroder, H.; Amiano, P.; Barricarte, A.; Huerta, J.-M.; Quirós, J.R.; Sánchez, M.-J.; González, C.A. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am. J. Epidemiol. 2011, 173, 448–458. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Verburg, B.O.; Steegers, E.A.; De Ridder, M.; Snijders, R.J.; Smith, E.; Hofman, A.; Moll, H.A.; Jaddoe, V.W.; Witteman, J.C. New charts for ultrasound dating of pregnancy and assessment of fetal growth: Longitudinal data from a population-based cohort study. Ultrasound Obstet. Gynecol. 2008, 31, 388–396. [Google Scholar] [CrossRef]
- Godfrey, K.; Robinson, S.; Barker, D.J.; Osmond, C.; Cox, V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 1996, 312, 410–414. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Peral-Sanchez, I.; Hojeij, B.; Ojeda, D.A.; Steegers-Theunissen, R.P.M.; Willaime-Morawek, S. Epigenetics in the Uterine Environment: How Maternal Diet and ART May Influence the Epigenome in the Offspring with Long-Term Health Consequences. Genes 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Nutrition of the human fetus during the first trimester—A review. Placenta 2001, 22 (Suppl. A), S70–S77. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, I.F.; Mulders, A.; van der Windt, M.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: A systematic review. Hum. Reprod. Update 2019, 25, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2021, 119, 428–442. [Google Scholar] [CrossRef]
- Crozier, S.R.; Robinson, S.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Women’s dietary patterns change little from before to during pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef]
- Widyawati, S.A.; Suhartono, S.; Mexitalia, M.; Soejoenoes, A. The Relationship between Pesticide Exposure and Umbilical Serum IGF-1 Levels and Low-birth Weight: A Case-control Study in Brebes, Indonesia. Int. J. Occup. Environ. Med. 2020, 11, 15–23. [Google Scholar] [CrossRef]
- Petit, C.; Chevrier, C.; Durand, G.; Monfort, C.; Rouget, F.; Garlantezec, R.; Cordier, S. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities: A prospective cohort study in Brittany, France. Environ. Health 2010, 9, 71. [Google Scholar] [CrossRef]
- Beyene, T. Veterinary drug residues in food-animal products: Its risk factors and potential effects on public health. J. Vet. Sci. Technol. 2016, 7, 285. [Google Scholar] [CrossRef]

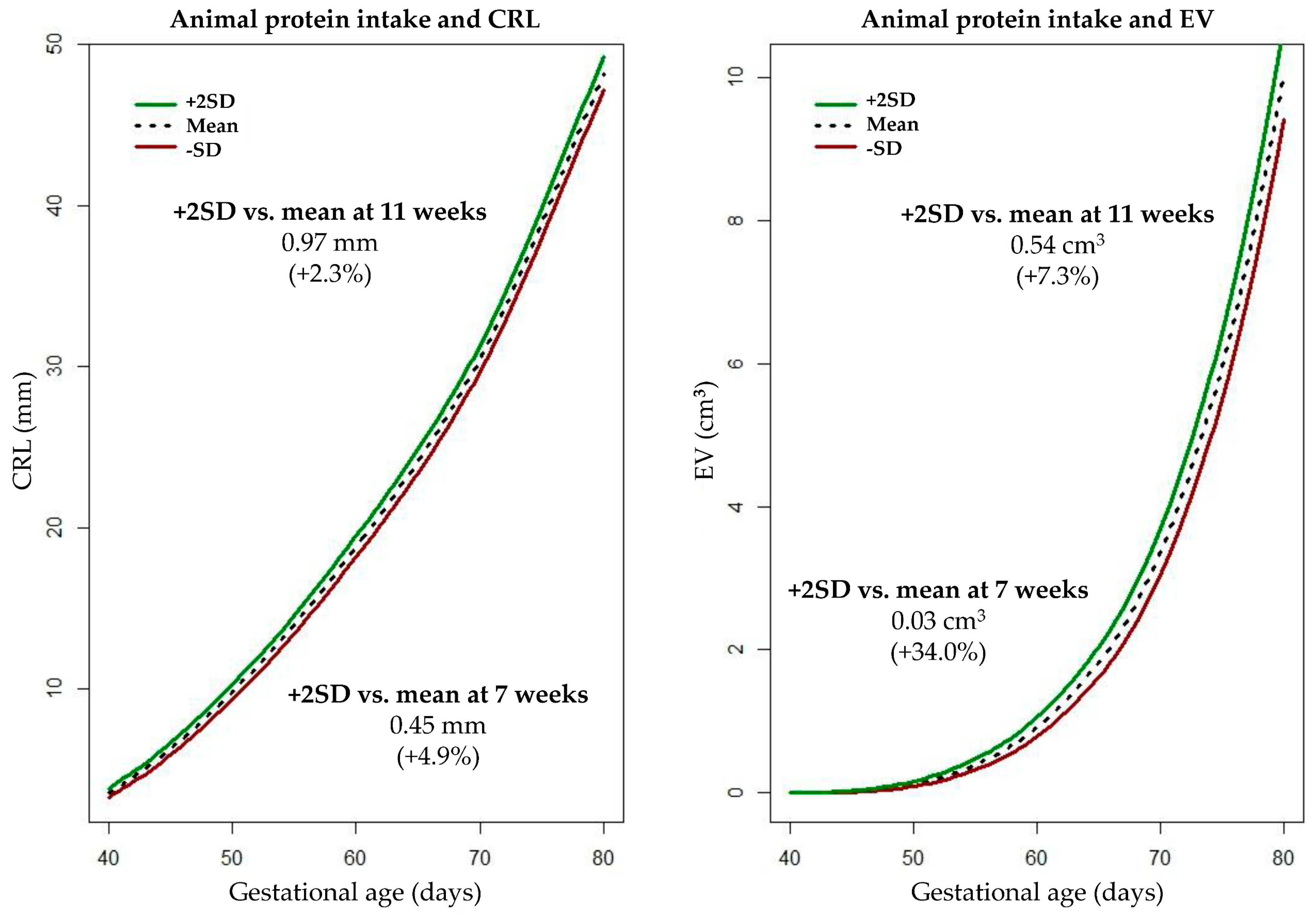
| Maternal Characteristics | Total Study Population (n = 501) | Tertile 1 (n = 167) 8.3–14.1% | Tertile 2 (n = 167) 14.2–16.0% | Tertile 3 (n = 167) 16.1–23.4% |
|---|---|---|---|---|
| Age at conception (years) | ||||
| Mean (SD) | 32.7 (4.3) | 32.1 (4.5) | 32.8 (4.4) | 33.3 (3.9) |
| Missing | 0 | 0 | 0 | 0 |
| Geographical background | ||||
| Non-western | 50 (10.4%) | 21 (13.0%) | 14 (8.8%) | 15 (9.4%) |
| Western | 431 (89.6%) | 141 (87.0%) | 145 (91.2%) | 145 (90.6%) |
| Missing | 20 | 5 | 8 | 7 |
| Educational level | ||||
| Low | 33 (6.9%) | 20 (12.3%) | 9 (5.6%) | 4 (2.5%) |
| Medium | 159 (33.0%) | 64 (39.5%) | 45 (28.1%) | 50 (31.3%) |
| High | 290 (60.2%) | 78 (48.1%) | 106 (66.3%) | 106 (66.3%) |
| Missing | 19 | 5 | 7 | 7 |
| Parity | ||||
| Nulliparous | 205 (42.4%) | 64 (39.5%) | 69 (42.9%) | 72 (45.0%) |
| Multiparous | 278 (57.6%) | 98 (60.5%) | 92 (57.1%) | 88 (55.0%) |
| Missing | 18 | 5 | 6 | 7 |
| Conception mode | ||||
| IVF/ICSI | 271 (54.1%) | 87 (52.1%) | 87 (52.1%) | 96 (57.5%) |
| Natural | 230 (45.9%) | 80 (47.9%) | 80 (47.9%) | 71 (42.5%) |
| Missing | 0 | 0 | 0 | 0 |
| Body Mass Index (kg/m2) | ||||
| Mean (SD) | 24.7 (4.1) | 24.7 (4.2) | 24.6 (4.5) | 24.6 (3.5) |
| Missing | 0 | 0 | 0 | 0 |
| Folic acid supplement use | ||||
| Inadequate | 62 (12.9%) | 24 (14.8%) | 25 (15.5%) | 13 (8.2%) |
| Adequate | 420 (87.1%) | 138 (85.2%) | 136 (84.5%) | 146 (91.8%) |
| Missing | 19 | 5 | 6 | 8 |
| Smoking | ||||
| Yes | 66 (13.7%) | 29 (17.9%) | 23 (14.3%) | 14 (8.8%) |
| No | 417 (86.3%) | 133 (82.1%) | 138 (85.7%) | 146 (91.3%) |
| Missing | 18 | 5 | 6 | 7 |
| Alcohol | ||||
| Yes | 139 (28.8%) | 39 (24.1%) | 60 (37.3%) | 40 (25.0%) |
| No | 344 (71.2%) | 123 (75.9%) | 101 (62.7%) | 120 (75.0%) |
| Missing | 18 | 5 | 6 | 7 |
| Drugs | ||||
| Yes | 10 (2.1%) | 6 (3.7%) | 3 (1.9%) | 1 (0.6%) |
| No | 473 (97.9%) | 156 (96.3%) | 158 (98.1%) | 159 (99.4%) |
| Missing | 18 | 5 | 6 | 7 |
| Energy intake (kcal/day) | ||||
| Mean (SD) | 1940 (562) | 2100 (699) | 1990 (498) | 1740 (383) |
| Missing | 0 | 0 | 0 | 0 |
| Maternal Nutrient Intake | g/Day | % of Energy | % of Protein |
|---|---|---|---|
| Total protein | 72.8 (20.5) | 15.2 (2.4) | |
| Animal protein | 41.4 (15.8) | 8.6 (2.6) | 56.0 (10.6) |
| Plant protein | 31.4 (10.1) | 6.5 (1.3) | 44.0 (10.6) |
| RDI first trimester | 59.3 (10.5) | ||
| RDI second trimester | 67.3 (10.5) | ||
| RDI third trimester | 86.3 (10.5) |
| Total Study Population | Model 1 | Model 2 | ||
|---|---|---|---|---|
| (n = 501) | (95% CI) | p-Value | (95% CI) | p-Value |
| CRL (mm) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.024 (0.002, 0.046) | 0.031 | 0.022 (−0.001, 0.045) | 0.061 |
| Animal protein intake | 0.025 (0.003, 0.047) | 0.027 | 0.023 (−0.0002, 0.046) | 0.052 |
| Plant protein intake | 0.011 (−0.031, 0.054) | 0.597 | 0.0001 (−0.045, 0.046) | 0.998 |
| ∛EV (cm3) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.011 (0.001, 0.022) | 0.033 | 0.014 (0.003, 0.025) | 0.017 |
| Animal protein intake | 0.012 (0.002, 0.023) | 0.024 | 0.015 (0.003, 0.026) | 0.012 |
| Plant protein intake | −0.004 (−0.024, 0.016) | 0.698 | −0.01 (−0.028, 0.016) | 0.617 |
| Total Study Population | Model 1 | Model 2 | ||
|---|---|---|---|---|
| (n = 501) | (95% CI) | p-Value | (95% CI) | p-Value |
| CRL (mm) | ||||
| For each 10% increase | ||||
| Total protein intake | 0.118 (0.015, 0.222) | 0.025 | 0.119 (0.009, 0.229) | 0.035 |
| Animal protein intake | 0.113 (0.009, 0.216) | 0.034 | 0.011 (0.004, 0.220) | 0.049 |
| Plant protein intake | 0.083 (−0.127, 0.294) | 0.437 | 0.043 (−0.187, 0.273) | 0.716 |
| ∛EV (cm3) | ||||
| For each 10% increase | ||||
| Total protein intake | 0.048 (−0.0004, 0.097) | 0.052 | 0.061 (0.008, 0.114) | 0.024 |
| Animal protein intake | 0.050 (0.002, 0.099) | 0.043 | 0.062 (0.009, 0.115) | 0.021 |
| Plant protein intake | −0.020 (−0.120, 0.081) | 0.701 | −0.028 (−0.138, 0.083) | 0.623 |
| Total Study Population | Model 2 | Model 3 | ||
|---|---|---|---|---|
| (n = 501) | (95% CI) | p-Value | (95% CI) | p-Value |
| CRL (mm) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.022 (−0.001, 0.045) | 0.061 | 0.028 (0.003, 0.054) | 0.027 |
| Animal protein intake | 0.023 (−0.0002, 0.046) | 0.052 | 0.027 (0.001, 0.053) | 0.040 |
| Plant protein intake | 0.0001 (−0.045, 0.046) | 0.998 | 0.0001 (−0.046, 0.046) | 0.998 |
| ∛EV (cm3) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.014 (0.003, 0.025) | 0.017 | 0.018 (0.006, 0.031) | 0.004 |
| Animal protein intake | 0.015 (0.003, 0.026) | 0.012 | 0.015 (0.032, 0.026) | 0.012 |
| Plant protein intake | −0.01 (−0.028, 0.016) | 0.617 | −0.006 (−0.028, 0.016) | 0.617 |
| Women with a BMI: 18.5–25 kg/m2 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| (n = 286) | (95% CI) | p-Value | (95% CI) | p-Value |
| CRL (mm) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.046 (0.017, 0.076) | 0.002 | 0.044 (0.012, 0.076) | 0.007 |
| Animal protein intake | 0.047 (0.017, 0.077) | 0.002 | 0.044 (0.012, 0.075) | 0.007 |
| Plant protein intake | 0.036 (−0.021, 0.094) | 0.212 | 0.020 (−0.042, 0.082) | 0.519 |
| ∛EV (cm3) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.016 (0.002, 0.031) | 0.021 | 0.017 (0.002, 0.033) | 0.024 |
| Animal protein intake | 0.016 (0.003, 0.030) | 0.021 | 0.018 (0.003, 0.033) | 0.023 |
| Plant protein intake | 0.001 (−0.027, 0.029) | 0.939 | 0.001 (−0.030, 0.031) | 0.973 |
| Total Study Population | Model 1 | Model 2 | ||
|---|---|---|---|---|
| (95% CI) | p-Value | (95% CI) | p-Value | |
| Standardized EFW (n = 422) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.062 (−0.025, 0.150) | 0.161 | 0.060 (−0.029, 0.150) | 0.186 |
| Animal protein intake | 0.064 (−0.024, 0.151) | 0.155 | 0.062 (−0.028, 0.151) | 0.178 |
| Plant protein intake | 0.032 (−0.131, 0.194) | 0.702 | 0.021 (−0.151, 0.193) | 0.810 |
| Birthweight (n = 501) | ||||
| For each additional 10 g/day | ||||
| Total protein intake | 0.049 (−0.036, 0.134) | 0.259 | 0.019 (−0.069, 0.107) | 0.667 |
| Animal protein intake | 0.049 (−0.036, 0.134) | 0.256 | 0.019 (−0.069, 0.107) | 0.675 |
| Plant protein intake | 0.038 (−0.123, 0.198) | 0.647 | −0.001 (−0.174, 0.173) | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Zundert, S.; van der Padt, S.; Willemsen, S.; Rousian, M.; Mirzaian, M.; van Schaik, R.; Steegers-Theunissen, R.; van Rossem, L. Periconceptional Maternal Protein Intake from Animal and Plant Sources and the Impact on Early and Late Prenatal Growth and Birthweight: The Rotterdam Periconceptional Cohort. Nutrients 2022, 14, 5309. https://doi.org/10.3390/nu14245309
van Zundert S, van der Padt S, Willemsen S, Rousian M, Mirzaian M, van Schaik R, Steegers-Theunissen R, van Rossem L. Periconceptional Maternal Protein Intake from Animal and Plant Sources and the Impact on Early and Late Prenatal Growth and Birthweight: The Rotterdam Periconceptional Cohort. Nutrients. 2022; 14(24):5309. https://doi.org/10.3390/nu14245309
Chicago/Turabian Stylevan Zundert, Sofie, Simone van der Padt, Sten Willemsen, Melek Rousian, Mina Mirzaian, Ron van Schaik, Régine Steegers-Theunissen, and Lenie van Rossem. 2022. "Periconceptional Maternal Protein Intake from Animal and Plant Sources and the Impact on Early and Late Prenatal Growth and Birthweight: The Rotterdam Periconceptional Cohort" Nutrients 14, no. 24: 5309. https://doi.org/10.3390/nu14245309
APA Stylevan Zundert, S., van der Padt, S., Willemsen, S., Rousian, M., Mirzaian, M., van Schaik, R., Steegers-Theunissen, R., & van Rossem, L. (2022). Periconceptional Maternal Protein Intake from Animal and Plant Sources and the Impact on Early and Late Prenatal Growth and Birthweight: The Rotterdam Periconceptional Cohort. Nutrients, 14(24), 5309. https://doi.org/10.3390/nu14245309







