The Relationship between Nutrient Intake and Cataracts in the Older Adult Population of Korea
Abstract
1. Introduction
2. Materials and Methods
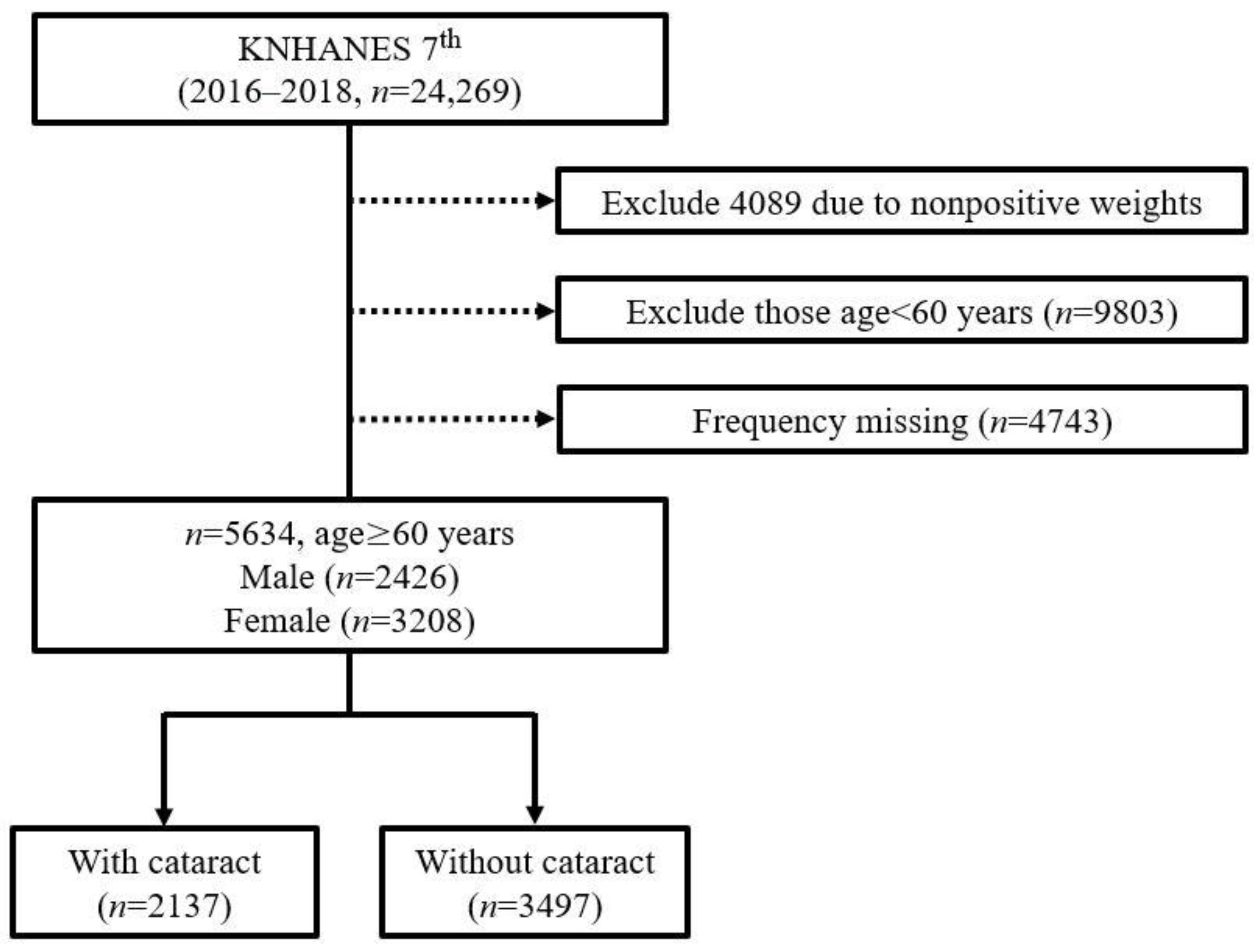
2.1. Study Population
2.2. Study Variables
2.3. Statistical Analysis
3. Results
3.1. Basic Characteristics of the Study Population
3.2. Associations between Nutrients and Cataract
3.3. Nutrient Factors Associated with Cataracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Bourne, R.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Suh, L.H.; Kane, S.A. Cataract. In The Columbia Guide to Basic Elements of Eye Care; Springer: Berlin/Heidelberg, Germany, 2019; pp. 117–136. [Google Scholar]
- Song, P.; Wang, H.; Theodoratou, E.; Chan, K.Y.; Rudan, I. The national and subnational prevalence of cataract and cataract blindness in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010804. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Sándor, G.L.; Tóth, G.; Pék, A.; Lukács, R.; Szalai, I.; Tóth, G.Z.; Papp, A.; Nagy, Z.Z.; Limburg, H. Visual impairment and blindness in Hungary. Acta Ophthalmol. 2018, 96, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pardhan, S.; Kulothungan, V.; Swaminathan, G.; Ravichandran, J.S.; Ganesan, S.; Sharma, T.; Raman, R. The prevalence and risk factors for cataract in rural and urban India. Indian J. Ophthalmol. 2019, 67, 477. [Google Scholar]
- HEALTH INSURANCE REVIEW & ASSESSMENT SERVICE, National Health Insurance Service. National Health Insurance Statistical Yearbook 2020; HEALTH INSURANCE REVIEW & ASSESSMENT SERVICE: Wonju-Si, Korea, 2020; p. 670. ISSN 1738-8945. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAJ030000007001 (accessed on 21 July 2022).
- Korean Statistical Information Service Home Page; Main Surgery Statistical Yearbook: Number of Patients per 100,000 population by Age, Gender and Type of Medical Institution. Available online: https://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01 (accessed on 21 July 2022).
- Taylor, A.; Jacques, P.F.; Epstein, E.M. Relations among aging, antioxidant status, and cataract. Am. J. Clin. Nutr. 1995, 62, 1439S–1447S. [Google Scholar] [CrossRef]
- Caird, F.; Hutchinson, M.; Pirie, A. Cataract and diabetes. Br. Med. J. 1964, 2, 665. [Google Scholar] [CrossRef]
- Taylor, A. Cataract: Relationship between nutrition and oxidation. J. Am. Coll. Nutr. 1993, 12, 138–146. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Samoli, E.; Theodossiadis, P.G.; Papathanassiou, M.; Lagiou, A.; Lagiou, P.; Tzonou, A. Diet and cataract: A case–control study. Int. Ophthalmol. 2014, 34, 59–68. [Google Scholar] [CrossRef]
- Wu, C.; Han, X.; Yan, X.; Keel, S.; Shang, X.; Zhang, L.; He, M. Impact of diet on the incidence of cataract surgery among diabetic patients: Findings from the 45 and up study. Curr. Eye Res. 2019, 44, 385–392. [Google Scholar] [CrossRef]
- Rautiainen, S.; Lindblad, B.E.; Morgenstern, R.; Wolk, A. Total antioxidant capacity of the diet and risk of age-related cataract: A population-based prospective cohort of women. JAMA Ophthalmol. 2014, 132, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, H.; Li, P.; Gao, T.; Lin, J.; Yang, J.; Wu, Y.; Ye, J. Association between dietary carbohydrate intake and dietary glycemic index and risk of age-related cataract: A meta-analysis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3660–3668. [Google Scholar] [CrossRef]
- Bae, J.H.; Shin, D.S.; Lee, S.C.; Hwang, I.C. Sodium intake and socioeconomic status as risk factors for development of age-related cataracts: The Korea National Health and Nutrition Examination Survey. PLoS ONE 2015, 10, e0136218. [Google Scholar] [CrossRef]
- Rodriguez-Sargent, C.; Cangiano, J.; Berríos Cabán, G.; Marrero, E.; Martínez-Maldonado, M. Cataracts and hypertension in salt-sensitive rats. A possible ion transport defect. Hypertension 1987, 9, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Ghanavati, M.; Hajian, P.N.; Hajishirazi, S.; Ehteshami, M.; Rashidkhani, B. Nutrient patterns and risk of cataract: A case-control study. Int. J. Ophthalmol. 2017, 10, 586. [Google Scholar] [PubMed]
- Choi, J.-H.; Lee, E.; Heo, Y.-R. The Association between Dietary Vitamin A and C Intakes and Cataract: Data from Korea National Health and Nutrition Examination Survey 2012. Clin. Nutr. Res. 2020, 9, 163. [Google Scholar] [CrossRef]
- Wang, A.; Han, J.; Jiang, Y.; Zhang, D. Association of vitamin A and β-carotene with risk for age-related cataract: A meta-analysis. Nutrition 2014, 30, 1113–1121. [Google Scholar] [CrossRef]
- Frederikse, P.H.; Farnsworth, P.; Zigler Jr, J.S. Thiamine Deficiencyin VivoProduces Fiber Cell Degeneration in Mouse Lenses. Biochem. Biophys. Res. Commun. 1999, 258, 703–707. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.-j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.-K. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- Kim, Y. The Korea National Health and nutrition examination survey (KNHANES): Current status and challenges. Epidemiol. Health 2014, 36, e2014002. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.D.; Vashist, P.; Gupta, S.K.; Young, I.S.; Maraini, G.; Camparini, M.; Jayanthi, R.; John, N.; Fitzpatrick, K.E.; Chakravarthy, U. Inverse association of vitamin C with cataract in older people in India. Ophthalmology 2011, 118, 1958–1965.e1952. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.-P.; Wang, P.; Xu, Y.; Pan, C.-W. Cataract and depressive symptoms among older Chinese adults. Optom. Vis. Sci. 2016, 93, 1479–1484. [Google Scholar] [CrossRef]
- Vashist, P.; Talwar, B.; Gogoi, M.; Maraini, G.; Camparini, M.; Ravindran, R.D.; Murthy, G.V.; Fitzpatrick, K.E.; John, N.; Chakravarthy, U. Prevalence of cataract in an older population in India: The India study of age-related eye disease. Ophthalmology 2011, 118, 272–278.e272. [Google Scholar] [CrossRef]
- Castell, G.S.; Serra-Majem, L.; Ribas-Barba, L. What and how much do we eat? 24-hour dietary recall method. Nutr. Hosp. 2015, 31, 46–48. [Google Scholar] [CrossRef]
- Karvetti, R.-L. Validity of the 24-hour dietary recall. J. Am. Diet. Assoc. 1985, 85, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Roth, D.L.; Ritchie, C.S.; Burgio, K.L.; Locher, J.L. Reliability and predictive validity of energy intake measures from the 24-hour dietary recalls of homebound older adults. J. Am. Diet. Assoc. 2010, 110, 773–778. [Google Scholar] [CrossRef]
- Greger, J.; Etnyre, G. Validity of 24-hour dietary recalls by adolescent females. Am. J. Public Health 1978, 68, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Kesse, E.; Slimani, N.; Bueno-De-Mesquita, H.; Ocké, M.; Skeie, G.; Kumle, M.; Iraeta, M.D.; Gómez, P.M.; Janzon, L. Meat consumption in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts: Results from 24-hour dietary recalls. Public Health Nutr. 2002, 5, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Sciences, Rural Development Administration National Institute of Agricultural Sciences. Korea Food Composition Database. Available online: http://koreanfood.rda.go.kr/eng/fctFoodSrchEng/list (accessed on 22 July 2022).
- Liu, Y.-T.; Hung, T.-Y.; Lee, Y.-K.; Huang, M.-Y.; Hsu, C.-Y.; Su, Y.-C. Association between chronic kidney disease and risk of cataract: A nationwide retrospective cohort study. Am. J. Nephrol. 2017, 45, 524–531. [Google Scholar] [CrossRef]
- Jeon, Y.; Song, Y. Association between Vitamin D and Allergic Disease and Cataract in Korean Adults. Korean J. Fam. Pract. 2020, 10, 223–230. [Google Scholar] [CrossRef]
- Prokofyeva, E.; Wegener, A.; Zrenner, E. Cataract prevalence and prevention in Europe: A literature review. Acta Ophthalmol. 2013, 91, 395–405. [Google Scholar] [CrossRef]
- Maralani, H.G.; Tai, B.C.; Wong, T.Y.; Tai, E.S.; Li, J.; Wang, J.J.; Mitchell, P. Metabolic syndrome and risk of age-related cataract over time: An analysis of interval-censored data using a random-effects model. Investig. Ophthalmol. Vis. Sci. 2013, 54, 641–646. [Google Scholar] [CrossRef]
- Nemet, A.; Vinker, S.; Levartovsky, S.; Kaiserman, I. Is cataract associated with cardiovascular morbidity? Eye 2010, 24, 1352–1358. [Google Scholar] [CrossRef]
- Hiratsuka, Y.; Ono, K.; Murakami, A. Alcohol use and cataract. Curr. Drug Abus. Rev. 2009, 2, 226–229. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Williams, P.T. Walking and running are associated with similar reductions in cataract risk. Med. Sci. Sport. Exerc. 2013, 45, 1089. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef]
- Grice, J.W.; Barrett, P.T. A note on Cohen’s overlapping proportions of normal distributions. Psychol. Rep. 2014, 115, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.; Burt, B.; Taylor, G. Prevalence and trends in periodontitis in the USA: From the NHANES III to the NHANES, 1988 to 2000. J. Dent. Res. 2005, 84, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lucas Jr, H.C.; Shmueli, G. Research commentary—Too big to fail: Large samples and the p-value problem. Inf. Syst. Res. 2013, 24, 906–917. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- DiGangi, E.A.; Hefner, J.T. Ancestry estimation. Res. Methods Hum. Skelet. Biol. 2013, 12, 117–149. [Google Scholar] [CrossRef]
- Leon, A.C. 3.12—Descriptive and Inferential Statistics. In Comprehensive Clinical Psychology; Bellack, A.S., Hersen, M., Eds.; Pergamon: Oxford, UK, 1998; pp. 243–285. [Google Scholar] [CrossRef]
- Hoffman, J.I.E. Chapter 33—Logistic Regression. In Basic Biostatistics for Medical and Biomedical Practitioners (Second Edition); Hoffman, J.I.E., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 581–589. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 1–8. [Google Scholar] [CrossRef]
- Ratner, B. Variable selection methods in regression: Ignorable problem, outing notable solution. J. Target. Meas. Anal. Mark. 2010, 18, 65–75. [Google Scholar] [CrossRef]
- Mundy, K.M.; Nichols, E.; Lindsey, J. Socioeconomic disparities in cataract prevalence, characteristics, and management. Semin. Ophthalmol. 2016, 31, 358–363. [Google Scholar] [CrossRef]
- Park, Y.-H.; Shin, J.A.; Han, K.; Yim, H.W.; Lee, W.-C.; Park, Y.-M. Gender difference in the association of metabolic syndrome and its components with age-related cataract: The Korea National Health and Nutrition Examination Survey 2008-2010. PLoS ONE 2014, 9, e85068. [Google Scholar] [CrossRef]
- Clayman, R.V. Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. J. Urol. 2005, 174, 600–601. [Google Scholar] [CrossRef]
- O’brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Mansfield, E.R.; Helms, B.P. Detecting multicollinearity. Am. Stat. 1982, 36, 158–160. [Google Scholar] [CrossRef]
- Senaviratna, N.; Cooray, T. Diagnosing multicollinearity of logistic regression model. Asian J. Probab. Stat. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Ruscio, J.; Roche, B. Determining the number of factors to retain in an exploratory factor analysis using comparison data of known factorial structure. Psychol. Assess. 2012, 24, 282. [Google Scholar] [CrossRef] [PubMed]
- Nugraheni, R.; Adnan, Z.A.; Nuhriawangsa, A.M.P. The correlation between dietary fats intake with total cholesterol and triglycerides levels in patients with coronary heart disease. AIP Conf. Proc. 2019, 2120, 080001. [Google Scholar] [CrossRef]
- Baltaci, D.; Kutlucan, A.; Turker, Y.; Yilmaz, A.; Karacam, S.; Deler, H.; Ucgun, T.; Kara, I.H. Association of vitamin B12 with obesity, overweight, insulin resistance and metabolic syndrome, and body fat composition; primary care-based study. Med. Glas (Zenica) 2013, 10, 203–210. [Google Scholar]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.-A.; Biesalski, H.K. β-Carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-I.; Lee, H.S.; Kim, B.H.; Jang, Y.A.; Suh, H.J. Change in nutritional status of the elderly population in Korea. J. Food Compos. Anal. 2004, 17, 449–457. [Google Scholar] [CrossRef]
- Manippa, V.; Padulo, C.; Van der Laan, L.N.; Brancucci, A. Gender differences in food choice: Effects of superior temporal sulcus stimulation. Front. Hum. Neurosci. 2017, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G.; Mitchell, P.; Smith, W. Diet and cataract: The blue mountains eye study. Ophthalmology 2000, 107, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Tarwadi, K.; Agte, V. Potential of commonly consumed green leafy vegetables for their antioxidant capacity and its linkage with the micronutrient profile. Int. J. Food Sci. Nutr. 2003, 54, 417–425. [Google Scholar] [CrossRef]
- Chang, D.; Rong, S.; Zhang, Y.; Sha, Q.; Liang, M.; Zhang, X.; Li, M.; Pan, H. Serum free fatty acids level in senile cataract. J. Am. Coll. Nutr. 2014, 33, 406–411. [Google Scholar] [CrossRef]
- Hatcher, H.; Andrews, J.S. Changes in lens fatty acid composition during galactose cataract formation. Investig. Ophthalmol. Vis. Sci. 1970, 9, 801–806. [Google Scholar]
- Hutton, J.; Schofield, P.; Williams, J.; Regtop, H.; Hollows, F. The effect of an unsaturated-fat diet on cataract formation in streptozotocin-induced diabetic rats. Br. J. Nutr. 1976, 36, 161–177. [Google Scholar] [CrossRef]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Stampfer, M.J.; Seddon, J.M.; Colditz, G.A.; Rosner, B.; Speizer, F.E.; Willett, W.C. Nutrient intake and cataract extraction in women: A prospective study. Br. Med. J. 1992, 305, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, J.; Moslen, M.T.; Haque, A.K.; McCleery, R.; Rassin, D.K. Total parenteral nutrition-associated alterations in hepatobiliary function and histology in rats: Is light exposure a clue? Pediatr. Res. 1993, 33, 487–492. [Google Scholar] [CrossRef]
- Chessex, P.; Lavoie, J.-C.; Rouleau, T.; Brochu, P.; St-Louis, P.; Lévy, E.; Alvarez, F. Photooxidation of parenteral multivitamins induces hepatic steatosis in a neonatal guinea pig model of intravenous nutrition. Pediatr. Res. 2002, 52, 958–963. [Google Scholar] [CrossRef]
- Kale, H.; Harikumar, P.; Kulkarni, S.; Nair, P.; Netrawali, M. Assessment of the genotoxic potential of riboflavin and lumiflavin: B. Effect of light. Mutat. Res./Genet. Toxicol. 1992, 298, 17–23. [Google Scholar] [CrossRef]
- Jernigan Jr, H.M. Role of hydrogen peroxide in riboflavin-sensitized photodynamic damage to cultured rat lenses. Exp. Eye Res. 1985, 41, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Eckhert, C.; Hsu, M.; Pang, N. Photoreceptor damage following exposure to excess riboflavin. Experientia 1993, 49, 1084–1087. [Google Scholar] [CrossRef]
- Majchrzak, D.; Fabian, E.; Elmadfa, I. Vitamin A content (retinol and retinyl esters) in livers of different animals. Food Chem. 2006, 98, 704–710. [Google Scholar] [CrossRef]
- Hägg, M.; Kumpulainen, J. Thiamine and riboflavin contents in finnish pig, heifer, and cow livers and in pork loin. J. Food Compos. Anal. 1994, 7, 301–306. [Google Scholar] [CrossRef]
- Aldrich, J. Correlations genuine and spurious in Pearson and Yule. Stat. Sci. 1995, 10, 364–376. [Google Scholar] [CrossRef]
| Variables | Unit | Cataract (n = 2137) | Non-Cataract (n = 3497) | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Age [n (%)] | |||||
| 60–69 | 218 (7.9) | 391 (14.2) | 977 (35.5) | 1167 (42.4) | |
| 70–79 | 546 (18.9) | 982 (34.1) | 685 (23.8) | 668 (23.2) | |
| Education [n (%)] | |||||
| Less than middle school | 312 (8.4) | 1121 (30.3) | 874 (23.6) | 1394 (37.7) | |
| Higher than high school | 427 (23.2) | 204 (11.1) | 774 (42.2) | 432 (23.5) | |
| National basic livelihood [n (%)] | |||||
| Non-beneficiaries | 74 (13.1) | 205 (36.3) | 120 (21.3) | 165 (29.3) | |
| Beneficiaries | 690 (13.7) | 1168 (23.0) | 1542 (30.4) | 1669 (32.9) | |
| Marital status [n (%)] | |||||
| Single | 8 (15.4) | 12 (23.1) | 19 (36.5) | 13 (25.0) | |
| Married | 756 (13.5) | 1361 (24.4) | 1643 (29.4) | 1822 (23.7) | |
| Obesity [n (%)] | |||||
| Absence | 357 (14.4) | 573 (23.0) | 777 (31.3) | 779 (31.3) | |
| Presence | 225 (13.4) | 404 (24.0) | 500 (29.8) | 552 (32.8) | |
| Hypertension [n (%)] | |||||
| Absence | 293 (12.6) | 432 (18.6) | 762 (32.8) | 837 (36.0) | |
| Presence | 469 (14.3) | 938 (28.4) | 898 (27.3) | 995 (29.0) | |
| Diabetes [n (%)] | |||||
| Absence | 325 (11.7) | 556 (20.1) | 893 (32.2) | 995 (36.0) | |
| Presence | 439 (15.3) | 817 (28.5) | 769 (26.8) | 840 (29.4) | |
| Asthma [n (%)] | |||||
| Absence | 704 (13.2) | 1252 (23.4) | 1626 (30.4) | 1760 (33.0) | |
| Presence | 41 (16.9) | 90 (37.2) | 36 (14.9) | 75 (31.0) | |
| Sinusitis [n (%)] | |||||
| Absence | 701 (13.2) | 1262 (23.8) | 1592 (30.0) | 1756 (33.0) | |
| Presence | 44 (16.2) | 79 (29.0) | 70 (25.7) | 79 (29.1) | |
| Allergic rhinitis [n (%)] | |||||
| Absence | 690 (13.4) | 1252 (24.1) | 1560 (30.0) | 1688 (32.5) | |
| Presence | 55 (14.0) | 89 (22.6) | 102 (26.0) | 147 (37.4) | |
| Hyperlipidemia [n (%)] | |||||
| Absence | 554 (14.8) | 805 (21.5) | 1267 (33.9) | 1109 (30.0) | |
| Presence | 209 (11.0) | 568 (30.0) | 395 (20.8) | 726 (38.2) | |
| Heart failure [n (%)] | |||||
| Absence | 735 (13.2) | 1331 (24.0) | 1652 (30.0) | 1828 (32.8) | |
| Presence | 10 (27.0) | 10 (27.0) | 10 (27.0) | 7 (19.0) | |
| Physical activity [n (%)] | |||||
| Absence | 486 (13.0) | 975 (26.1) | 1033 (27.7) | 1239 (33.2) | |
| Presence | 246 (13.7) | 352 (20.0) | 617 (34.0) | 581 (32.3) | |
| Smoke [n (%)] | |||||
| Non-smoker | 662 (13.1) | 1323 (26.2) | 1284 (25.4) | 1779 (35.3) | |
| Smoker | 137 (23.4) | 34 (5.8) | 367 (62.6) | 48 (8.2) | |
| Heavy drinking [n (%)] | |||||
| Non-heavy drinking | 707 (13.4) | 1344 (25.5) | 1429 (27.1) | 1797 (34.0) | |
| Heavy drinking | 50 (15.7) | 13 (4.1) | 225 (70.5) | 31 (9.7) | |
| Nutrient intake (Mean ± SD) | |||||
| Food intake | g | 1394.1 ± 29.4 | 1105.0 ± 19.5 | 1576.9 ± 25.1 | 1261.8 ± 19.9 |
| Energy | kcal | 1892.8 ± 28.7 | 1421.0 ± 18.9 | 2026.6 ± 24.0 | 1558.1 ± 18.9 |
| Water | g | 874.3 ± 22.1 | 722.9 ± 16.1 | 1003.3 ± 20.1 | 847.1 ± 16.6 |
| Protein | g | 63.7 ± 1.1 | 46.5 ± 0.7 | 69.3 ± 1.0 | 52.3 ± 0.8 |
| Fat | g | 31.6 ± 1.0 | 23.0 ± 0.6 | 33.9 ± 0.8 | 26.9 ± 0.6 |
| Saturated fatty acids | g | 9.7 ± 0.3 | 7.0 ± 0.2 | 10.4 ± 0.2 | 8.2 ± 0.2 |
| Monounsaturated fatty acids | g | 9.4 ± 0.4 | 6.9 ± 0.2 | 10.4 ± 0.3 | 8.2 ± 0.2 |
| Polyunsaturated fatty acids | g | 9.4 ± 0.3 | 7.0 ± 0.2 | 9.9 ± 0.2 | 8.0 ± 0.2 |
| Omega-3 fatty acid | g | 1.9 ± 0.1 | 1.4 ± 0.06 | 1.9 ± 0.07 | 1.6 ± 0.05 |
| Omega-6 fatty acid | g | 7.5 ± 0.3 | 5.5 ± 0.2 | 7.9 ± 0.2 | 6.5 ± 0.2 |
| Cholesterol | mg | 162.3 ± 6.1 | 118.5 ± 4.3 | 183.3 ± 5.7 | 138.8 ± 4.5 |
| Carbohydrates | g | 315.2 ± 4.8 | 254.0 ± 3.5 | 329.5 ± 3.9 | 272.9 ± 3.4 |
| Dietary fiber | g | 27.6 ± 0.7 | 23.0 ± 0.5 | 29.4 ± 0.5 | 25.7 ± 0.4 |
| Sugar | g | 51.9 ± 1.6 | 45.2 ± 1.2 | 56.7 ± 1.2 | 52.7 ± 1.1 |
| Calcium | mg | 493.8 ± 13.0 | 400.9 ± 9.8 | 541.6 ± 10.3 | 428.9 ± 7.9 |
| Phosphorus | mg | 1026.4 ± 17.9 | 778.8 ± 12.6 | 1103.6 ± 15.5 | 860.9 ± 12.2 |
| Iron | mg | 12.4 ± 0.3 | 9.7 ± 0.2 | 13.3 ± 0.2 | 10.6 ± 0.2 |
| Sodium | mg | 3310.7 ± 76.2 | 2314.8 ± 46.8 | 3549.6 ± 63.6 | 2533.6 ± 50.1 |
| Potassium | mg | 2842.4 ± 53.0 | 2284.2 ± 43.0 | 3074.1 ± 46.6 | 2583.1 ± 41.4 |
| Vitamin A | μg | 319.2 ± 12.1 | 261.1 ± 8.3 | 371.2 ± 17.6 | 299.7 ± 8.6 |
| Carotene | μg | 2809.1 ± 115.2 | 2248.3 ± 81.2 | 3151.1 ± 94.8 | 2630.7 ± 79.5 |
| Vitamin B1 | mg | 1.3 ± 0.02 | 1.0 ± 0.02 | 1.4 ± 0.02 | 1.1 ± 0.01 |
| Vitamin B2 | mg | 1.3 ± 0.03 | 1.0 ± 0.02 | 1.5 ± 0.03 | 1.2 ± 0.02 |
| Vitamin B3 | mg | 11.9 ± 0.3 | 8.7 ± 0.2 | 13.2 ± 0.2 | 10.0 ± 0.2 |
| Vitamin B9 | mg | 372.7 ± 7.3 | 271.6 ± 5.5 | 372.7 ± 6.0 | 300.4 ± 4.6 |
| Vitamin C | mg | 55.7 ± 2.5 | 53.1 ± 2.3 | 63.7 ± 2.6 | 58.7 ± 1.8 |
| Nutrition | Unadjusted Model | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||
| Water | Low–mid | 1.130 (0.839–1.523) | 0.966 (0.677–1.379) | 1.182 (0.789–1.770) | 1.230 (0.800–1.892) |
| High–mid | 0.726 (0.548–0.962) * | 0.740 (0.517–1.061) | 0.681 (0.435–1.067) | 0.669 (0.429–1.045) | |
| Protein | Low–mid | 0.930 (0.697–1.239) | 0.975 (0.713–1.332) | 1.199 (0.830–1.732) | 1.148 (0.790–1.669) |
| High–mid | 0.959 (0.662–1.390) | 1.083 (0.724–1.622) | 0.979 (0.607–1.581) | 0.934 (0.576–1.515) | |
| Monounsaturated fatty acids | Low–mid | 1.073 (0.833–1.382) | 1.025 (0.778–1.350) | 0.920 (0.657–1.289) | 0.935 (0.668–1.308) |
| High–mid | 1.022 (0.663–1.576) | 1.146 (0.703–1.868) | 1.029 (0.594–1.783) | 1.075 (0.622–1.858) | |
| Dietary fiber | Low–mid | 0.839 (0.591–1.191) | 0.906 (0.614–1.335) | 0.854 (0.547–1.335) | 0.867 (0.548–1.373) |
| High–mid | 0.996 (0.759–1.306) | 1.023 (0.769–1.360) | 1.114 (0.791–1.569) | 1.063 (0.755–1.498) | |
| Calcium | Low–mid | 1.147 (0.848–1.551) | 0.962 (0.696–1.328) | 0.889 (0.609–1.300) | 0.891 (0.607–1.308) |
| High–mid | 1.004 (0.749–1.348) | 1.009 (0.753–1.353) | 1.134 (0.803–1.602) | 1.185 (0.838–1.676) | |
| Potassium | Low–mid | 0.776 (0.552–1.091) | 0.795 (0.552–1.143) | 0.954 (0.625–1.459) | 0.977 (0.636–1.502) |
| High–mid | 1.095 (0.819–1.463) | 1.087 (0.798–1.479) | 1.151 (0.784–1.690) | 1.182 (0.803–1.740) | |
| Vitamin B1 | Low–mid | 1.083 (0.812–1.444) | 1.142 (0.832–1.567) | 1.115 (0.769–1.618) | 1.100 (0.755–1.603) |
| High–mid | 0.762 (0.561–1.035) | 0.763 (0.551–1.057) | 0.685 (0.478–0.981) * | 0.673 (0.468–0.968) * | |
| Vitamin B2 | Low–mid | 0.989 (0.735–1.330) | 0.967 (0.698–1.342) | 1.152 (0.784–1.693) | 1.152 (0.788–1.685) |
| High–mid | 1.010 (0.742–1.374) | 0.998 (0.733–1.358) | 1.046 (0.728–1.504) | 1.010 (0.697–1.461) | |
| Vitamin B3 | Low–mid | 1.202 (0.899–1.606) | 1.103 (0.814–1.495) | 0.873 (0.616–1.237) | 0.860 (0.602–1.227) |
| High–mid | 0.871 (0.636–1.191) | 0.914 (0.645–1.294) | 0.924 (0.623–1.370) | 0.913 (0.613–1.358) |
| Nutrition | Unadjusted Model | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||
| Water | Low–mid | 1.034 (0.798–1.341) | 0.928 (0.668–1.290) | 0.839 (0.560–1.257) | 0.809 (0.537–1.219) |
| High–mid | 1.009 (0.774–1.316) | 1.174 (0.780–1.766) | 1.027 (0.628–1.681) | 1.032 (0.632–1.685) | |
| Protein | Low–mid | 1.049 (0.782–1.408) | 1.086 (0.780–1.513) | 1.004 (0.688–1.463) | 0.992 (0.678–1.453) |
| High–mid | 0.724 (0.512–1.024) | 0.755 (0.527–1.080) | 0.707 (0.459–1.088) | 0.721 (0.467–1.113) | |
| Fat | Low–mid | 1.058 (0.718–1.560) | 0.997 (0.660–1.504) | 1.090 (0.654–1.817) | 1.112 (0.670–1.846) |
| High–mid | 0.780 (0.448–1.360) | 0.652 (0.355–1.196) | 0.632 (0.311–1.282) | 0.629 (0.310–1.274) | |
| Saturated fatty acids | Low–mid | 0.958 (0.726–1.264) | 0.902 (0.658–1.237) | 0.925 (0.634–1.349) | 0.880 (0.607–1.275) |
| High–mid | 0.970 (0.630–1.493) | 1.024 (0.643–1.630) | 0.840 (0.463–1.523) | 0.857 (0.471–1.557) | |
| Monounsaturated fatty acids | Low–mid | 0.802 (0.566–1.137) | 0.774 (0.537–1.116) | 0.739 (0.490–1.115) | 0.745 (0.493–1.126) |
| High–mid | 0.886 (0.550–1.428) | 0.943 (0.554–1.605) | 0.991 (0.521–1.886) | 1.002 (0.528–1.900) | |
| Polyunsaturated fatty acids | Low–mid | 1.588 (1.113–2.266) * | 1.555 (1.029–2.349) * | 2.026 (1.227–3.346) * | 2.063 (1.256–3.388) * |
| High–mid | 1.343 (0.795–2.266) | 1.192 (0.668–2.129) | 1.047 (0.517–2.120) | 1.064 (0.522–2.169) | |
| Omega-3 fatty acid | Low–mid | 0.918 (0.714–1.181) | 0.967 (0.730–1.280) | 0.919 (0.669–1.263) | 0.904 (0.656–1.246) |
| High–mid | 1.099 (0.849–1.422) | 1.099 (0.835–1.446) | 1.259 (0.909–1.743) | 1.244 (0.894–1.732) | |
| Omega-6 fatty acid | Low–mid | 0.753 (0.529–1.072) | 0.779 (0.538–1.127) | 0.677 (0.437–1.049) | 0.657 (0.423–1.021) |
| High–mid | 0.659 (0.401–1.082) | 0.794 (0.453–1.390) | 0.845 (0.437–1.635) | 0.837 (0.432–1.624) | |
| Cholesterol | Low–mid | 1.095 (0.873–1.374) | 0.944 (0.741–1.203) | 1.066 (0.799–1.422) | 1.051 (0.786–1.405) |
| High–mid | 1.135 (0.845–1.525) | 1.118 (0.822–1.520) | 1.082 (0.740–1.582) | 1.053 (0.716–1.550) | |
| Carbohydrates | Low–mid | 1.035 (0.765–1.400) | 1.163 (0.822–1.646) | 1.211 (0.798–1.839) | 1.108 (0.726–1.692) |
| High–mid | 0.929 (0.730–1.182) | 0.905 (0.681–1.203) | 0.897 (0.644–1.248) | 0.927 (0.662–1.296) | |
| Dietary fiber | Low–mid | 1.102 (0.815–1.491) | 0.990 (0.716–1.369) | 0.936 (0.623–1.405) | 1.012 (0.669–1.529) |
| High–mid | 0.901 (0.696–1.168) | 0.875 (0.664–1.152) | 0.960 (0.694–1.327) | 0.956 (0.692–1.323) | |
| Sugar | Low–mid | 1.260 (1.006–1.578) * | 1.221 (0.965–1.546) | 1.272 (0.961–1.684) | 1.318 (0.995–1.747) |
| High–mid | 0.941 (0.722–1.227) | 1.008 (0.762–1.334) | 0.987 (0.711–1.371) | 0.990 (0.714–1.373) | |
| Calcium | Low–mid | 0.829 (0.636–1.081) | 0.800 (0.599–1.068) | 0.941 (0.664–1.335) | 0.965 (0.681–1.368) |
| High–mid | 0.931 (0.722–1.199) | 0.949 (0.717–1.255) | 0.928 (0.670–1.286) | 0.938 (0.679–1.296) | |
| Phosphorus | Low–mid | 0.824 (0.596–1.138) | 0.787 (0.546–1.135) | 0.815 (0.527–1.260) | 0.822 (0.527–1.283) |
| High–mid | 1.184 (0.842–1.665) | 1.193 (0.829–1.716) | 1.201 (0.797–1.809) | 1.173 (0.774–1.779) | |
| Iron | Low–mid | 0.907 (0.672–1.225) | 0.946 (0.678–1.320) | 0.898 (0.625–1.290) | 0.874 (0.605–1.262) |
| High–mid | 1.206 (0.931–1.563) | 1.291 (0.973–1.712) | 1.346 (0.979–1.849) | 1.351 (0.977–1.867) | |
| Potassium | Low–mid | 1.118 (0.796–1.571) | 1.105 (0.756–1.615) | 1.153 (0.732–1.816) | 1.151 (0.726–1.826) |
| High–mid | 0.935 (0.717–1.219) | 0.983 (0.741–1.304) | 0.999 (0.711–1.403) | 1.002 (0.713–1.407) | |
| Vitamin A | Low–mid | 1.333 (1.010–1.760) * | 1.504 (1.109–2.039) * | 1.416 (1.007–1.992) * | 1.430 (1.015–2.014) * |
| High–mid | 1.173 (0.876–1.571) | 1.073 (0.796–1.448) | 1.074 (0.741–1.557) | 1.047 (0.719–1.524) | |
| Carotene | Low–mid | 1.052 (0.833–1.330) | 0.957 (0.738–1.240) | 1.003 (0.726–1.387) | 1.002 (0.723–1.390) |
| High–mid | 0.929 (0.710–1.217) | 1.034 (0.785–1.363) | 0.982 (0.718–1.343) | 0.986 (0.720–1.350) | |
| Vitamin B1 | Low–mid | 0.961 (0.719–1.286) | 1.098 (0.807–1.496) | 0.929 (0.640–1.348) | 0.976 (0.672–1.419) |
| High–mid | 1.164 (0.908–1.491) | 1.076 (0.819–1.413) | 1.122 (0.809–1.555) | 1.126 (0.809–1.568) | |
| Vitamin B2 | Low–mid | 1.254 (0.935–1.680) | 1.144 (0.846–1.547) | 1.022 (0.726–1.438) | 1.011 (0.718–1.425) |
| High–mid | 1.199 (0.911–1.580) | 1.364 (1.024–1.815) * | 1.626 (1.149–2.302) * | 1.639 (1.160–2.317) * | |
| Vitamin B3 | Low–mid | 1.382 (1.085–1.761) * | 1.234 (0.964–1.579) | 1.048 (0.786–1.399) | 1.075 (0.805–1.435) |
| High–mid | 0.898 (0.673–1.196) | 1.047 (0.754–1.452) | 0.889 (0.606–1.306) | 0.850 (0.577–1.252) | |
| Vitamin B9 | Low–mid | 0.916 (0.666–1.261) | 0.806 (0.574–1.130) | 0.902 (0.620–1.314) | 0.890 (0.609–1.299) |
| High–mid | 1.009 (0.776–1.313) | 1.026 (0.774–1.359) | 0.977 (0.698–1.368) | 0.983 (0.701–1.378) | |
| Vitamin C | Low–mid | 0.996 (0.773–1.284) | 1.039 (0.793–1.362) | 0.996 (0.716–1.386) | 0.995 (0.713–1.387) |
| High–mid | 1.119 (0.892–1.403) | 1.159 (0.910–1.476) | 1.078 (0.806–1.441) | 1.084 (0.809–1.453) |
| Rotated Factor Pattern | |||
|---|---|---|---|
| Variables | Factor 1 | Factor 2 | Factor 3 |
| Fat | 0.95034 | 0.15504 | 0.05081 |
| Monounsaturated fatty acids | 0.91289 | 0.09431 | 0.05992 |
| Saturated fatty acids | 0.86574 | 0.09477 | 0.01638 |
| Omega-6 fatty acid | 0.80332 | 0.26433 | 0.03012 |
| Polyunsaturated fatty acids | 0.79808 | 0.28216 | 0.07921 |
| Protein | 0.74213 | 0.46094 | 0.19707 |
| Cholesterol | 0.68389 | 0.14459 | 0.28426 |
| Vitamin B2 | 0.65169 | 0.44709 | 0.33082 |
| Phosphorus | 0.64615 | 0.61353 | 0.25723 |
| Vitamin B3 | 0.60773 | 0.45240 | 0.25044 |
| Vitamin B1 | 0.52272 | 0.46360 | 0.30187 |
| Omega-3 fatty acid | 0.47650 | 0.25455 | 0.25092 |
| Dietary fiber | 0.09142 | 0.87826 | 0.11848 |
| Potassium | 0.35470 | 0.80338 | 0.26611 |
| Carbohydrates | 0.27792 | 0.79487 | −0.02306 |
| Vitamin B9 | 0.20651 | 0.75798 | 0.34327 |
| Water | 0.25510 | 0.70240 | 0.19341 |
| Sugar | 0.27692 | 0.62612 | −0.07567 |
| Iron | 0.36783 | 0.60880 | 0.27743 |
| Sodium | 0.45774 | 0.51476 | 0.16612 |
| Calcium | 0.33192 | 0.51375 | 0.34266 |
| Vitamin C | −0.01679 | 0.49206 | 0.13408 |
| Vitamin A | 0.27720 | 0.13055 | 0.84083 |
| Carotene | 0.02572 | 0.28761 | 0.77332 |
| Groups | Variables | Unadjusted Model | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||
| Male Group | Factor 1 | 0.650 (0.302–1.397) | 3.945 (1.560–9.975) ** | 3.367 (1.248–9.088) * | 3.169 (1.175–8.551) * |
| Factor 2 | 0.490 (0.294–0.816) ** | 3.470 (1.740–6.917) ** | 2.783 (1.275–6.074) * | 2.360 (1.038–5.369) * | |
| Factor 3 | 0.661 (0.379~1.152) | 0.750 (0.410–1.371) | 0.700 (0.373–1.312) | 0.692 (0.372–1.288) | |
| Female Group | Factor 1 | 0.278 (0.125–0.618) ** | 4.096 (1.733–9.684) ** | 2.395 (0.851–6.735) | 2.456 (0.871–6.921) |
| Factor 2 | 0.371 (0.228–0.604) ** | 6.874 (3.402–13.889) ** | 5.278 (2.400–11.607) ** | 5.074 (1.050–3.519) ** | |
| Factor 3 | 0.765 (0.435–1.343) | 1.337 (0.766–2.332) | 1.347 (0.703–2.580) | 1.281 (0.667–2.460) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, S.; Jeong, M.; Jung, S.; Lee, M.; Yoo, S. The Relationship between Nutrient Intake and Cataracts in the Older Adult Population of Korea. Nutrients 2022, 14, 4962. https://doi.org/10.3390/nu14234962
Lee S, Lee S, Jeong M, Jung S, Lee M, Yoo S. The Relationship between Nutrient Intake and Cataracts in the Older Adult Population of Korea. Nutrients. 2022; 14(23):4962. https://doi.org/10.3390/nu14234962
Chicago/Turabian StyleLee, Sangyun, Soyeon Lee, Myeonghyeon Jeong, Sunwoo Jung, Myoungjin Lee, and Sunyong Yoo. 2022. "The Relationship between Nutrient Intake and Cataracts in the Older Adult Population of Korea" Nutrients 14, no. 23: 4962. https://doi.org/10.3390/nu14234962
APA StyleLee, S., Lee, S., Jeong, M., Jung, S., Lee, M., & Yoo, S. (2022). The Relationship between Nutrient Intake and Cataracts in the Older Adult Population of Korea. Nutrients, 14(23), 4962. https://doi.org/10.3390/nu14234962





