Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Prostate Tissues
2.2. Organ Bath
2.3. Cell Culture
2.4. Proliferation Assay
2.5. Cell Apoptosis and Cell Death Analysis
2.6. Viability Assay
2.7. Drugs and Nomenclature
2.8. Statistical Analyses
3. Results
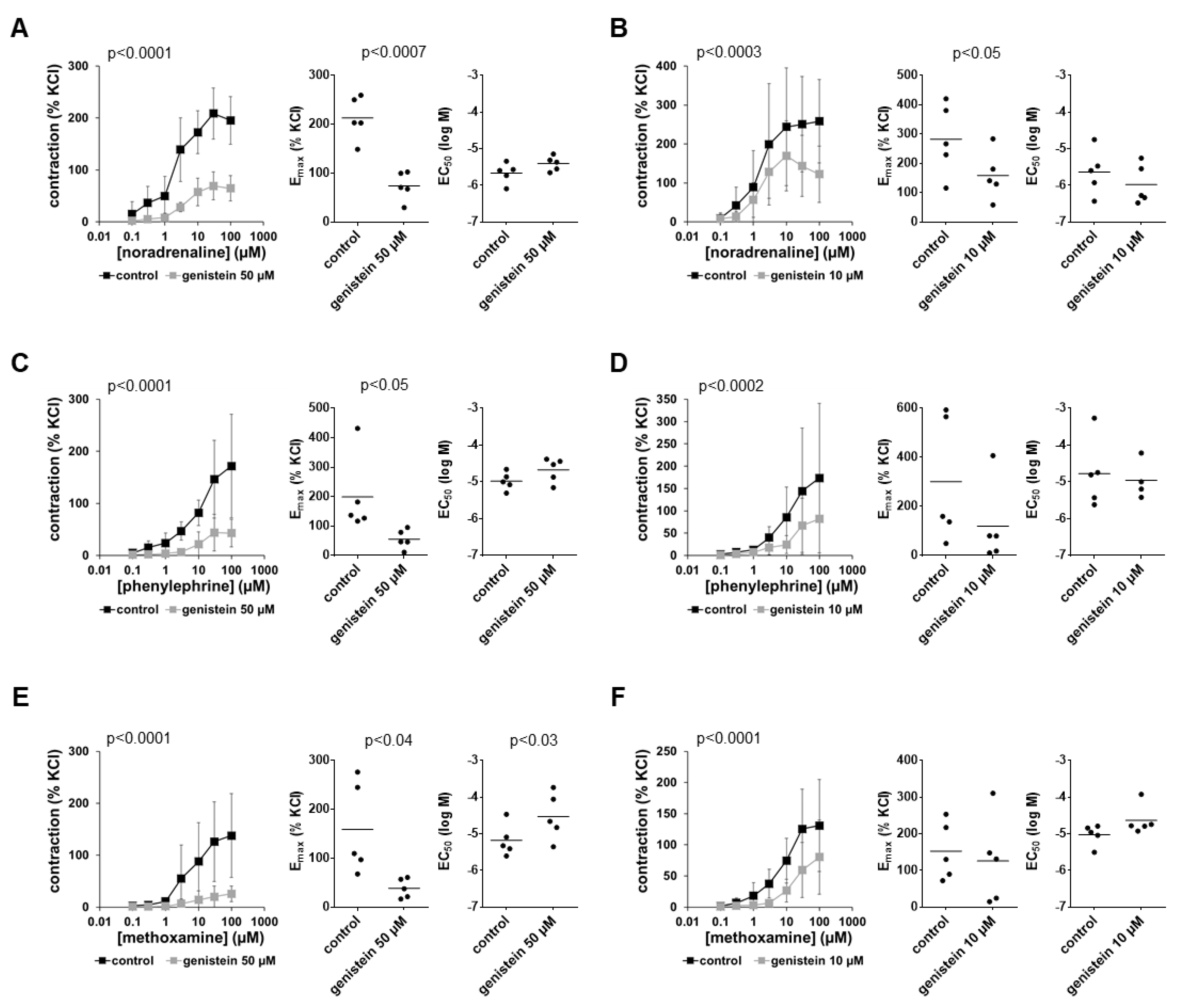
3.1. Effects of Genistein on α1-Adrenergic Contractions
3.2. Effects of Genistein on EFS-Induced Contractions
3.3. Effects of Genistein on Non-Adrenergic Contractions
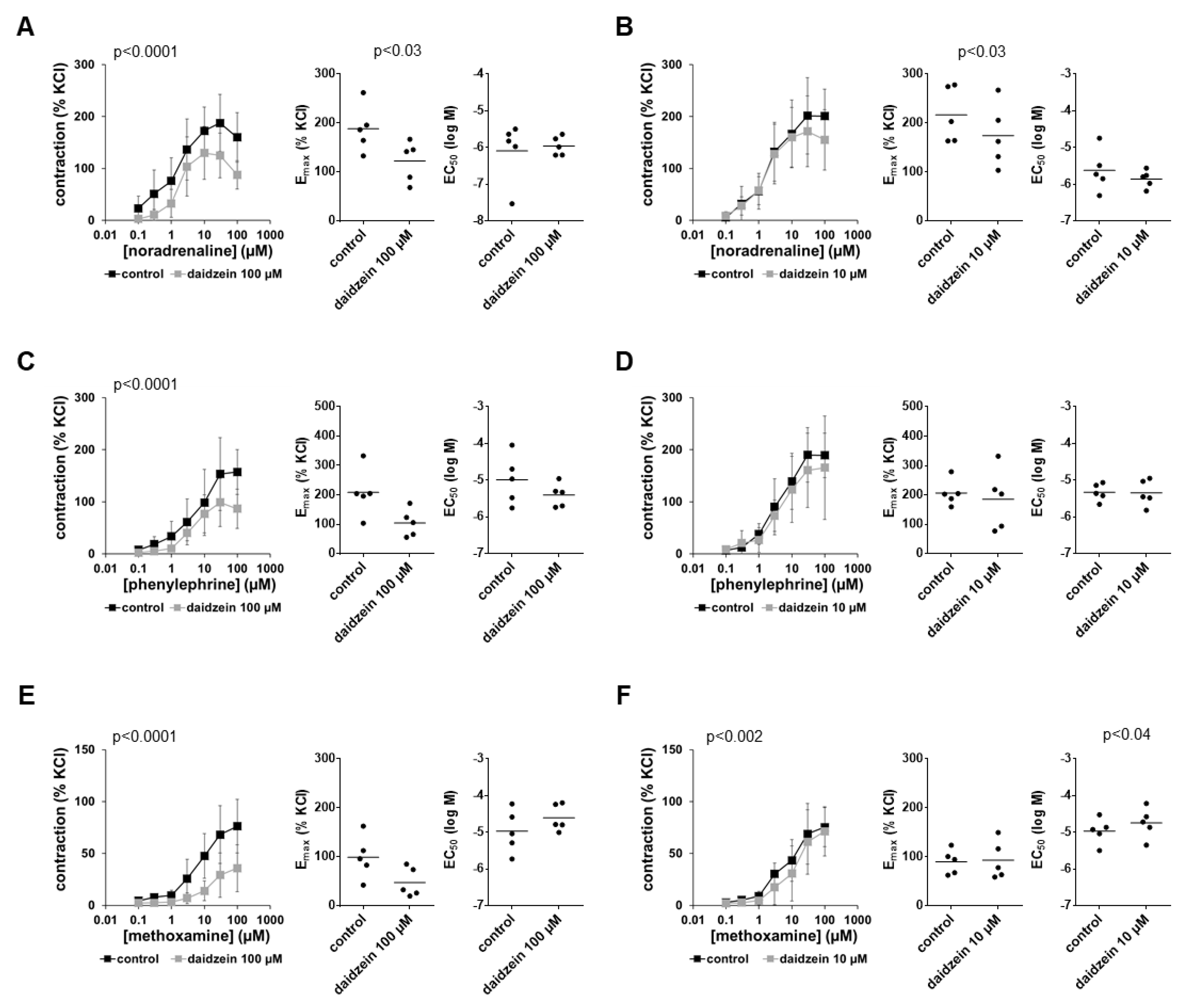
3.4. Effects of Daidzein on α1-Adrenergic Contractions
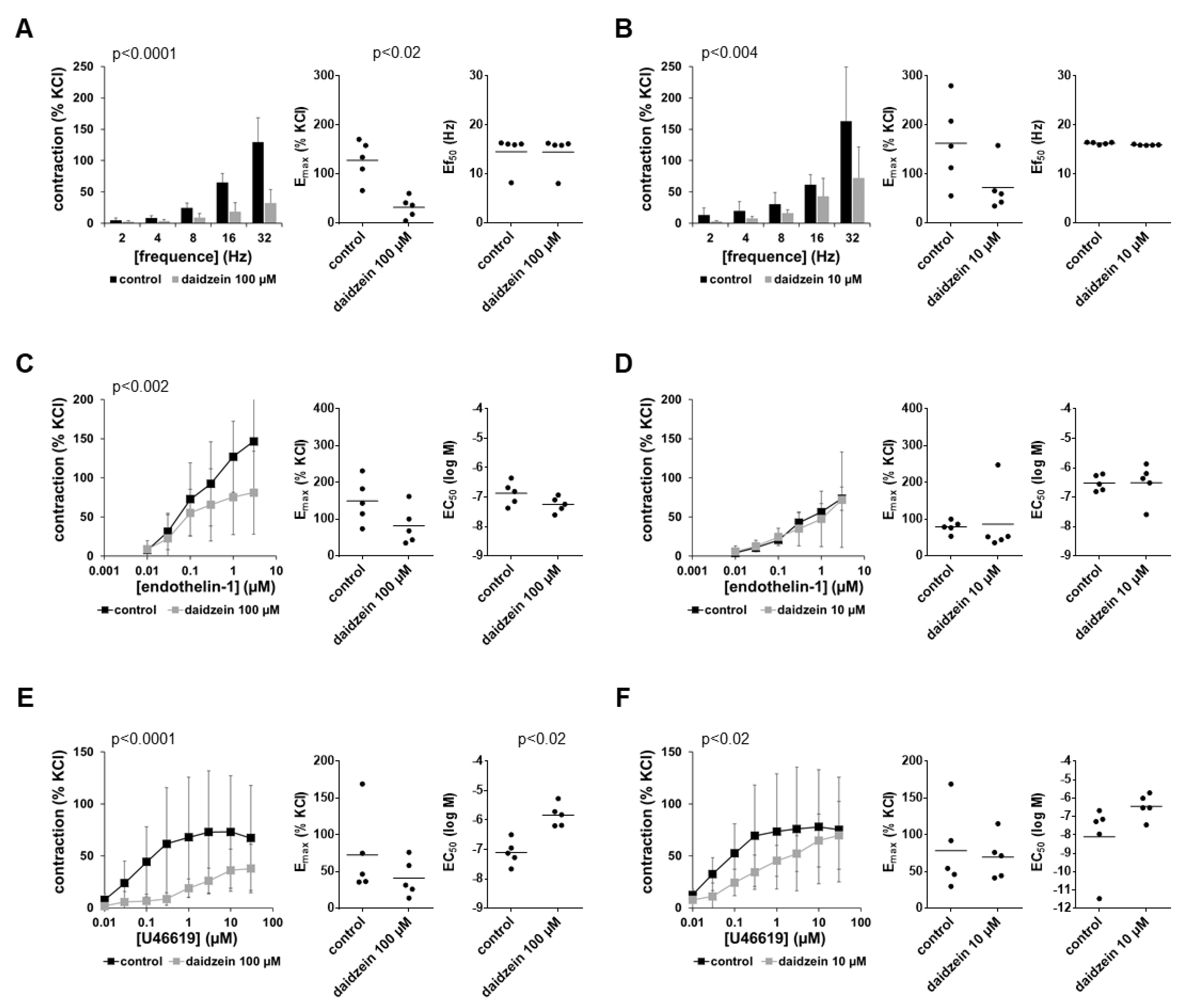
3.5. Effects of Daidzein on EFS-Induced Contractions
3.6. Effects of Daidzein on Non-Adrenergic Contractions
3.7. Effects of Combined Genistein and Daidzein on Contractions
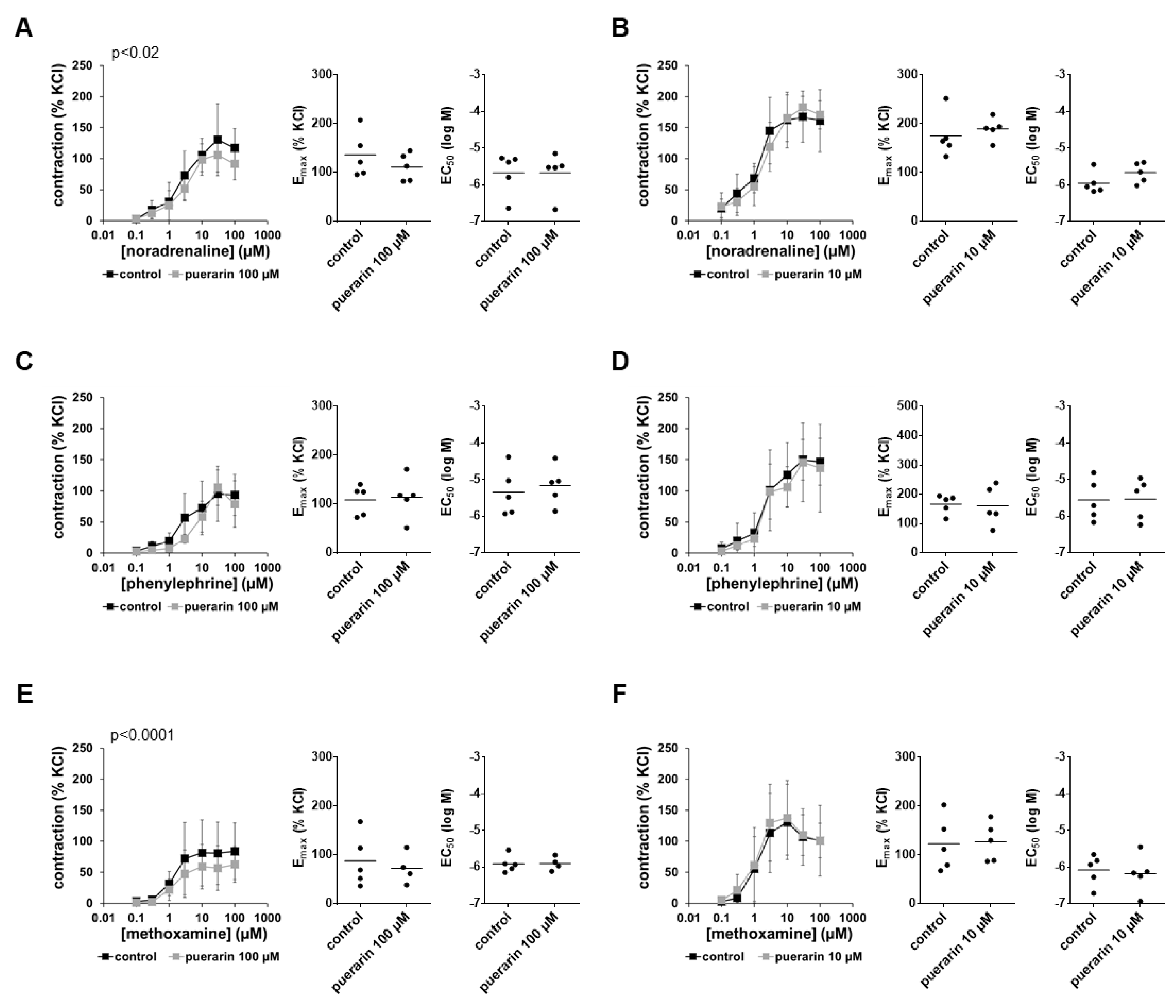
3.8. Effects of Puerarin on α1-Adrenergic Contractions
3.9. Effects of Puerarin on EFS-Induced Contractions
3.10. Effects of Puerarin on Non-Adrenergic Contractions
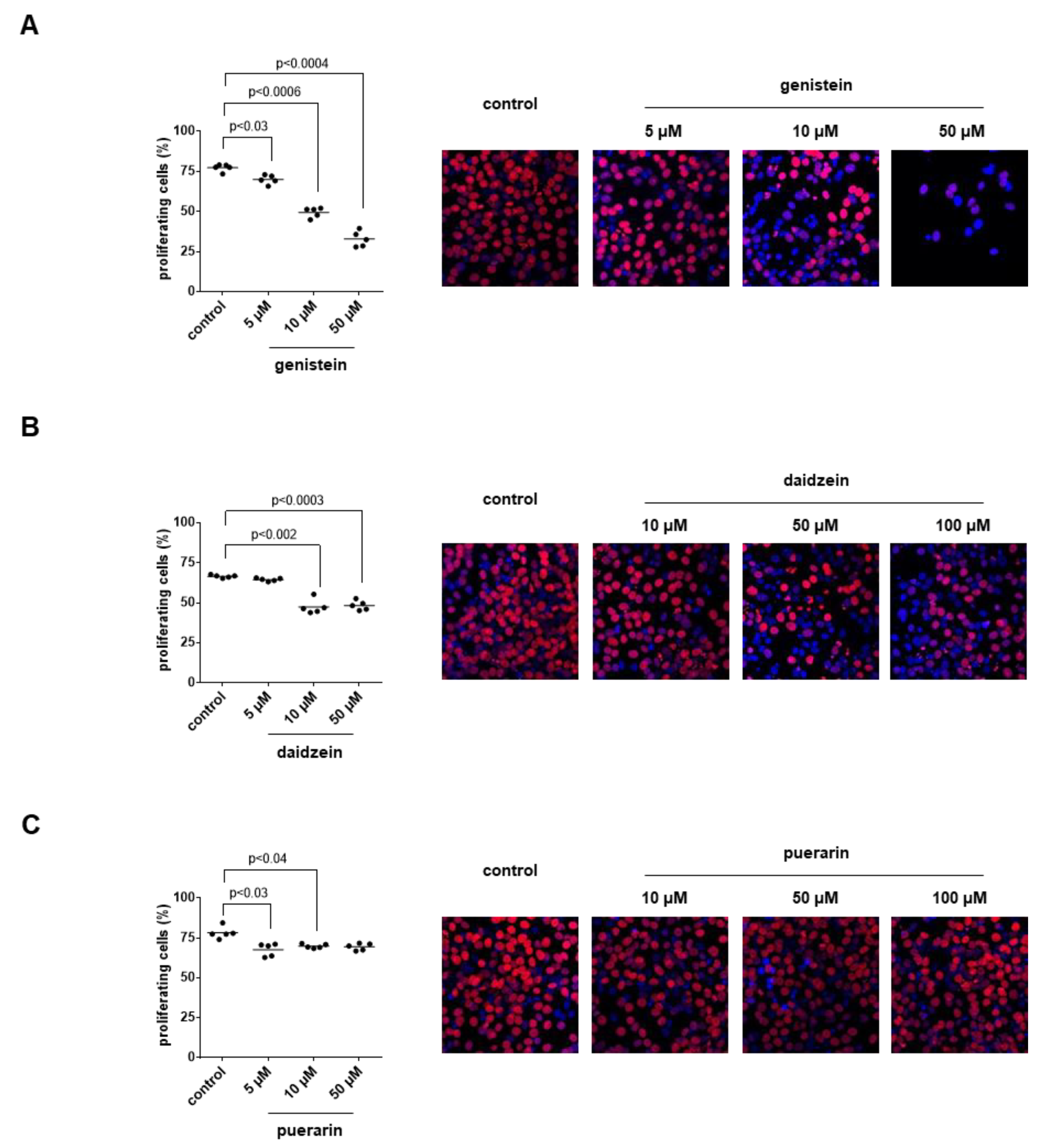
3.11. Effects of Genistein, Daidzein and Puerarin on the Proliferation of Stromal Cells
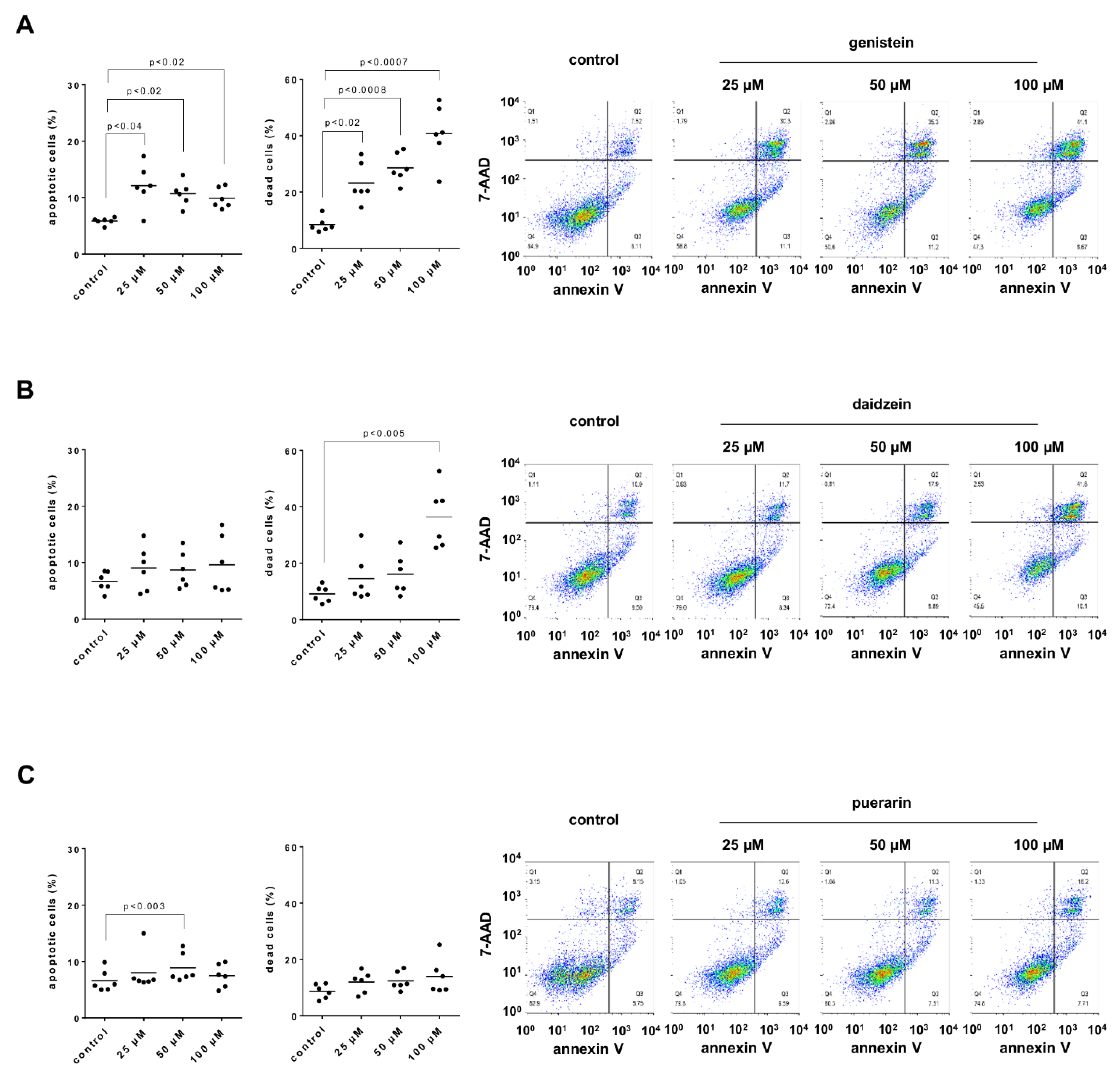
3.12. Effects of Genistein, Daidzein and Puerarin on Apoptosis and Cell Death of Stromal Cells
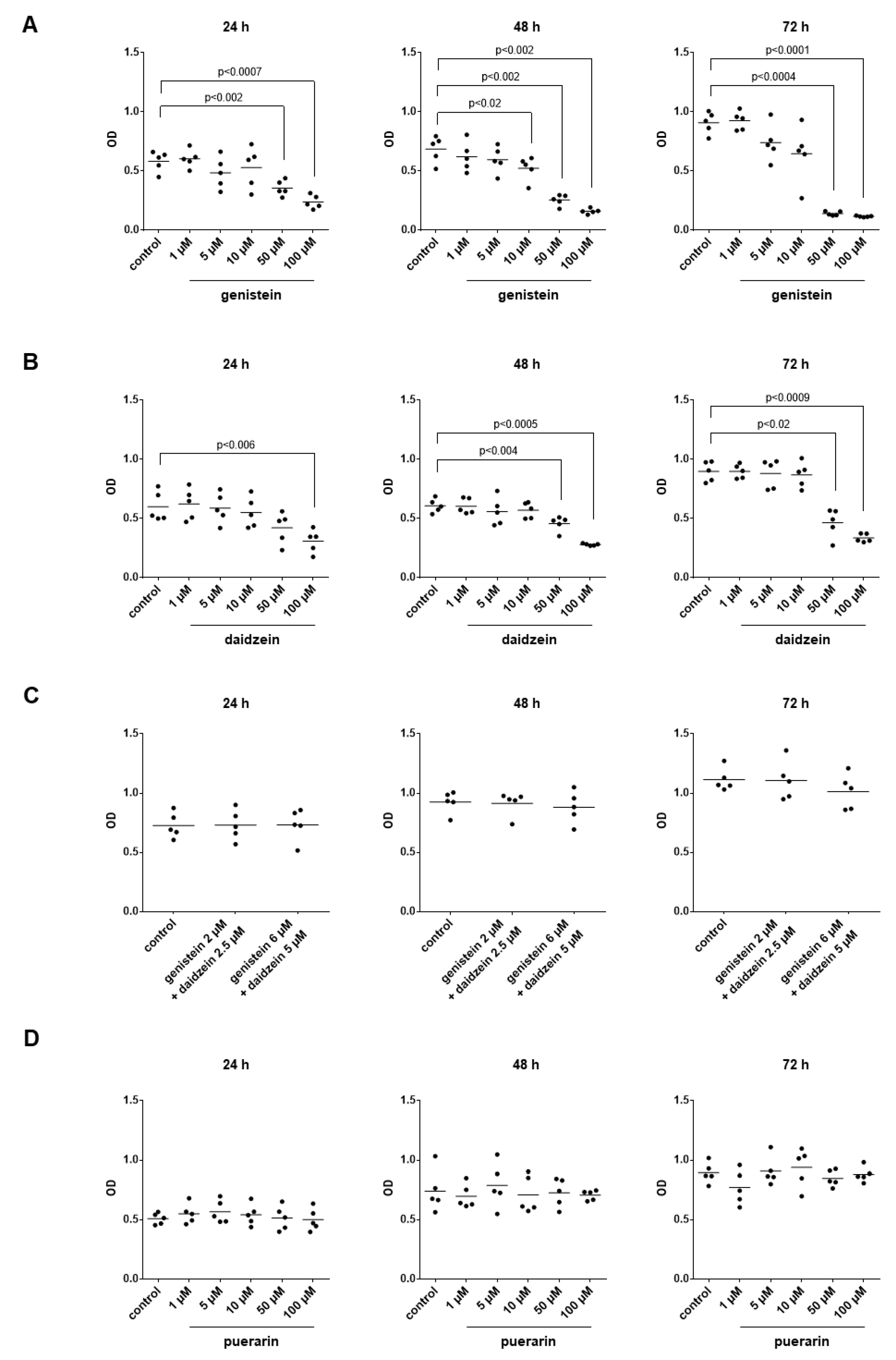
3.13. Effects of Genistein, Daidzein and Puerarin on the Viability of Stromal Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burdette, C.Q.; Marcus, R.K. Determination of isoflavone content in soy, red clover, and kudzu dietary supplement materials by liquid chromatography-particle beam/electron ionization mass spectrometry. J. AOAC Int. 2013, 96, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef]
- Silva, H. The Vascular Effects of Isolated Isoflavones-A Focus on the Determinants of Blood Pressure Regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, P.; Perry, J.; Rader, J.I. Determination of isoflavones in dietary supplements containing soy, Red Clover and kudzu: Extraction followed by basic or acid hydrolysis. J. Chromatogr. A 2006, 1107, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mamagkaki, A.; Bouris, I.; Parsonidis, P.; Vlachou, I.; Gougousi, M.; Papasotiriou, I. Genistein as a dietary supplement; formulation, analysis and pharmacokinetics study. PLoS ONE 2021, 16, e0250599. [Google Scholar] [CrossRef]
- Abdi, F.; Rahnemaei, F.A.; Roozbeh, N.; Pakzad, R. Impact of phytoestrogens on treatment of urogenital menopause symptoms: A systematic review of randomized clinical trials. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 261, 222–235. [Google Scholar] [CrossRef]
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Z.; Ye, D.; Ma, B.L. Constituents, Pharmacokinetics, and Pharmacology of Gegen-Qinlian Decoction. Front. Pharm. 2021, 12, 668418. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef]
- Zhang, Z.; Lam, T.N.; Zuo, Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharm. 2013, 53, 787–811. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; de Klerk, N.H.; Mackerras, D.; Leavy, J.; Fritschi, L. Dietary patterns and surgically treated benign prostatic hyperplasia: A case control study in Western Australia. BJU Int. 2008, 101, 853–860. [Google Scholar] [CrossRef]
- Denis, L.; Morton, M.S.; Griffiths, K. Diet and its preventive role in prostatic disease. Eur. Urol. 1999, 35, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Lau, W.W.; Leung, P.C.; Leung, J.C.; Woo, J. The association between isoflavone and lower urinary tract symptoms in elderly men. Br. J. Nutr. 2007, 98, 1237–1242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennetau-Pelissero, C. Risks and benefits of phytoestrogens: Where are we now? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 477–483. [Google Scholar] [CrossRef]
- Lepor, H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev. Urol. 2004, 6 (Suppl. S9), S3–S10. [Google Scholar] [PubMed]
- Strand, D.W.; Costa, D.N.; Francis, F.; Ricke, W.A.; Roehrborn, C.G. Targeting phenotypic heterogeneity in benign prostatic hyperplasia. Differentiation 2017, 96, 49–61. [Google Scholar] [CrossRef]
- Oelke, M.; Bachmann, A.; Descazeaud, A.; Emberton, M.; Gravas, S.; Michel, M.C.; N’Dow, J.; Nordling, J.; de la Rosette, J.J.; European Association of Urology. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 2013, 64, 118–140. [Google Scholar] [CrossRef]
- Cindolo, L.; Pirozzi, L.; Fanizza, C.; Romero, M.; Tubaro, A.; Autorino, R.; De Nunzio, C.; Schips, L. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: Population-based cohort study. Eur. Urol. 2015, 68, 418–425. [Google Scholar] [CrossRef]
- Cindolo, L.; Pirozzi, L.; Sountoulides, P.; Fanizza, C.; Romero, M.; Castellan, P.; Antonelli, A.; Simeone, C.; Tubaro, A.; de Nunzio, C.; et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: Is combination therapy better than monotherapy? BMC Urol. 2015, 15, 96. [Google Scholar] [CrossRef]
- Hennenberg, M.; Acevedo, A.; Wiemer, N.; Kan, A.; Tamalunas, A.; Wang, Y.; Yu, Q.; Rutz, B.; Ciotkowska, A.; Herlemann, A.; et al. Non-Adrenergic, Tamsulosin-Insensitive Smooth Muscle Contraction is Sufficient to Replace alpha1 -Adrenergic Tension in the Human Prostate. Prostate 2017, 77, 697–707. [Google Scholar] [CrossRef]
- Hennenberg, M.; Stief, C.G.; Gratzke, C. Prostatic alpha1-adrenoceptors: New concepts of function, regulation, and intracellular signaling. Neurourol. Urodyn. 2014, 33, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Pradidarcheep, W.; Wallner, C.; Dabhoiwala, N.F.; Lamers, W.H. Anatomy and histology of the lower urinary tract. Handb. Exp. Pharm. 2011, 117–148. [Google Scholar] [CrossRef]
- Shaikhibrahim, Z.; Lindstrot, A.; Ellinger, J.; Rogenhofer, S.; Buettner, R.; Perner, S.; Wernert, N. The peripheral zone of the prostate is more prone to tumor development than the transitional zone: Is the ETS family the key? Mol. Med. Rep. 2012, 5, 313–316. [Google Scholar] [CrossRef]
- Alcaraz, A.; Hammerer, P.; Tubaro, A.; Schroder, F.H.; Castro, R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur. Urol. 2009, 55, 864–873. [Google Scholar] [CrossRef]
- Orsted, D.D.; Bojesen, S.E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 2013, 10, 49–54. [Google Scholar] [CrossRef]
- Grabbert, M.; Buchner, A.; Butler-Ransohoff, C.; Kretschmer, A.; Stief, C.G.; Bauer, R.M. Long-term functional outcome analysis in a large cohort of patients after radical prostatectomy. Neurourol. Urodyn. 2018, 37, 2263–2270. [Google Scholar] [CrossRef]
- Wang, Y.; Kunit, T.; Ciotkowska, A.; Rutz, B.; Schreiber, A.; Strittmatter, F.; Waidelich, R.; Liu, C.; Stief, C.G.; Gratzke, C.; et al. Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of Rac, NSC23766 and EHT1864. Br. J. Pharm. 2015, 172, 2905–2917. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Lee, S.; Exintaris, B. Generation and Regulation of Spontaneous Contractions in the Prostate. Adv. Exp. Med. Biol. 2019, 1124, 195–215. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Y.; Li, B.; Wang, R.; Tamalunas, A.; Waidelich, R.; Strittmatter, F.; Stief, C.G.; Hennenberg, M. Inhibition of human prostate smooth muscle contraction by the inhibitors of protein kinase C, GF109203X, and Go6983. Prostate 2022, 82, 59–77. [Google Scholar] [CrossRef]
- Angulo, J.; Cuevas, P.; Fernandez, A.; La Fuente, J.M.; Allona, A.; Moncada, I.; Saenz de Tejada, I. Tadalafil enhances the inhibitory effects of tamsulosin on neurogenic contractions of human prostate and bladder neck. J. Sex. Med. 2012, 9, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.; Li, B.; Rutz, B.; Ciotkowska, A.; Huang, R.; Liu, Y.; Wang, R.; Strittmatter, F.; Waidelich, R.; Stief, C.G.; et al. Purinergic smooth muscle contractions in the human prostate: Estimation of relevance and characterization of different agonists. Naunyn. Schmiedebergs. Arch. Pharm. 2021, 394, 1113–1131. [Google Scholar] [CrossRef]
- Webber, M.M.; Trakul, N.; Thraves, P.S.; Bello-DeOcampo, D.; Chu, W.W.; Storto, P.D.; Huard, T.K.; Rhim, J.S.; Williams, D.E. A human prostatic stromal myofibroblast cell line WPMY-1: A model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis 1999, 20, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gratzke, C.; Tamalunas, A.; Wiemer, N.; Ciotkowska, A.; Rutz, B.; Waidelich, R.; Strittmatter, F.; Liu, C.; Stief, C.G.; et al. P21-Activated Kinase Inhibitors FRAX486 and IPA3: Inhibition of Prostate Stromal Cell Growth and Effects on Smooth Muscle Contraction in the Human Prostate. PLoS ONE 2016, 11, e0153312. [Google Scholar] [CrossRef]
- Michel, M.C.; Murphy, T.J.; Motulsky, H.J. New Author Guidelines for Displaying Data and Reporting Data Analysis and Statistical Methods in Experimental Biology. Mol. Pharm. 2020, 97, 49–60. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharm. 2015, 172, 3461–3471. [Google Scholar] [CrossRef]
- Wong, W.C.; Wong, E.L.; Li, H.; You, J.H.; Ho, S.; Woo, J.; Hui, E. Isoflavones in treating watchful waiting benign prostate hyperplasia: A double-blinded, randomized controlled trial. J. Altern. Complement. Med. 2012, 18, 54–60. [Google Scholar] [CrossRef]
- Mohamad, J.; Masrudin, S.S.; Alias, Z.; Muhamad, N.A. The effects of Pueraria mirifica extract, diadzein and genistein in testosterone-induced prostate hyperplasia in male Sprague Dawley rats. Mol. Biol. Rep. 2019, 46, 1855–1871. [Google Scholar] [CrossRef]
- Weber, K.S.; Setchell, K.D.; Stocco, D.M.; Lephart, E.D. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J. Endocrinol. 2001, 170, 591–599. [Google Scholar] [CrossRef]
- Masrudin, S.S.; Mohamad, J. Preventive effect of Pueraria mirifica on testosterone-induced prostatic hyperplasia in Sprague Dawley rats. Andrologia 2015, 47, 1153–1159. [Google Scholar] [CrossRef]
- Brandli, A.; Simpson, J.S.; Ventura, S. Isoflavones isolated from red clover (Trifolium pratense) inhibit smooth muscle contraction of the isolated rat prostate gland. Phytomedicine 2010, 17, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Bae, W.J.; Yuk, S.M.; Han, D.S.; Ha, U.S.; Hwang, S.Y.; Yoon, S.H.; Kim, S.W.; Han, C.H. Seoritae extract reduces prostate weight and suppresses prostate cell proliferation in a rat model of benign prostate hyperplasia. Evid. Based. Complement. Altern. Med. 2014, 2014, 475876. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Q.; Cai, J.; Wu, W.; Zhu, Y.S. 17alpha-Estradiol and genistein inhibit high fat diet induced prostate gene expression and prostate growth in the rat. J. Urol. 2011, 186, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Ciotkowska, A.; Rutz, B.; Erlander, M.G.; Ridinger, M.; Wang, R.; Tamalunas, A.; Waidelich, R.; Stief, C.G.; et al. Onvansertib, a polo-like kinase 1 inhibitor, inhibits prostate stromal cell growth and prostate smooth muscle contraction, which is additive to inhibition by alpha1-blockers. Eur. J. Pharm. 2020, 873, 172985. [Google Scholar] [CrossRef]
- Nomura, H.; Kawashima, H.; Masaki, S.; Hosono, T.Y.; Matsumura, K.; Tamada, S.; Tanaka, T.; Nakatani, T. Effect of selective estrogen receptor modulators on cell proliferation and estrogen receptor activities in normal human prostate stromal and epithelial cells. Prostate. Cancer Prostatic. Dis. 2009, 12, 375–381. [Google Scholar] [CrossRef][Green Version]
- Rao, A.; Woodruff, R.D.; Wade, W.N.; Kute, T.E.; Cramer, S.D. Genistein and vitamin D synergistically inhibit human prostatic epithelial cell growth. J. Nutr. 2002, 132, 3191–3194. [Google Scholar] [CrossRef]
- Wang, X.; Clubbs, E.A.; Bomser, J.A. Genistein modulates prostate epithelial cell proliferation via estrogen- and extracellular signal-regulated kinase-dependent pathways. J. Nutr. Biochem. 2006, 17, 204–210. [Google Scholar] [CrossRef]
- Hedlund, T.E.; van Bokhoven, A.; Johannes, W.U.; Nordeen, S.K.; Ogden, L.G. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2006, 66, 557–566. [Google Scholar] [CrossRef]
- Geller, J.; Sionit, L.; Partido, C.; Li, L.; Tan, X.; Youngkin, T.; Nachtsheim, D.; Hoffman, R.M. Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture. Prostate 1998, 34, 75–79. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Wang, H.; Frydenberg, M.; Husband, A. The in vivo effect of red clover diet on ventral prostate growth in adult male mice. Reprod. Fertil. Dev. 2001, 13, 325–329. [Google Scholar] [CrossRef]
- Semenov, A.L.; Gubareva, E.A.; Ermakova, E.D.; Dorofeeva, A.A.; Tumanyan, I.A.; Radetskaya, E.A.; Yurova, M.N.; Aboushanab, S.A.; Kanwugu, O.N.; Fedoros, E.I.; et al. Astaxantin and Isoflavones Inhibit Benign Prostatic Hyperplasia in Rats by Reducing Oxidative Stress and Normalizing Ca/Mg Balance. Plants 2021, 10, 2735. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, P.F.; Riedl, C.R. Effects of one-year treatment with isoflavone extract from red clover on prostate, liver function, sexual function, and quality of life in men with elevated PSA levels and negative prostate biopsy findings. Urology 2008, 71, 185–190; discussion 190. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Williford, W.O.; Chang, Y.; Machi, M.; Jones, K.M.; Walker-Corkery, E.; Lepor, H. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995, 154, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.D.; Roehrborn, C.G.; Bautista, O.M.; Andriole, G.L., Jr.; Dixon, C.M.; Kusek, J.W.; Lepor, H.; McVary, K.T.; Nyberg, L.M., Jr.; Clarke, H.S.; et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003, 349, 2387–2398. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Siami, P.; Barkin, J.; Damiao, R.; Major-Walker, K.; Nandy, I.; Morrill, B.B.; Gagnier, R.P.; Montorsi, F.; Comb, A.T.S.G. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur. Urol. 2010, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharm. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Guy, L.; Vedrine, N.; Urpi-Sarda, M.; Gil-Izquierdo, A.; Al-Maharik, N.; Boiteux, J.P.; Scalbert, A.; Remesy, C.; Botting, N.P.; Manach, C. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr. Cancer 2008, 60, 461–468. [Google Scholar] [CrossRef]
- Gardner, C.D.; Oelrich, B.; Liu, J.P.; Feldman, D.; Franke, A.A.; Brooks, J.D. Prostatic soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate 2009, 69, 719–726. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Nielsen, I.L.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar] [CrossRef]
- Stanislawska, I.J.; Figat, R.; Kiss, A.K.; Bobrowska-Korczak, B. Essential Elements and Isoflavonoids in the Prevention of Prostate Cancer. Nutrients 2022, 14, 1225. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, M.; Li, Z.; Jiang, H.; Shi, J.; Shi, X.; Liu, S.; Zhao, J.; Kong, L.; Zhang, W.; et al. Intake of Soy, Soy Isoflavones and Soy Protein and Risk of Cancer Incidence and Mortality. Front. Nutr. 2022, 9, 847421. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Inoue, M.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Soy and isoflavone consumption and subsequent risk of prostate cancer mortality: The Japan Public Health Center-based Prospective Study. Int. J. Epidemiol. 2020, 49, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin; Taneja, I.; Arora, S.; Raju, K.S.; Siddiqui, N. Disposition of pharmacologically active dietary isoflavones in biological systems. Curr. Drug Metab. 2013, 14, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Liu, Y.; Hu, S.; Tamalunas, A.; Waidelich, R.; Strittmatter, F.; Stief, C.G.; Hennenberg, M. Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein. Nutrients 2022, 14, 4943. https://doi.org/10.3390/nu14234943
Huang R, Liu Y, Hu S, Tamalunas A, Waidelich R, Strittmatter F, Stief CG, Hennenberg M. Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein. Nutrients. 2022; 14(23):4943. https://doi.org/10.3390/nu14234943
Chicago/Turabian StyleHuang, Ru, Yuhan Liu, Sheng Hu, Alexander Tamalunas, Raphaela Waidelich, Frank Strittmatter, Christian G. Stief, and Martin Hennenberg. 2022. "Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein" Nutrients 14, no. 23: 4943. https://doi.org/10.3390/nu14234943
APA StyleHuang, R., Liu, Y., Hu, S., Tamalunas, A., Waidelich, R., Strittmatter, F., Stief, C. G., & Hennenberg, M. (2022). Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein. Nutrients, 14(23), 4943. https://doi.org/10.3390/nu14234943







