Abstract
Obesity is a worldwide epidemic associated with many health problems. One of the new trends in health care is the emphasis on regular exercise and a healthy diet. Zeaxanthin (Zea) is a carotenoid with many beneficial effects on human health. The aim of this study was to investigate whether the combination of Zea and exercise had therapeutic effects on obesity induced by an HFD in rats. Sixty male Wistar rats were randomly divided into five groups of twelve: rats fed a standard diet; rats fed a high-fat diet (HFD); rats fed an HFD with Zea; rats fed an HFD with Exc; and rats fed an HFD with both Zea and Exc. To induce obesity, rats were fed an HFD for twelve weeks. Then, Zea and exercise were introduced with the HFD for five weeks. The results showed that the HFD significantly increased visceral adipose tissue, oxidative stress, and inflammation biomarkers and reduced insulin, high-density lipoprotein, and antioxidant parameters. Treatments with Zea, Exc, and Zea plus Exc reduced body weight gain, triacylglycerol, glucose, total cholesterol, and nitric oxide levels and significantly increased catalase and insulin compared with the HFD group. This study demonstrated that Zea administration and Exc performance appeared to effectively alleviate the metabolic alterations induced by an HFD. Furthermore, Zea and Exc together had a better effect than either intervention alone.
Keywords:
zeaxanthin; exercise; swimming; anti-obesity; high-fat diet; antioxidants; adipokines; anti-inflammatory; lipid profiles 1. Introduction
Obesity is an alarmingly increasing global public health problem. In addition, it is becoming a significant economic and health burden [1]. Obesity is a complicated disease that is characterized by a significant increase in body fat mass. It is responsible for increasing the risk of developing diseases such as dyslipidemia, type 2 diabetes mellitus, myocardial infarction, coronary heart disease, arterial hypertension, ovarian polycytosis, fatty liver disease, and various cancers; consequently, it results in a shorter life expectancy [2,3]. Obesity results from a multifaceted and complex interaction between environmental factors, human behavior, and genetic susceptibility. Unhealthy lifestyles (such as a sedentary lifestyle and a high-fat diet) are strong predictors of obesity, type 2 diabetes, and insulin resistance [4]. Obesity is a critical factor in the development of metabolic abnormalities in glucose, lipid metabolism, dyslipidemia, oxidative stress, and chronic inflammation [5,6,7,8]. These metabolic abnormalities of obesity may be exacerbated by an abnormal inflammatory response and the increased production of reactive oxygen species. It is well-known that a proinflammatory response is associated with the production of toxic reactive oxygen species and the subsequent generation of oxidative stress. Adipocytes from obese individuals exhibit an altered adipokine profile with increased expression and secretion of proinflammatory cytokines [9].
Due to the undesirable side effects of anti-obesity medications and the numerous risks associated with surgical interventions, the combination of regular exercise and healthy diet remains the safest and most cost-effective method for obesity management [10,11]. Furthermore, because natural products are safe and nontoxic, consuming functional foods is one of the best treatment options for weight loss [12]. In addition, the high safety margins of functional foods, especially when used in long-term regimens, generates interest in their use for managing obesity compared with synthetic medications [10].
Carotenoids are widely used terpenoid pigments. They are endowed with a system of conjugated double bonds, which yield their colors. Carotenoids are categorized into oxygen-free carotenes and their oxygenated derivatives, the xanthophylls. Furthermore, they are differentiated according to the structures of their end groups (acyclic or linear, monocyclic, and bicyclic) [13]. Zeaxanthin (Zea), from the xanthophyll family, is a carotenoid pigment that can be found in a wide range of fruits and vegetables and is more abundant in eggs, yellow corn, orange bell pepper, and green leafy vegetables [14,15,16]. Zea has also been proven to exert a variety of beneficial effects on human health due to its capability to scavenge free radicals, exhibit antioxidant effects, and reduce inflammation [16]. The oral administration of Zea to obese mice has improved dyslipidemia and decreased the progression of obesity [17]. Tuzcu et al. found that rats fed an HFD showed increased oxidative stress and the upregulation of inflammatory markers. However, the administration of Zea significantly reduced oxidative damage by increasing antioxidant enzyme activities and reducing malondialdehyde (MDA) concentrations.
Physical exercise (Exc) is an important intervention for treating and preventing various diseases such as osteoarthritis, obesity, Alzheimer’s disease, and hypertension [18]. In addition, regular exercise training improves lipid metabolism and oxidative capacity and lowers blood pressure, insulin resistance, and serum triglycerides [19,20,21].
Recent research on healthcare emphasizes regular exercise and a healthy diet. How diet and exercise training interact to produce synergistic or antagonistic effects on physiological functions that lead to health promotion and the possible mechanisms underlying such interactions are still poorly understood [22]. The combination of functional food consumption and exercise can cause changes in body metabolism [23]. However, there are no data on the therapeutic impact of zeaxanthin supplementation in combination with exercise for weight loss or to reduce the problems associated with obesity. This study investigated the effects of Zea, Exc, and the combination of Zea and Exc on obesity induced by an HFD in male Wistar rats.
2. Materials and Methods
2.1. Animals and Diets
Sixty seven-week-old male Wistar rats weighing (200 ± 10) g were obtained from the animal house unit of King Fahad Medical Research Center (KFMRC), Jeddah, Saudi Arabia. The animals were kept in ambient conditions (22 ± 2 °C, 50% humidity, 12/12 h light/dark) for one week before the initiation of the experiments. A balanced commercial diet and tap water were provided throughout the experiment. The study was approved by the Ethics Committee of King Abdulaziz University (approval No. 518-20) and compiled with the rules and regulations of the Animal Care and Use Committee at KFMRC. A standard chow diet (Grain Silos & Flour Mills Organization, KSA) was obtained from the KFMRC. The standard diet (387 kcal/100 g) contained 67.8% carbohydrates, 20% protein, 4% fat, 3.5% fiber, 0.3% choline chloride, and 4.5% vitamins and minerals. The high-fat diet (HFD) was prepared by mixing the standard diet (59.6%) with commercial butter (29.79%), casein (7.45%), DL-methionine (0.2%), and a vitamin and mineral mix (2.97%) [24]. The HFD (529 kcal/100 g) contained 40.4% carbohydrates, 19.4% protein, 32.2% fat, 2.1% fiber, 0.1% choline chloride, 0.2% methionine, and 5.6% vitamins and minerals. Zeaxanthin (purity of 98%) extracted from marigold flowers was provided by Xi’an Tongze Biotech Co., Ltd., Xi’an, China.
2.2. Experimental Design
The experimental design is shown in Figure 1. After a one-week acclimation period, 60 mice were randomly divided into two groups: the normal group was fed a standard diet (control, n = 12), and the experimental group was fed a high-fat diet (HFD, n = 48). At week 12, after confirming the body weight difference, the 48 obese rats were divided into four groups (n = 12/group): group I—positive control group (HFD); group II—HFD with the daily oral administration of 100 mg/kg of zeaxanthin (HFD + Zea); group III—HFD with exercise (HFD + Exc); and group IV—HFD with the daily oral administration of 100 mg/kg of zeaxanthin combined with exercise (HFD + Zea/Exc). All obese groups were continually fed the HFD during the experimental period (17 weeks). Sunflower oil was used as a vehicle; all groups received the same volume of sunflower oil (~2 mL/kg/day) [25,26]. A dosage of 100 mg/kg of zeaxanthin was selected because many in vivo studies have shown that it is an antioxidant at this dosage [27,28,29]. At the end of the experiment, rats fasted overnight and were anesthetized with isoflurane (induced with 5% isoflurane and maintained with 3% in a 2 L/min oxygen flow in a sealed container). Blood was drained from the retro-orbital veins of the rats, and serum was obtained from the blood by centrifugation at 3000 rpm for 15 min and was stored at −80 °C for biochemical analysis. Liver samples were frozen in liquid nitrogen and stored at −80 °C until analysis.
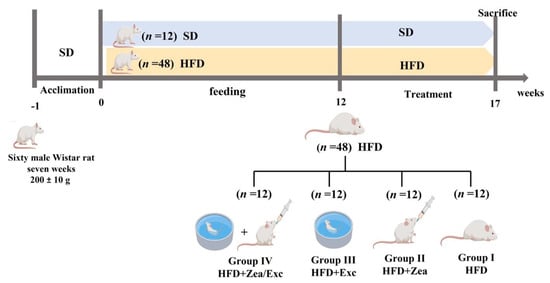
Figure 1.
Experimental design.
2.3. Exercise Protocol
In this study, swimming was used as a model exercise intervention. Rats in the swimming groups (HFD + Exc and HFD + Zea/Exc) swam in a plastic swimming tank with dimensions of 78 × 56 × 48 cm. The depth of the water was sufficient to preclude resting and prevent bobbing. To avoid hypothermia, the water temperature was set at 32 ± 2 °C. The rats swam for 10 min on the first day. The exercise time was increased by 10 min each day. In the following weeks, the rats were allowed to swim for 60 min once per day for five weeks [30,31,32].
2.4. Measurement of Body Weight, Food Intake, and Relative Weight of Visceral Adipose Tissue
During the experiment, the body weights of all experimental rats were recorded at the beginning of the experiment (time 0) and then weekly. The weight gain of the rats was also monitored weekly. The food intake (difference between the food offered and the food remaining) was measured daily, and the energy intake was calculated by multiplying the food intake by the total energy of the standard diet and the HFD [33]. At the end of the study, the weights of the visceral adipose tissues of the control and experimental groups were determined and noted. The ratio of visceral adipose tissue to body weight was expressed as the relative weight per 100 g of body weight [34]. The following formula was used to calculate the relative visceral adipose tissue:
2.5. Determination of Serum Biochemical Parameters
The levels of serum glucose, triacylglycerol (TGA), total cholesterol (TC), and high-density lipoprotein (HDL) were estimated using specific standard diagnostic kits with an automated modular analyzer (COBAS® 8000 series) at Alnoor Specialist Hospital, Makkah, Saudi Arabia. The levels of very low density lipoprotein (VLDL) and low-density lipoprotein (LDL) were calculated based on Friedewald’s equation [35]:
Serum insulin and the inflammatory markers leptin, resistin, and adiponectin were determined in serum using enzyme-linked immunosorbent assay (ELISA) kits (Solarbio Science & Technology, Beijing, China). The oxidative stress markers malondialdehyde (MDA) and nitric oxide (NO) as well as the activities of the antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) were evaluated in hepatic tissue using colorimetric assay kits from Solarbio Science & Technology (Beijing, China).
2.6. Histological Analysis of Visceral Adipose Tissue
Visceral adipose tissues were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 4 μm thick sections. These sections were stained with hematoxylin and eosin (H & E), and histological images were examined through a light microscope (Olympus BX51) under 20× magnification. Adipocyte size and diameter were calculated using ImageJ version 1.53t (National Institutes of Health).
2.7. Statistical Analysis
The data were analyzed using GraphPad Prism 8 (GraphPad Inc., La Jolla, CA, USA). Data are shown as the means ± standard errors of the means (means ± SEM). A one-way ANOVA was used to assess the significant differences between different groups, followed by Tukey’s post hoc test. Differences with p < 0.05 were considered statistically significant.
3. Results
3.1. Effects of Zea and Exc on Body Weight, Weight Gain, Food Intake, and Relative Visceral Adipose Tissue in HFD-Induced Obese Rats
As shown in Table 1, at the end of 12 weeks of obesity induction, rats fed an HFD showed a significant increase in body weight (p < 0.01) compared with the rats fed a standard diet (Figure 2). After five weeks of treatment, the final body weight and weight gain had decreased, but not statistically significantly, compared with the HFD group. Moreover, groups that underwent Exc (HFD + Exc and HFD + Zea/Ex) showed decreases in their body weights compared with HFD + Zea, but no significant difference was observed. Food and energy intake were significantly increased in the control group compared with the HFD-fed groups due to the low calorie content in the standard diet. Additionally, the HFD groups had no significant differences in food and energy intake. Furthermore, the relative weights of visceral adipose tissue were increased in the HFD group and slightly decreased in the treated groups (Table 1).

Table 1.
Effects of Zea and Exc on body weight, weight gain, food intake, and relative visceral adipose tissue in HFD-induced obese rats.
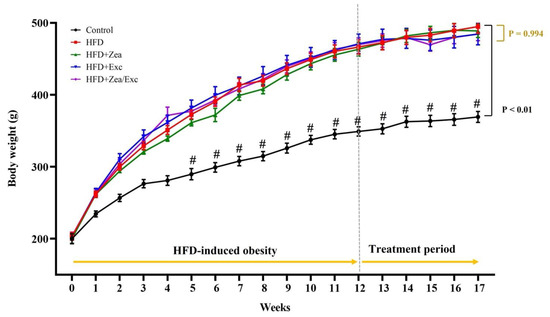
Figure 2.
Effects of Zea and Exc on body weight in HFD-induced obese rats. Data are expressed as means ± SEM, n = 12. # p < 0.05 vs. control.
3.2. Effects of Zea and Exc on Blood Glucose and Insulin Levels
The determination of blood glucose and insulin levels in control and obese rats is shown in Figure 3. There was a highly significant increase (p < 0.001) in blood glucose levels in the HFD group compared with the control group. On the other hand, obese rats treated with Zea and/or Exc showed a significant reduction in glucose levels compared with the HFD group (Figure 3A). In addition, serum insulin levels were decreased in the HFD group compared with the control group, with no significant differences. Interestingly, all treated groups (HFD + Zea, HFD + Exc, and HFD + Zea/Exc) exhibited significantly elevated (p < 0.05) insulin levels compared with the HFD group (Figure 3B).
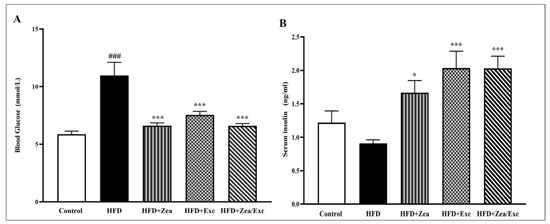
Figure 3.
Effects of Zea and Exc on blood glucose levels (A) and insulin levels (B) in HFD-induced obese rats. Data are expressed as means ± SEM, n = 7. ### p < 0.001 vs. control; * p < 0.05, *** p < 0.001 vs. HFD.
3.3. Effects of Zea and Exc on Serum Lipid Profiles
The effects of Zea and Exc on the serum lipids of HFD-fed rats are illustrated in Figure 4. It was found that the HDL levels in the HFD group were lower than in the control group but did not differ significantly. Notably, the HDL levels among the treated groups (HFD + Zea, HFD + Exc, and HFD + Zea/Exc) were increased compared with the HFD group and were statistically elevated (p < 0.01) in the Exc groups (HFD + Exc and HFD + Zea/Exc) (Figure 4A). In addition, the group that did not perform Exc (HFD + Zea) had significantly lower (p < 0.05) HDL levels than the group that underwent Exc.
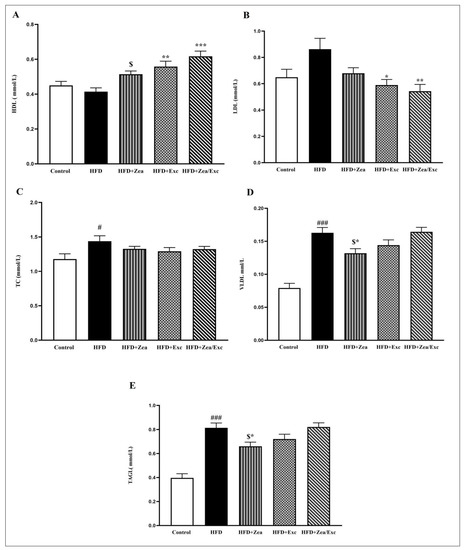
Figure 4.
Effects of Zea and Exc on the serum lipid profiles in HFD-induced obese rats. (A) High-density lipoprotein (HDL); (B) low-density lipoprotein (LDL); (C) total cholesterol (TC); (D) very low density lipoprotein (VLDL); (E) triacylglycerol (TAG). Data are expressed as means ± SEM, n = 12. # p < 0.05, ### p < 0.001 vs. control; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. HFD; $ p < 0.05 vs. HFD + Zea/Ex.
The LDL levels in the HFD group were higher than in the control group, without significant differences. In contrast, all the treated groups had low LDL levels compared with the HFD group, especially the Exc groups (HFD + Exc and HFD + Zea/Exc), which were significantly decreased (p < 0.05) compared with the HFD group (Figure 4B). Interestingly, the HFD + Zea/Exc group had lower LDL levels than HFD + Exc alone, and there was no significant difference.
Furthermore, the TC levels in the HFD group were significantly elevated (p < 0.05) compared with the control group, and the TC levels were slightly reduced in all treated groups, without significant differences (Figure 4C). Finally, the TAG and VLDL levels showed highly significant increases in the HFD group compared with the control group (p < 0.001). On the other hand, the HFD + Zea and HFD + Exc groups had low levels of TAG and VLDL compared with the HFD group, especially HFD + Zea, which was significantly decreased (p < 0.05). In addition, the levels of TGA and VLDL in the HFD + Zea group were significantly decreased (p < 0.05) compared with the HFD + Zea/Exc group, which was similar to the HFD group (Figure 4D,E).
3.4. Effects of Zea and Exc on Serum Inflammatory Markers
As illustrated in Figure 5, there was a significant increase (p < 0.01) in the leptin levels in the HFD group compared with the control group. Furthermore, the groups treated with Zea exhibited low levels of leptin compared with the HFD group, especially HFD + Zea/Exc, which was significantly decreased (p < 0.05) compared with the HFD group and exercise alone (Figure 5A). Meanwhile, the resistin levels were significantly increased (p < 0.001) in the HFD group compared with the control group. Additionally, the resistin levels in the HFD + Zea and HFD + Zea/Exc groups were significantly reduced (p < 0.01). However, there was no statistical decrease in the HFD + Exc group compared with the HFD group (Figure 5B). On the other hand, the adiponectin levels were reduced in the HFD group compared with the control group, with no significant difference. Nevertheless, increased adiponectin levels were observed in all treated groups compared with the HFD group (Figure 5).
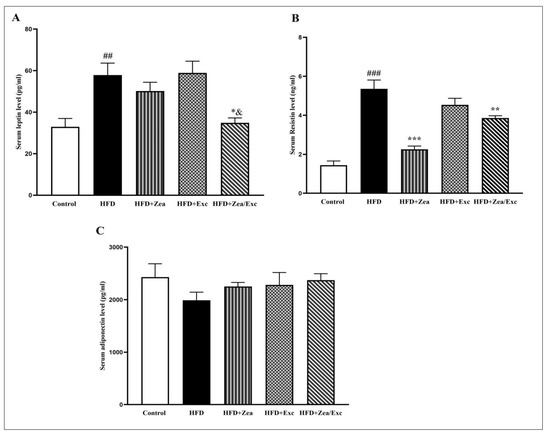
Figure 5.
Effects of Zea and Exc on serum leptin levels (A), serum resistin levels (B), and adiponectin levels (C) in HFD-induced obese rats. Data are expressed as means ± SEM, n = 7. ## p < 0.01, ### p < 0.001 vs. control; * p < 0.05, ** p < 0.01, *** p≤ 0.001 vs. HFD; & p ≤ 0.05 vs. HFD + Ex.
3.5. Effects of Zea and Exc on Oxidative Stress and Antioxidant Markers in Liver Tissue
The oxidative stress and antioxidant markers were measured in hepatic tissue (Figure 6). CAT activity was significantly reduced (p < 0.05) in the HFD group compared with the control group. Moreover, the levels of CAT in the HFD + Zea, HFD + Exc, and HFD + Zea/Exc treated groups were significantly increased (p < 0.001) compared with the HFD group (Figure 6A). Additionally, the SOD activity was lower than in the control group, with no significant difference. Intriguingly, groups receiving Zea (HFD + Zea and HFD + Zea/Exc) exhibited significant increases in SOD levels (p < 0.01) compared with the HFD group and the HFD + Exc group (Figure 6B).
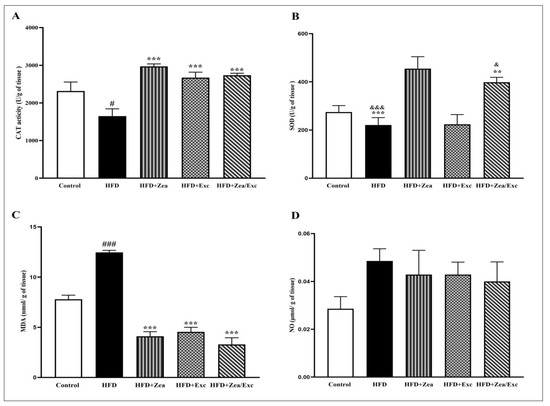
Figure 6.
Effects of Zea and Exc on hepatic tissue oxidative stress and antioxidant markers in HFD-induced obese rats. (A) Catalase (CAT); (B) superoxide dismutase (SOD); (C) malondialdehyde (MDA); (D) nitric oxide (NO). Data are expressed as means ± SEM, n = 7. # p < 0.05, ### p< 0.001 vs. control; ** p <0.01, *** p <0.001 vs. HFD; & p < 0.05, &&& p < 0.001 vs. HFD + Exc.
In the current study, the hepatic MDA levels were significantly increased (p < 0.001) in the HFD group compared with the control group. However, after intervention with Zea and Exc, the level of MDA was significantly reduced (p < 0.001) compared with the HFD group. Additionally, it was noted that groups treated with Zea (HFD + Zea and HFD + Zea/Exc) exhibited lower levels of MDA compared with the HFD + Exc group, with no significant difference (Figure 6C). No significant increase was observed in the levels of nitric oxide (NO) in the HFD group in comparison with the control group. Additionally, all treated groups had reduced NO levels compared with the HFD group (Figure 6D).
3.6. Effects of Zea and Exc on Visceral Adipose Tissue
As shown in Figure 7A, the HFD group exhibited an accumulation of visceral adipose tissue compared with the control group. However, the rats treated with either Zea or Exc showed no significant decrease in visceral adipose tissue accumulation compared with the HFD control group. The degree of lipid accumulation in the white adipose tissue is proportional to the size of the tissue [36,37]. The histological analysis of visceral adipose tissue showed that adipocytes were enlarged in the HFD group compared with the control group, and this effect was visible with the naked eye. In addition, the groups administered Zea and Exc (HFD + Zea, HFD + Exc, and HFD + Zea/Exc) showed smaller adipocytes and similar histology to the control group (Figure 7B). The sizes and diameters of the adipocytes in visceral adipose tissues were significantly increased (p< 0.001) in the HFD group compared with the control group. In contrast, it was found that the groups treated with Zea and Exc (HFD + Zea, HFD + Exc, and HFD + Zea/Exc) had significantly decreased (p < 0.001) adipocyte sizes and diameters compared with the HFD group (Figure 7C,D). Notably, there were no differences in the sizes and diameters of adipocytes between treated groups.
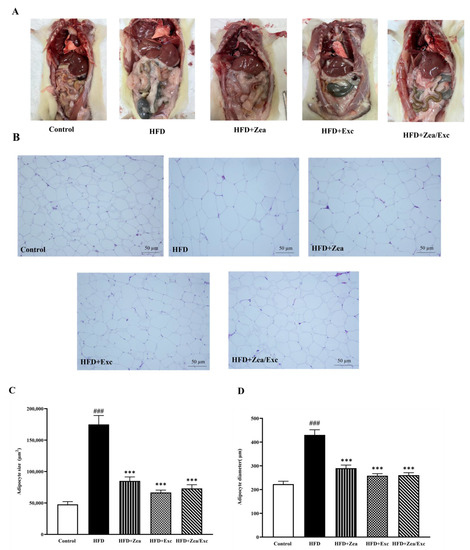
Figure 7.
Effects of Zea and Exc on visceral adipose tissue in HFD-induced obese rats. (A) Abdominal cavity dissection of visceral adipose tissue; (B) histology of visceral adipose tissue; (C) adipocyte size; (D) adipocyte diameter. Data are expressed as means ± SEM, n = 4. ### p < 0.001 vs. control; *** p < 0.001 vs. HFD; 20× magnification.
4. Discussion
Obesity is a pathological problem caused by an unhealthy diet and lack of exercise. A healthy low-fat diet, more physical activity, and behavioral changes are all part of the current best practices for treating obesity [38]. Recently, there has been extensive research on using natural products to treat obesity and reduce its complications instead of medical drugs with unwanted side effects. Additionally, physical exercise can reduce the adverse effects of obesity, even if weight loss does not occur [39]. In this study, the anti-obesity effects of Zea and Exc in HFD-induced obese rats were investigated.
HFD-induced obesity in rodents is considered a suitable model to study the potential roles of dietary fat in the incidence and progression of obesity because they are reportedly very similar to human obesity [40,41]. In this study, the induction of obesity by feeding rats an HFD was successful after 12 weeks, consistent with a previous study [42]. All rats fed an HFD during the 12 weeks of induction had significantly increased body weight compared with the control group. However, after treatment with Zea and/or Exc from the end of the 12th week to the 17th week of the experiment, rats in the HFD + Zea, HFD + Exc, and HFD + Zea/Exc groups had slower rates of weight gain, lower body weights, and less fat mass. The results of the current study suggest that Zea and Exc inhibit HFD-induced obesity by preventing increased body weight and visceral fat. According to a previous study [17], treatment with Zea for four weeks significantly decreased body weight, weight gain, and adipose mass in HFD-induced obese mice. Additionally, the groups that underwent Exc (HFD + Exc and HFD + Zea/Exc) exhibited lower body mass and weight gain than those only treated with Zea (HFD + Zea), which suggests that physical exercise can prevent the progression of obesity or at least mitigate it. This result agrees with previous studies reporting that physical activity and regular exercise can increase energy metabolism in the body, helping to control body weight and reduce obesity [43,44].
The reduction in body weight reflects the negative status of energy expenditure, which could result from a reduction in food intake or an increase in energy expenditure [45]. In our study, the Exc groups (HFD +Exc and HFD + Zea/Exc) showed less feed intake and subsequently reduced caloric intake; however, no significant difference was observed when compared with the HFD group. Similarly, other studies found that feeding rats a high-fat diet causes them to feed less and consume fewer calories when performing physical exercise [46,47,48]. One reason for this might be that physical exercise, which is a stress agent, may affect the production of anorectic hormones and increase the sensitivity of their receptors, thereby suppressing the sensation of hunger and controlling energy balance during physical exercise [49,50]. Moreover, other studies have shown that physical activity can reduce food intake and increase energy expenditure, which was also the case in this study [51,52]. Several studies found that regular exercise can improve some of the metabolic complications of obesity, even in the absence of weight loss [39].
Obesity is associated with over 200 health complications, such as type 2 diabetes mellitus and insulin resistance, and it is the fifth largest cause of mortality globally [53]. Previous reports have shown that blood glucose levels were significantly elevated in rats fed an HFD compared with a normal healthy group [54,55].
In the current study, the HFD group exhibited significantly higher blood glucose levels than the control group. However, the supplementation of Zea and/or the induction of Exc led to a significant decrease in blood glucose levels in the HFD + Zea, HFD + Exc, and HFD + Zea/Exc groups. This finding agrees with Tuzcu et al., who showed that Zea reduced glucose levels in rats fed an HFD [28]. Zea has also been proven to lower blood sugar levels, relieve diabetes symptoms, and improve cognitive deficits in diabetic animals [56,57]. In addition, exercise training has been reported to improve glucose levels, insulin sensitivity, and pancreatic β-cell function [58,59,60]. Similar to the current findings, previous studies have demonstrated that glucose uptake efficiency improves with exercise and is closely related to physical load and exercise intensity. Furthermore, Exc increases glucose absorption by stimulating insulin and regulating the response of cells to insulin [61,62]. On the other hand, this study found that insulin levels had decreased in the HFD group and increased in the control and treated groups. This result is in contrast to what was expected and what was demonstrated in previous studies. A possible explanation for these results could be a defect in pancreatic β-cells leading to a decrease in insulin production. According to previous research by Gupta et al., the function and viability of β cells depend on insulin signaling; obesity-related abnormalities (lipotoxicity, glucotoxicity, increased oxidative stress, and inflammation) are known to lead to impaired insulin secretion and hyperglycemia [63]. However, it is imperative to point out that the proper diagnosis of insulin resistance would require performing glucose tolerance tests (GTT) and insulin tolerance tests (ITT). Therefore, these findings lay a foundation for future investigations focusing on diabetes as a complication of obesity using HFD-induced obese rats as a diabetic model.
Serum lipid profiles are good predictors of the development of diseases related to metabolic disorders caused by obesity [64]. According to previous studies, an HFD has been associated with dyslipidemia, which is characterized by elevated total LDL, VLDL, TC, and TGA levels and decreased HDL [65,66]. In this study, rats fed an HFD had high levels of LDL, VLDL, TC, and TGA and low HDL levels, suggesting the development of dyslipidemia. In contrast, we found that the LDL, VLDL, TC, and TGA levels were reduced in the HFD + Zea and HFD + Exc groups, whereas HDL levels were increased, which is consistent with the findings of previous studies [28,67]. Furthermore, compared with the HFD and other treated groups, the combination of Zea and Exc had the highest HDL levels, whereas the LDL levels were significantly decreased. Moreover, the levels of TC in the combination group were low, as in the other treated group, without significant difference. However, in contrast to the HFD + Zea and HFD + Exc groups, the TGA levels in the HFD + Zea/Exc group were similar to the levels in the HFD group, in agreement with previous studies [17,21]. Our results demonstrate that Zea supplementation might prevent dyslipidemia and improve lipid profiles in HFD-fed rats. Furthermore, as one of the carotenoids, Zea might stimulate several nuclear receptors that regulate the transcription of several lipid metabolism pathways [68]. On the other hand, HFD-induced lipid metabolism disorders may be recovered with a suitable exercise intervention, which agrees with previously published research [44,69,70]. Mika et al. reported that exercise increases lipolysis, reduces the uptake of fatty acids by adipocytes, alters the composition of fatty acids in adipose tissue, and modifies the expression of the enzymes responsible for the synthesis, elongation, and desaturation of fatty acids [71]. Exercise also encourages the “beiging” of adipose tissue and helps to increase mitochondrial activity, which leads to a rise in fatty acid oxidation in the adipose tissue [71].
Obesity is characterized by alterations in circulating adipokine concentrations due to the excessive accumulation and dysfunction of adipose tissue [72]. Leptin, resistin, and adiponectin are major adipokines secreted from white adipose tissue, and they play an essential role in regulating metabolism [73,74]. Increased circulating leptin and resistin levels with decreased adiponectin levels are characteristics of obesity [75,76]. Leptin and resistin are proinflammatory adipokines associated with energy homeostasis, the central control of food intake, inflammation, cardiovascular disease, and insulin resistance [77,78,79], while adiponectin has antidiabetic, anti-inflammatory, and antiatherogenic properties [80]. In the current study, rats fed an HFD exhibited significantly increased leptin and resistin levels with a parallel decrease in adiponectin, which is in agreement with prior findings [81]. On the other hand, leptin levels decreased in groups that were provided Zea (HFD + Zea and HFD + Zea/Exc), especially the HFD + Zea/Exc group, which exhibited significantly reduced leptin levels compared with Exc alone. Zea and Exc significantly reduced resistin levels in all treated groups, particularly in the HFD + Zea and HFD + Zea/Exc groups. Additionally, adiponectin was increased in all treated groups compared with the HFD groups, with no significant difference. It has been reported that leptin, resistin, and adiponectin, which are involved in mediating obesity, have been linked to increased oxidative stress and inflammation, which may cause a global health risk leading to hyperglycemia, cancer, diabetes, and other diseases affecting vital organs [78,82]. Thus, from this point, we can explain the effect of Zea on leptin, resistin, and adiponectin levels by its activity as an antioxidant and anti-inflammatory agent, which agrees with previous studies [16,83]. On the other hand, the role of exercise in lowering leptin and resistin and increasing adiponectin can be explained by two reasons. First, physical exercise not only contributes to a reduction in adipose tissue mass but may also attenuate the inflammation caused by obesity through the dysregulated expression of adipokines secreted by adipose tissue [84]. Second, during exercise, muscles produce myokines and release them into the circulation, which may balance and counteract the harmful effects of proinflammatory adipokines [84,85,86,87].
Obesity and its associated disorders are closely associated with oxidative stress [88], which is defined as an imbalance between the oxidative and antioxidant systems that results in impaired redox signaling [89,90]. In addition, it is known that HFD-induced obesity leads to oxidative damage through lipid peroxidation by producing malondialdehyde, depleting endogenous antioxidants, and decreasing the activity of antioxidant enzymes such as SOD and CAT [91,92,93]. Moreover, nitric oxide and other oxidative stress markers appear to be important factors in the association between obesity and other diseases [94]. In the present study, after five weeks of administration of Zea and/or Exc performance, the levels of CAT and SOD were significantly increased, while the MDA levels were significantly decreased compared with the HFD group. Additionally, the groups treated with Zea (HFD + Zea and HFD + Zea/Exc) had the highest levels of SOD when compared with the HFD group. In addition, the NO levels were lower in all treated groups. From the current results, it appears that both Zea and Exc may improve oxidative stress by increasing the activity of CAT and SOD and reducing MDA and NO, which resulted from feeding the rats an HFD. However, it is worth noting that the effect of Zea was slightly greater than the effect of exercise (Figure 7). According to the findings of previous studies [28,95,96], Zea may exert its antioxidant properties by directly suppressing reactive oxygen species. Similar to other carotenoids, Zea has a chain of isoprene residues with conjugated double bonds, which allows it to accept an extra electron and absorb energy from excited molecules [96]. Additionally, the potential role of exercise in suppressing oxidative stress is notable. It has previously been reported that after exercise the body produces more endogenous antioxidants, which help protect cells from oxidative damage [97].
Obesity is initiated by the accumulation of free fatty acids in adipose tissue, which can lead to enlarged adipocytes [36,37]. In the present study, histological and statistical analyses of visceral adipose tissue showed that the sizes and diameters of adipocytes were smaller in rats treated with Zea and Exc (HFD + Zea, HFD + Exc, and HFD + Zea/Exc groups) compared with the HFD group. This is consistent with a previous study [17] in which the sizes of larger adipocytes were significantly reduced after four weeks of treatment with Zea. Furthermore, regular exercise training has been shown to reduce adipocyte hypertrophy in both subcutaneous and visceral adipose tissue [98,99,100], which is consistent with our findings in this study.
To the best of our knowledge, there was no previous study on the combination of zeaxanthin and exercise; thus, we can infer that the combination of zeaxanthin and exercise has greater efficacy in reversing the complications of obesity (Figure 8).
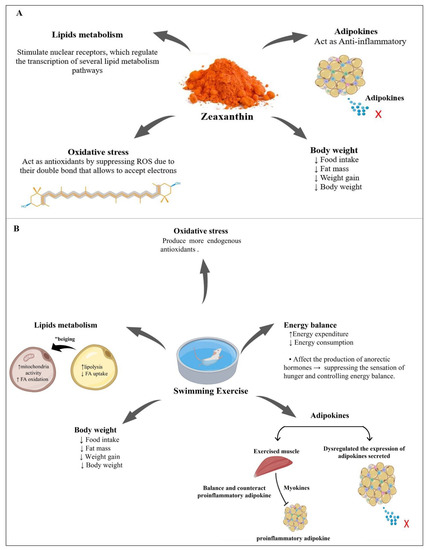
Figure 8.
Possible mechanisms of zeaxanthin (A) and exercise (B) leading to the reduction of the complications of obesity and contributing to its treatment. ↑ = increase, ↓ = decrease, x = no adipokine secreted.
5. Conclusions
This study showed that the induction of obesity by feeding an HFD to rats was responsible for significant weight gain, increased energy intake, and visceral adipose tissue accumulation. Moreover, HFD-induced obesity was associated with metabolic disorders. This resulted in abnormal values of most biochemical parameters that were studied, such as glucose, insulin, and lipid profiles, and disturbances in the inflammatory and oxidative stress parameters. After weeks of treatment, we found that zeaxanthin supplementation and exercise exerted an anti-obesity effect by reducing visceral fat mass, effectively improving dyslipidemia, and lowering blood glucose levels. Moreover, these interventions were able to suppress oxidative stress by increasing the activities of antioxidant enzymes and depleting ROS. Additionally, Zea and Exc decreased proinflammatory adipokines. In conclusion, the current study demonstrates that zeaxanthin and exercise have beneficial effects in treating and preventing obesity and its complications. Finally, zeaxanthin and exercise together had a better effect than either intervention alone. Future studies with a more extended treatment duration of more than five weeks are recommended. Further research could be conducted to determine the effect of Zea on other HFD complications, such as diabetes, cardiovascular disease, and renal dysfunction. In addition, it is essential to perform further studies on other types of exercise with adequate intensity and appropriate durations.
Author Contributions
Conceptualization, H.G., L.B., M.A.-t., S.A. and M.E.; methodology, H.G., L.B., M.A.-t., S.A. and M.E.; software, M.A.-t.; validation, H.G. and L.B.; formal analysis, H.G., L.B. and M.A.-t.; investigation, M.A.-t.; data curation, M.A.-t.; writing—original draft preparation, M.A.-t.; writing—review and editing, H.G. and L.B.; supervision, H.G. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the biomedical ethics research committee (approval No. 518-20, approval on 11 October 2020) at King Abdulaziz University and followed the rules and regulations of the Animal Care and Use Committee at the KFMRC, which complied with the “System of Ethics of Research on Living Creatures” guidelines prepared by King Abdulaziz City for Science and Technology and were approved by Royal Decree No. M/59, dated 24 August 2010.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Badrah S. Algamdi, Head of the Pre-Clinical Research Unit at the King Fahad Medical Research Center, Jeddah, for their infrastructure and support. We would also like to thank the staff of King Fahd Medical Research Center, KAU, Jeddah, KSA, for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayalon, I.; Bodilly, L.; Kaplan, J. The impact of obesity on critical illnesses. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2021, 56, 691–700. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why primary obesity is a disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, R.; Soufi, F.G.; hossein Somi, M.; Mohaddes, G.; Bavil, F.M.; Naderi, R.; Alipour, M.R. Swim training improves HOMA-IR in type 2 diabetes induced by high fat diet and low dose of streptozotocin in male rats. Adv. Pharm. Bull. 2015, 5, 379. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Castro Cabezas, M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Leung, M.-Y.M.; Pollack, L.M.; Colditz, G.A.; Chang, S.-H. Life years lost and lifetime health care expenditures associated with diabetes in the US, National Health Interview Survey, 1997–2000. Diabetes Care 2015, 38, 460–468. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 2014, 32, 3568. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Hill, J.A. Obesity, diabetes, and cardiovascular diseases: A compendium. Circ. Res. 2016, 118, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef]
- Ammar, N.M.; Farag, M.A.; Kholeif, T.E.; Metwally, N.S.; El-Sheikh, N.M.; El Gendy, A.N.; Abdel-Hamid, A.-H.Z. Serum metabolomics reveals the mechanistic role of functional foods and exercise for obesity management in rats. J. Pharm. Biomed. Anal. 2017, 142, 91–101. [Google Scholar] [CrossRef]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.-F.; Maratos-Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pan, M.-H. Natural products in anti-obesity therapy. Molecules 2016, 21, 1750. [Google Scholar] [CrossRef]
- Jia, K.-P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Johnson, E.J.; Maras, J.E.; Rasmussen, H.M.; Tucker, K.L. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J. Am. Diet. Assoc. 2010, 110, 1357–1362. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, properties, and antioxidant protection of eyes, heart, liver, and skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Xie, J.; Xu, Q.; Pan, C.; Wang, J.; Wu, X.; Zheng, M.; Liu, J. Anti-obesity effects of zeaxanthin on 3T3-L1 preadipocyte and high fat induced obese mice. Food Funct. 2017, 8, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of exercise intervention on various diseases in rats. J. Sport Health Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1. [Google Scholar]
- Snowling, N.J.; Hopkins, W.G. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: A meta-analysis. Diabetes Care 2006, 29, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Wang, R.; Li, M.; Li, D.; Xie, Z. Dietary supplement of Yunkang 10 green tea and treadmill exercise ameliorate high fat diet induced metabolic syndrome of C57BL/6 J mice. Nutr. Metab. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Oharomari, L.K.; Ikemoto, M.J.; Hwang, D.J.; Koizumi, H.; Soya, H. Benefits of Exercise and Astaxanthin Supplementation: Are There Additive or Synergistic Effects? Antioxidants 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Abdul Majid, N.; Abdul Hamid, A.; Salleh, S.Z.; Saari, N.; Abas, F.; Pak Dek, M.S.; Ramli, N.S.; Jaafar, A.H. Metabolomics approach to investigate the ergogenic effect of Morinda citrifolia L. leaf extract on obese Sprague dawley rats. Phytochem. Anal. 2020, 31, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Pierine, D.; Navarro, M.; Minatel, I.; Luvizotto, R.; Nascimento, A.; Ferreira, A.; Yeum, K.; Corrêa, C. Lycopene supplementation reduces TNF-α via RAGE in the kidney of obese rats. Nutr. Diabetes 2014, 4, e142. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, E.R.; Firdous, A.P.; Preethi, K.C.; Kuttan, R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride-and ethanol-induced hepatic damage. J. Pharm. Pharmacol. 2010, 62, 1054–1060. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Kuttan, R. Carotenoid lutein protects rats from gastric ulcer induced by ethanol. J. Basic Clin. Physiol. Pharmacol. 2012, 23, 33–37. [Google Scholar] [CrossRef]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef]
- Xue, C.; Rosen, R.; Jordan, A.; Hu, D.-N. Management of ocular diseases using lutein and zeaxanthin: What have we learned from experimental animal studies? J. Ophthalmol. 2015, 2015, 523027. [Google Scholar] [CrossRef]
- Maigoda, T.C.; Sulaeman, A.; Setiawan, B.; Wibawan, I.W.T. Effects of red dragon fruits (Hylocereus polyrhizus) powder and swimming exercise on inflammation, oxidative stress markers, and physical fitness in male obesity rats (Sprague dawley). IJSBAR 2016, 25, 123–141. [Google Scholar]
- Qi, J.; Luo, X.; Ma, Z.; Zhang, B.; Li, S.; Duan, X.; Yang, B.; Zhang, J. Swimming exercise protects against insulin resistance via regulating oxidative stress through Nox4 and AKT signaling in high-fat diet-fed mice. J. Diabetes Res. 2020, 2020, 2521590. [Google Scholar] [CrossRef] [PubMed]
- Riahi, F.; Riyahi, S. Effect of moderate swimming exercise on weight gain in high fat diet rats. Ann. Mil. Health Sci. Res. 2016, 14, e13819. [Google Scholar]
- Gong, H.; Han, Y.-W.; Sun, L.; Zhang, Y.; Zhang, E.-Y.; Li, Y.; Zhang, T.-M. The effects of energy intake of four different feeding patterns in rats. Exp. Biol. Med. 2016, 241, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L.; Yang, L.; Lǚ, H.; Wang, S.; Sun, G. Anti-obesity and Hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018, 15, 43. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Schadinger, S.E.; Bucher, N.L.; Schreiber, B.M.; Farmer, S.R. PPARγ2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1195–E1205. [Google Scholar] [CrossRef]
- Spiegelman, B.; Puigserver, P.; Wu, Z. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int. J. Obes. 2000, 24, S8–S10. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Shen, S.-W.; Shen, Z.-H.; Xu, M.; Yang, C.-J.; Li, F.; Feng, Y.-B.; Yun, J.-T.; Wang, L. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016, 15, 93. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Birari, R.; Kapat, K. Potential anti-obesity and lipid lowering natural products: A review. Nat. Prod. Commun. 2007, 2, 1934578X0700200316. [Google Scholar] [CrossRef]
- López, I.P.; Marti, A.; Milagro, F.I.; Zulet, M.d.l.A.; Moreno-Aliaga, M.J.; Martinez, J.A.; De Miguel, C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes. Res. 2003, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Jambocus, N.G.S.; Saari, N.; Ismail, A.; Khatib, A.; Mahomoodally, M.F.; Hamid, A.A. An investigation into the antiobesity effects of Morinda citrifolia L. leaf extract in high fat diet induced obese rats using a 1H NMR metabolomics approach. J. Diabetes Res. 2016, 2016, 2391592. [Google Scholar]
- Jakicic, J.M.; Otto, A.D. Treatment and prevention of obesity: What is the role of exercise? Nutr. Rev. 2006, 64, S57–S61. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, H.; Li, J.; Fan, J.; Jia, S.; Kou, X.; Chen, N. Swimming intervention mitigates HFD-induced obesity of rats through PGC-1α-irisin pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2123–2130. [Google Scholar] [PubMed]
- Stenman, L.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Levin, B.; Dunn-Meynell, A. Differential effects of exercise on body weight gain and adiposity in obesity-prone and-resistant rats. Int. J. Obes. 2006, 30, 722–727. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Higgins, J.A.; Wyatt, H.R.; Melanson, E.L.; Johnson, G.C.; Jackman, M.R.; Giles, E.D.; Brown, I.E.; Hill, J.O. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R793–R802. [Google Scholar]
- Rocha, G.L.d.; Crisp, A.H.; De Oliveira, M.R.; Silva, C.A.d.; Silva, J.O.; Duarte, A.C.; Sene-Fiorese, M.; Verlengia, R. Effect of high intensity interval and continuous swimming training on body mass adiposity level and serum parameters in high-fat diet fed rats. Sci. World J. 2016, 2016, 2194120. [Google Scholar] [CrossRef]
- Blundell, J.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef]
- Baek, K.-W.; Gim, J.-A.; Park, J.-J. Regular moderate aerobic exercise improves high-fat diet-induced nonalcoholic fatty liver disease via monoacylglycerol O-acyltransferase 1 pathway suppression. J. Sport Health Sci. 2020, 9, 472–478. [Google Scholar] [CrossRef]
- El Elj, N.; Lac, G.; Tabka, Z.; Gharbi, N.; El Fezaa, S. Effect of physical exercise on reducing food intake and weight gain. Procedia-Soc. Behav. Sci. 2011, 30, 2027–2031. [Google Scholar] [CrossRef][Green Version]
- Colleluori, G.; Perugini, J.; Giordano, A.; Cinti, S. From Obesity to Diabetes: The Role of the Adipose Organ. In From Obesity to Diabetes; Eckel, J., Clément, K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 75–92. [Google Scholar]
- Alkhudhayri, D.A.; Osman, M.A.; Alshammari, G.M.; Al Maiman, S.A.; Yahya, M.A. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. Saudi J. Biol. Sci. 2021, 28, 3333–3342. [Google Scholar] [CrossRef]
- Haque, M.R.; Ansari, H.S. Anti-obesity effect of arq zeera and its main components thymol and cuminaldehyde in high fat diet induced obese rats. Drug Res. 2018, 68, 637–647. [Google Scholar] [CrossRef]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Eong, K.-G.A. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S.; Ding, X.; Qin, L.; Mao, Y.; Chen, L.; Li, W.; Ying, C. Zeaxanthin improves diabetes-induced cognitive deficit in rats through activiting PI3K/AKT signaling pathway. Brain Res. Bull. 2017, 132, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-R.; Hou, P.-H.; Chen, W.-K.; Lin, C.-T.; Tsai, H.-P.; Mao, F.C. Exercise affects blood glucose levels and tissue chromium distribution in high-fat diet-fed C57BL6 mice. Molecules 2020, 25, 1658. [Google Scholar] [CrossRef]
- Corrêa, A.P.d.S.; Antunes, C.F.; Figueira, F.R.; de Castro, M.A.; Ribeiro, J.P.; Schaan, B.D.A. Effect of acute inspiratory muscle exercise on blood flow of resting and exercising limbs and glucose levels in type 2 diabetes. PLoS ONE 2015, 10, e0121384. [Google Scholar] [CrossRef]
- Nakhaei, H.; Mogharnasi, M.; Fanaei, H. Effect of swimming training on levels of asprosin, lipid profile, glucose and insulin resistance in rats with metabolic syndrome. Obes. Med. 2019, 15, 100111. [Google Scholar] [CrossRef]
- Farman, M.; Ghaffar, K. The Impact of Diet and Exercise on Diabetic Patients. J. Med. Biol. 2020, 2, 25–30. [Google Scholar]
- Roy, A.; Parker, R.S. Dynamic modeling of exercise effects on plasma glucose and insulin levels. J. Diabetes Sci. Technol. 2007, 1, 338–347. [Google Scholar] [CrossRef]
- Gupta, D.; Krueger, C.B.; Lastra, G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr. Diabetes Rev. 2012, 8, 76–83. [Google Scholar] [CrossRef]
- Che, D.N.; Kang, H.J.; Cho, B.O.; Shin, J.Y.; Jang, S.I. Combined effects of Diospyros lotus leaf and grape stalk extract in high-fat-diet-induced obesity in mice. Food Sci. Biotechnol. 2019, 28, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.; Al, A.; Faruk, M.; Rahman, M.; Nahar, K.; Kabir, F.; Alam, M.A.; Subhan, N. High carbohydrate high fat diet induced hepatic steatosis and dyslipidemia were ameliorated by Psidium guajava leaf powder supplementation in rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 1897237. [Google Scholar] [CrossRef]
- Sifat, N.; Zihad, S.N.K.; Lovely, F.; Rouf, R.; Shajib, G.M.A.; Alam, M.A.; Shilpi, J.A.; Uddin, S.J. Supplementation of Heliotropium indicum Linn attenuates obesity and associated metabolic disorders in high-carbohydrate-high-fat diet-induced obese rats. J. Food Biochem. 2020, 44, e13444. [Google Scholar] [CrossRef]
- Chang, F.-Y.; Ranganathan, A.; Li, B.; Bernstein, P.S. Effects of Carotenoid Supplementation on the Lipid Profile of the Serum of a Transgenic Mouse. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3880–A0082. [Google Scholar]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Mol. Nutr. Food Res. 2012, 56, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Elmas, M.A.; Cakıcı, S.E.; Dur, I.R.; Kozluca, I.; Arınc, M.; Binbuga, B.; Ozakpınar, O.B.; Kolgazi, M.; Sener, G.; Ercan, F. Protective effects of exercise on heart and aorta in high-fat diet-induced obese rats. Tissue Cell 2019, 57, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, D. Efectos del ejercicio aeróbico sobre lípidos y lipoproteínas. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.d.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019, 12, 1027. [Google Scholar] [CrossRef]
- Bradley, R.L.; Cleveland, K.A.; Cheatham, B. The adipocyte as a secretory organ: Mechanisms of vesicle transport and secretory pathways. Recent Prog. Horm. Res. 2001, 56, 329–358. [Google Scholar] [CrossRef]
- Klaus, S. Adipose tissue as a regulator of energy balance. Curr. Drug Targets 2004, 5, 241–250. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.S.M.; Sarhat, E.R.; Wadi, S.A. Effects of Extracted Phenolic Compounds from Grape Seeds on Leptin, Adiponectin and Resistin Levels in Rats Fed with High Fat Foods. Tikrit J. Pure Sci. 2018, 23, 75–77. [Google Scholar] [CrossRef]
- Friedman, J. The long road to leptin. J. Clin. Investig. 2016, 126, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Musovic, S.; Shrestha, M.M.; Komai, A.M.; Olofsson, C.S. Resistin is co-secreted with adiponectin in white mouse adipocytes. Biochem. Biophys. Res. Commun. 2021, 534, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Resistin in rodents and humans. Diabetes Metab. J. 2013, 37, 404–414. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: A population-based study. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef] [PubMed]
- Kilany, O.E.; Abdelrazek, H.M.; Aldayel, T.S.; Abdo, S.; Mahmoud, M.M. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J. Biol. Sci. 2020, 27, 2733–2746. [Google Scholar] [CrossRef]
- Riaz, M.; Ahmad, R.; Zia-Ul-Haq, M. Carotenoids as Antiobesity Agents. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 569–584. [Google Scholar]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Ogasawara, J.; Kizaki, T.; Sato, S.; Ishibashi, Y.; Takahashi, M.; Kobayashi, O.; Oh-Ishi, S.; Nagasawa, J.; Takahashi, K. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int. J. Endocrinol. 2013, 2013, 801743. [Google Scholar] [CrossRef] [PubMed]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and regulation of adipokine and myokine production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- So, B.; Kim, H.-J.; Kim, J.; Song, W. Exercise-induced myokines in health and metabolic diseases. Integr. Med. Res. 2014, 3, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Agil, R.; Patterson, Z.R.; Mackay, H.; Abizaid, A.; Hosseinian, F. Triticale bran alkylresorcinols enhance resistance to oxidative stress in mice fed a high-fat diet. Foods 2016, 5, 5. [Google Scholar] [CrossRef]
- Annamalai, S.; Mohanam, L.; Raja, V.; Dev, A.; Prabhu, V. Antiobesity, antioxidant and hepatoprotective effects of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomed. Pharmacother. 2017, 93, 81–87. [Google Scholar] [CrossRef]
- El Ayed, M.; Kadri, S.; Smine, S.; Elkahoui, S.; Limam, F.; Aouani, E. Protective effects of grape seed and skin extract against high-fat-diet-induced lipotoxicity in rat lung. Lipids Health Dis. 2017, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Correia-Costa, L.; Sousa, T.; Morato, M.; Cosme, D.; Afonso, J.; Areias, J.C.; Schaefer, F.; Guerra, A.; Afonso, A.C.; Azevedo, A. Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. Br. J. Nutr. 2016, 116, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The hypoglycemic, hypolipidemic, and anti-diabetic nephritic activities of zeaxanthin in diet-streptozotocin-induced diabetic Sprague dawley rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, O.; Yfanti, C. Antioxidants in Athlete’s Basic Nutrition: Considerations towards a Guideline for the Intake of Vitamin C and Vitamin E; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Despres, J.P.; Pouliot, M.C.; Moorjani, S.; Nadeau, A.; Tremblay, A.; Lupien, P.; Theriault, G.; Bouchard, C. Loss of abdominal fat and metabolic response to exercise training in obese women. Am. J. Physiol.-Endocrinol. Metab. 1991, 261, E159–E167. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Ai, L.; Wang, B.; Wang, L.; Gan, Y.; Liu, C.; Jensen, J.; Zhou, Y. Eccentric exercise and dietary restriction inhibits M1 macrophage polarization activated by high-fat diet-induced obesity. Life Sci. 2020, 243, 117246. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Shuman, W.P.; Larson, V.; Cain, K.C.; Fellingham, G.W.; Beard, J.C.; Kahn, S.E.; Stratton, J.R.; Cerqueira, M.D.; Abrass, I.B. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism 1991, 40, 545–551. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).