Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Bread Crust
2.3. RNA Extraction
2.4. Microarray Analyses
3. Results
3.1. Differentially Expressed RNAs of Control- vs. BC-Fed Mice
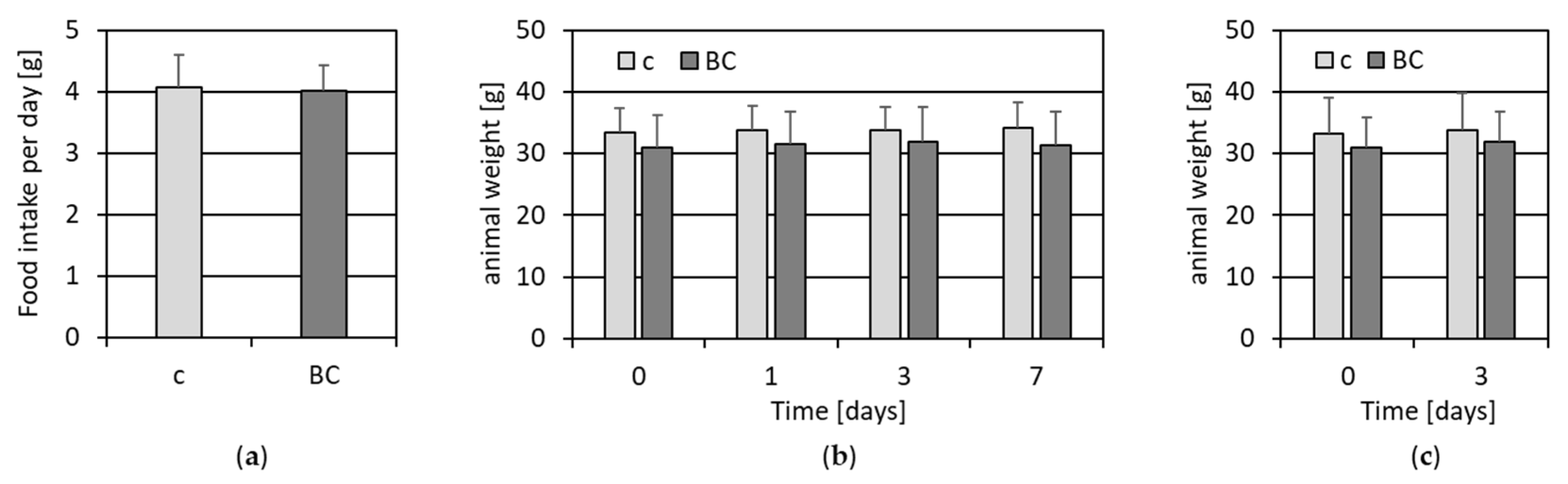
3.1.1. Food Intake and Animal Weight
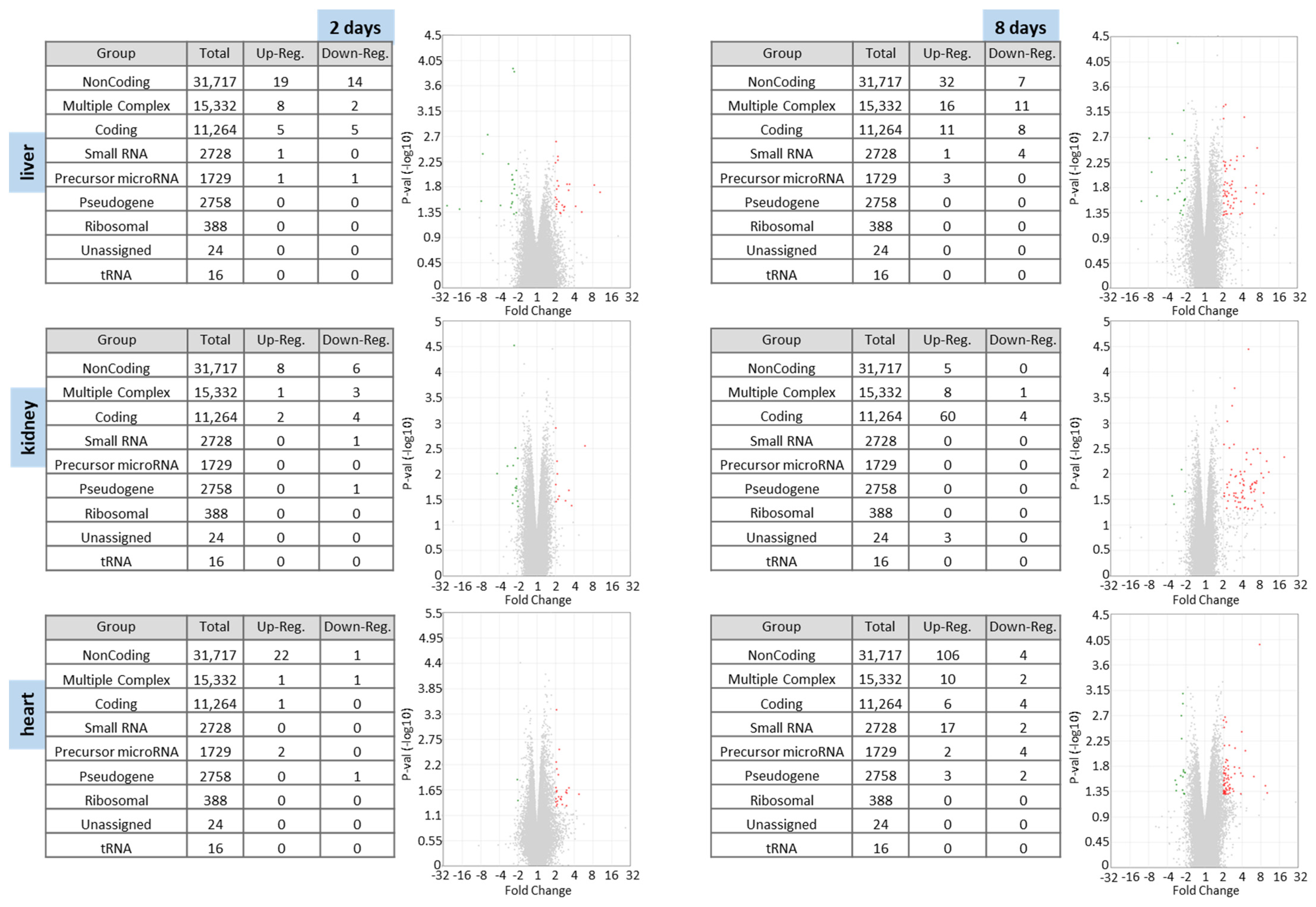
3.1.2. Microarray Analysis
Differentially Expressed RNAs in Liver, Kidney and Heart
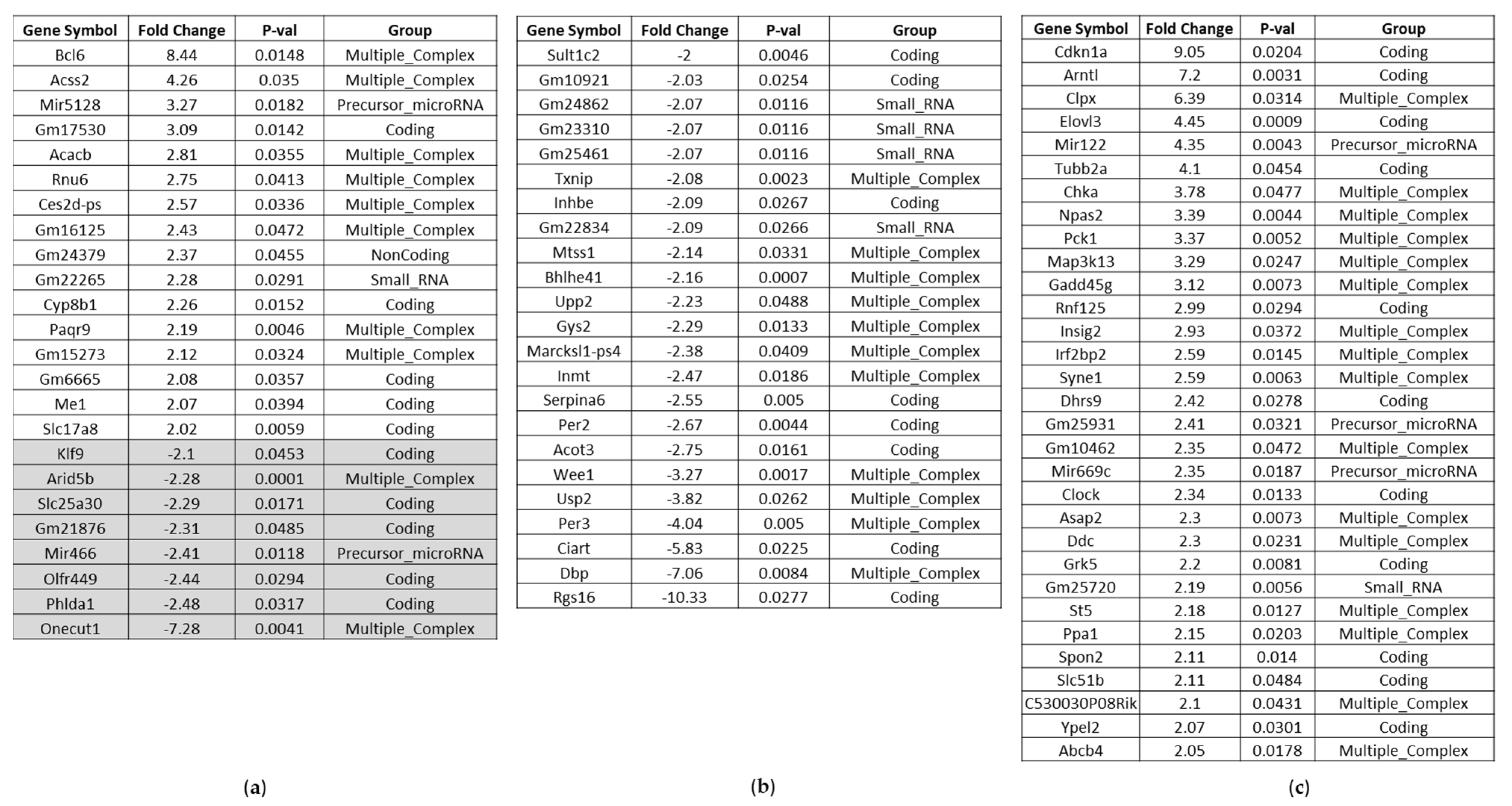
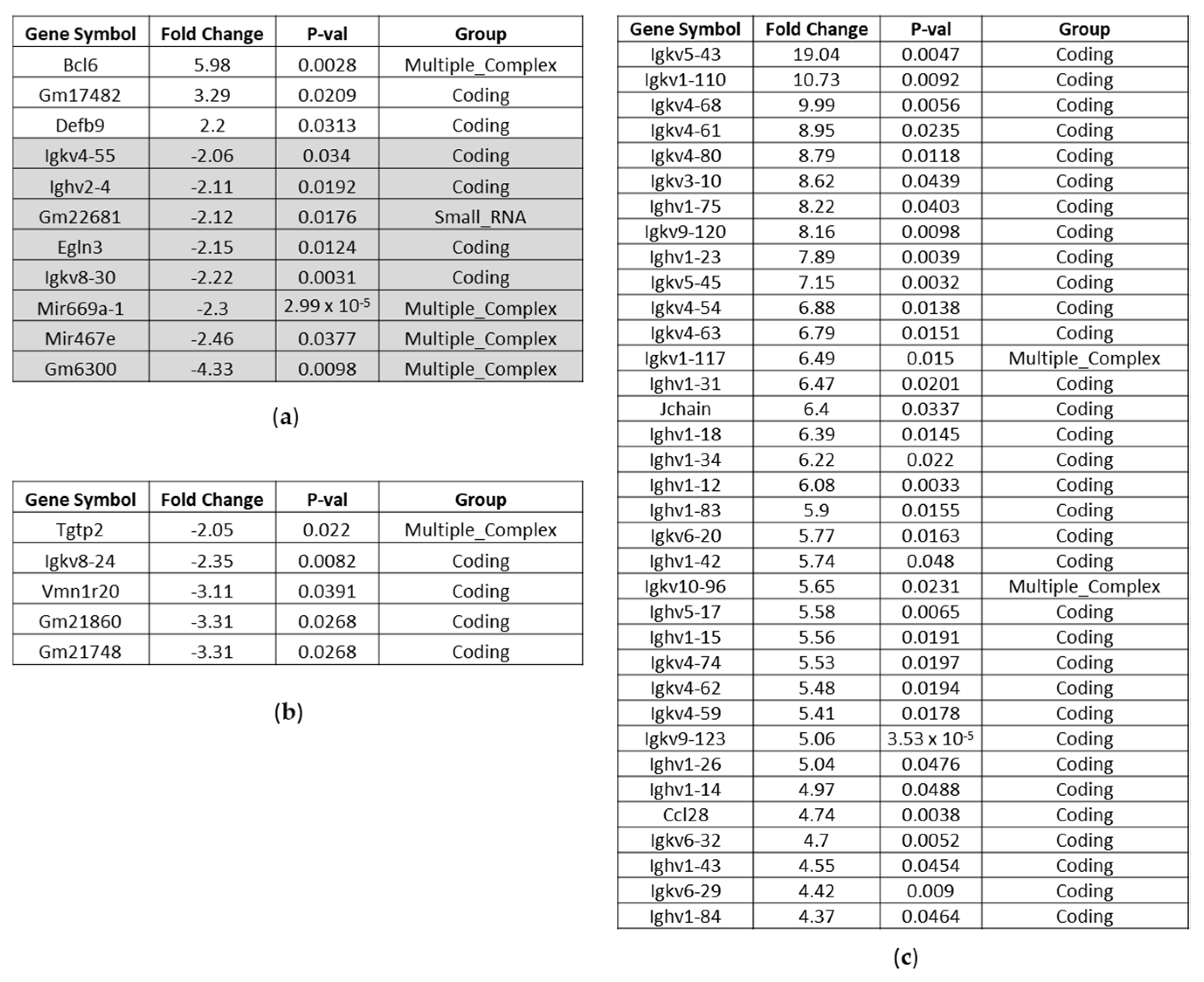
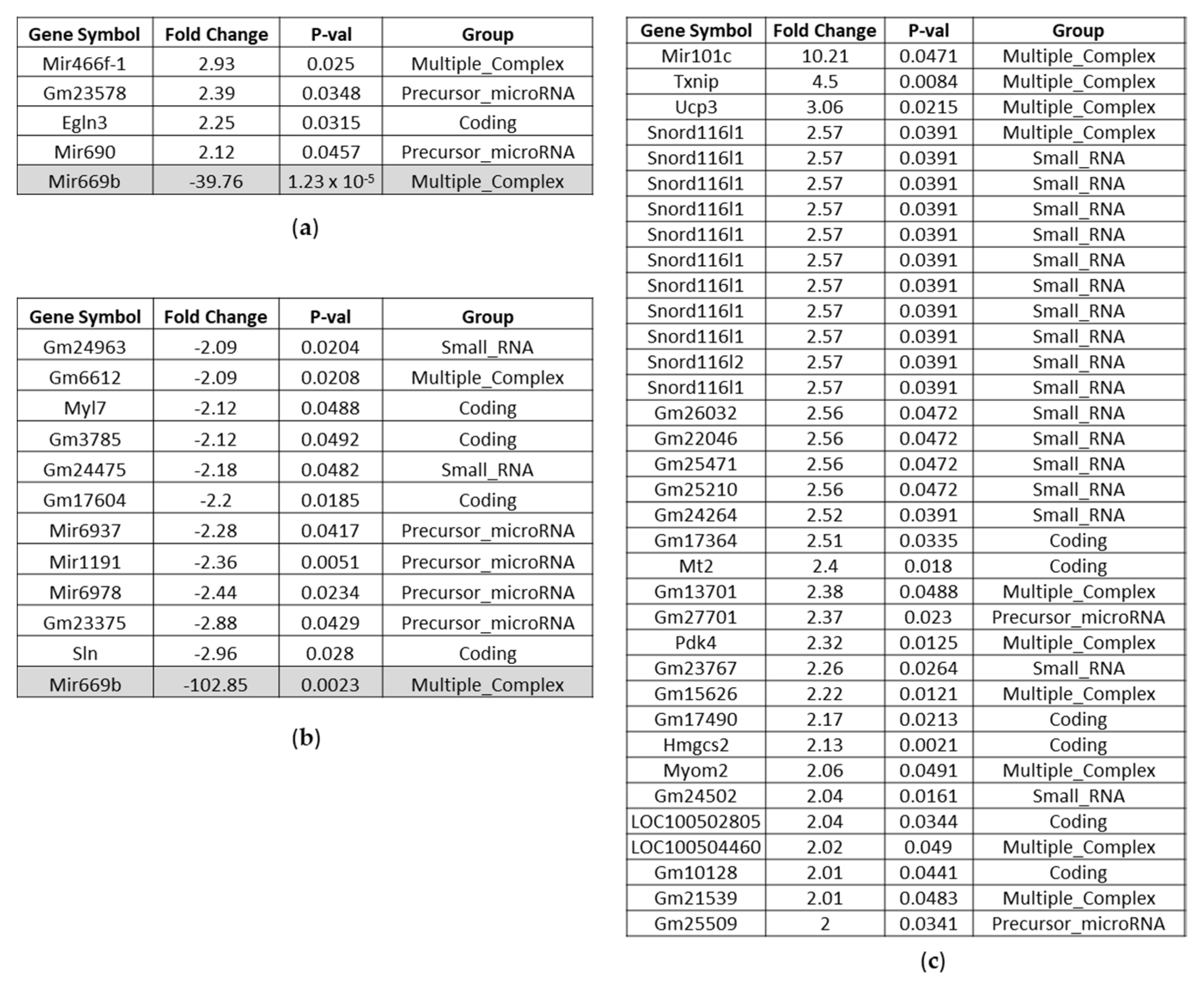
Affected Genes in the Liver of BC- vs. Control-Fed Mice
Affected Genes in the Kidney of BC- vs. Control-Fed Mice
Affected Genes in the Heart of BC- vs. Control-Fed Mice
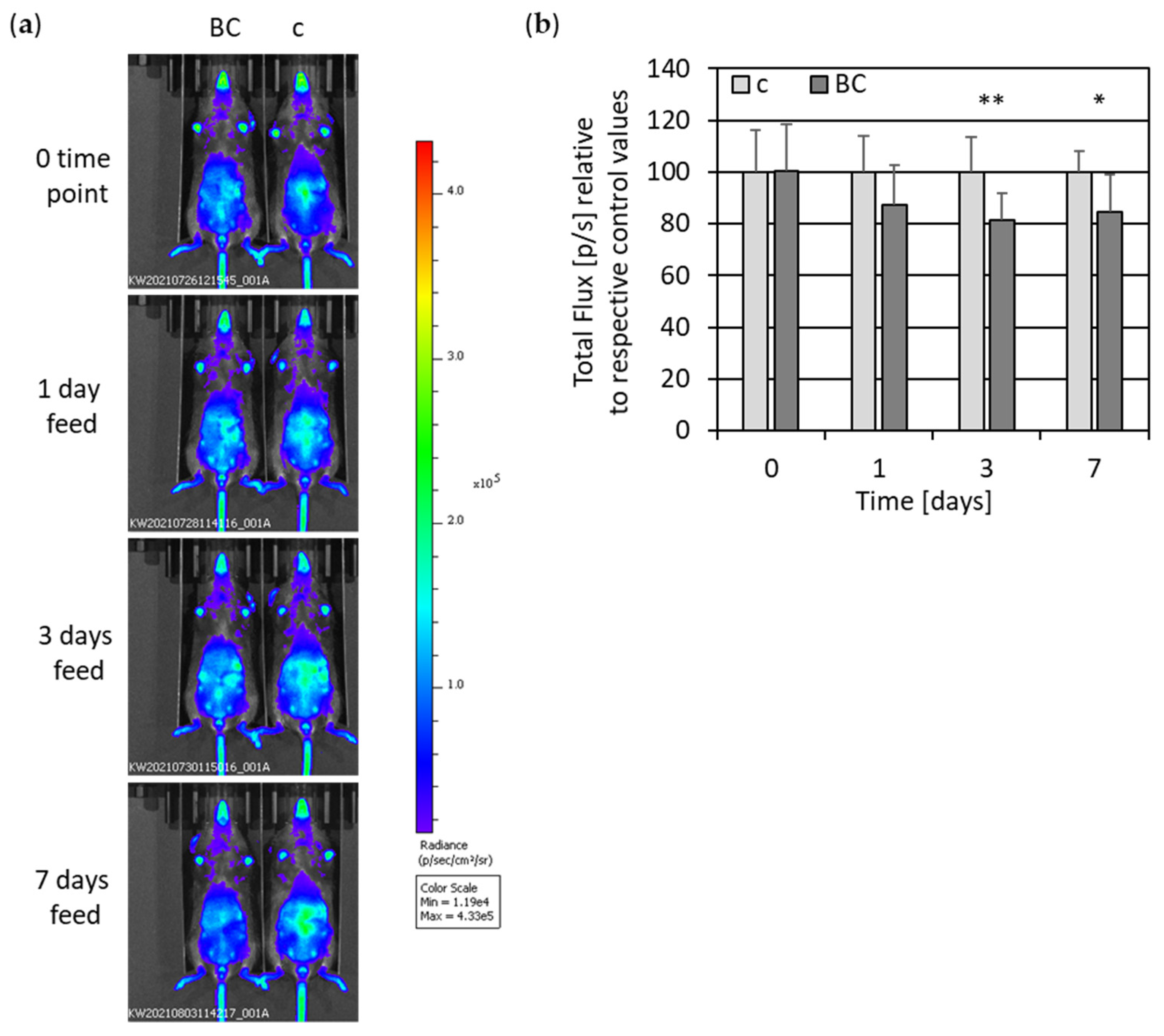
3.2. In Vivo Imaging of NF-κB-Luciferase-Reporter Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| AVG | average |
| ARE | antioxidant response element |
| Arntl | aryl hydrocarbon receptor nuclear translocator-like |
| BC | bread crust |
| Bhlhe41 | basic helix-loop-helix family, member e41 |
| Bmal1 | basic helix-loop-helix ARNT like 1 |
| Cdkn1a | cyclin-dependent kinase inhibitor 1A |
| Chka | choline kinase alpha |
| Ciart | circadian associated repressor of transcription |
| Clock | circadian locomotor output cycles kaput |
| Cyp8b1 | cytochrome P450, family 8, subfamily b, polypeptide 1 |
| Dbp | D site albumin promoter binding protein |
| Egln3 | egl-9 family hypoxia-inducible factor 3 |
| Elovl3 | elongation of very long chain fatty acids |
| Gadd45g | growth arrest and DNA-damage-inducible 45 gamma |
| Gclc | glutamate-cysteine ligase catalytic subunit |
| HMOX1 | heme oxygenase 1 |
| IL | Interleukin |
| Insig2 | insulin induced gene 2 |
| Map3k13 | mitogen-activated protein kinase kinase kinase 13 |
| MNSOD | superoxide dismutase 2 |
| Mtss1 | MTSS I-BAR domain containing 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NLRP3 | NLR family pyrin domain containing 3 |
| Npas2 | neuronal PAS domain protein 2 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| NRF2 | nuclear factor E2-related factor 2 |
| NF-κB | nuclear factor kappa B |
| PBS | phosphate-buffered saline |
| Per2, 3 | period circadian clock 2 |
| Phlda1 | pleckstrin homology like domain, family A, member 1 |
| P65 | NF-kB subunit |
| Rgs16 | regulator of G-protein signaling 16 |
| RIN | RNA integrity number |
| Rnf125 | RING finger protein 125 |
| ROS | reactive oxygen species |
| Syne1 | spectrin repeat containing, nuclear envelope protein 1 |
| TNF | tumor necrosis factor a |
| TRAF6 | TNF receptor associated factor 6 |
| TRIF | Toll-like receptor adaptor molecule 1 |
| Txnip | thioredoxin interacting protein |
| Usp2 | ubiquitin specific peptidase 2 |
References
- Simm, A. Protein glycation during aging and in cardiovascular disease. J. Proteom. 2013, 92, 248–259. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeier, M.; Faist, V.; Hofmann, T. Structural and Functional Characterization of Pronyl-lysine, a Novel Protein Modification in Bread Crust Melanoidins Showing in Vitro Antioxidative and Phase I/II Enzyme Modulating Activity. J. Agric. Food Chem. 2002, 50, 6997–7006. [Google Scholar] [CrossRef] [PubMed]
- Somoza, V.; Wenzel, E.; Lindenmeier, M.; Grothe, D.; Erbersdobler, H.F.; Hofmann, T. Influence of Feeding Malt, Bread Crust, and a Pronylated Protein on the Activity of Chemopreventive Enzymes and Antioxidative Defense Parameters in Vivo. J. Agric. Food Chem. 2005, 53, 8176–8182. [Google Scholar] [CrossRef] [PubMed]
- Ruhs, S.; Nass, N.; Bartling, B.; Brömme, H.-J.; Leuner, B.; Somoza, V.; Friess, U.; Silber, R.-E.; Simm, A. Preconditioning with Maillard reaction products improves antioxidant defence leading to increased stress tolerance in cardiac cells. Exp. Gerontol. 2010, 45, 752–762. [Google Scholar] [CrossRef]
- Bartling, B.; Hofmann, H.-S.; Sohst, A.; Hatzky, Y.; Somoza, V.; Silber, R.-E.; Simm, A. Prognostic Potential and Tumor Growth-Inhibiting Effect of Plasma Advanced Glycation End Products in Non-Small Cell Lung Carcinoma. Mol. Med. 2011, 17, 980–989. [Google Scholar] [CrossRef]
- Pastoriza, S.; Roncero-Ramos, I.; Rufián-Henares, J.; Delgado-Andrade, C. Antioxidant balance after long-term consumption of standard diets including bread crust glycated compounds by adult rats. Food Res. Int. 2014, 64, 106–113. [Google Scholar] [CrossRef]
- Wächter, K.; Santos, A.N.; Großkopf, A.; Baldensperger, T.; Glomb, M.A.; Szabó, G.; Simm, A. AGE-Rich Bread Crust Extract Boosts Oxidative Stress Interception via Stimulation of the NRF2 Pathway. Nutrients 2021, 13, 3874. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Schwär, M.; Loganathan, S.; Wächter, K.; Georgevici, A.-I.; Kraft, P.; Mayer, T.; Simm, A.; Karck, M.; Szabó, G. Bread crust extract protects rats’ vascular grafts from in vitro ischemia/reperfusion injury. J. Funct. Foods 2022, 95, 105187. [Google Scholar] [CrossRef]
- Herwig, R.; Hardt, C.; Lienhard, M.; Kamburov, A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat. Protoc. 2016, 11, 1889–1907. [Google Scholar] [CrossRef]
- Chen, D.; Zang, Y.-H.; Qiu, Y.; Zhang, F.; Chen, A.-D.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. BCL6 Attenuates Proliferation and Oxidative Stress of Vascular Smooth Muscle Cells in Hypertension. Oxidative Med. Cell. Longev. 2019, 2019, 5018410. [Google Scholar] [CrossRef]
- Han, C.; Yan, P.; He, T.; Cheng, J.; Zheng, W.; Zheng, L.-T.; Zhen, X. PHLDA1 promotes microglia-mediated neuroinflammation via regulating K63-linked ubiquitination of TRAF6. Brain Behav. Immun. 2020, 88, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Huang, Z.-T.; Zong, K.-Z.; Cao, Z.-R.; Peng, D.-D.; Zhou, B.-Y.; Shen, A.; Yan, P.; Wu, Z.-J. miR-194 ameliorates hepatic ischemia/reperfusion injury via targeting PHLDA1 in a TRAF6-dependent manner. Int. Immunopharmacol. 2021, 96, 107604. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tu, S.; Lin, G.; Guo, H.; Yan, C.; Liu, Q.; Huang, L.; Tang, N.; Xiao, Y.; Pope, R.M.; et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J. Exp. Med. 2020, 217, e20182091. [Google Scholar] [CrossRef] [PubMed]
- Kolosowska, N.; Gotkiewicz, M.; Dhungana, H.; Giudice, L.; Giugno, R.; Box, D.; Huuskonen, M.T.; Korhonen, P.; Scoyni, F.; Kanninen, K.M.; et al. Intracerebral overexpression of miR-669c is protective in mouse ischemic stroke model by targeting MyD88 and inducing alternative microglial/macrophage activation. J. Neuroinflammation 2020, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Yuan, F.; Zhao, Z.; Zhang, B.; Zhang, J.; Li, W.; Liu, T. MiR-669b-3p regulates CD4(+) T cell function by down-regulating indoleamine-2, 3-dioxygenase. Transpl. Immunol. 2020, 62, 101320. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, N.; Cai, Z.; Lin, Q.; Wang, Z.; Zhangjing, W.; Wu, S.; Li, H. Paraquat and MPTP induce neurodegeneration and alteration in the expression profile of microRNAs: The role of transcription factor Nrf2. npj Park. Dis. 2017, 3, 31. [Google Scholar] [CrossRef]
- Tong, J.; Yu, F.-c.; Li, Y.; Wei, Q.; Li, C.; Zhang, P.; Zhang, G. Prolyl 4-Hydroxylase Domain Protein 3-Inhibited Smooth-Muscle-Cell Dedifferentiation Improves Cardiac Peri-vascular Fibrosis Induced by Obstructive Sleep Apnea. Biomed. Res. Int. 2019, 2019, 9174218. [Google Scholar] [CrossRef]
- Anedda, A.; López-Bernardo, E.; Acosta-Iborra, B.; Suleiman, M.S.; Landázuri, M.O.; Cadenas, S. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radic. Biol. Med. 2013, 61, 395–407. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Kuang, F.; Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Xie, Y.; Tang, D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021, 34, 108767. [Google Scholar] [CrossRef]
- Kang, Y.J. The Antioxidant Function of Metallothionein in the Heart. Proc. Soc. Exp. Boil. Med. 1999, 222, 263–273. [Google Scholar] [CrossRef]
- Sato, M.; Kondoh, M. Recent Studies on Metallothionein: Protection Against Toxicity of Heavy Metals and Oxygen Free Radicals. Tohoku J. Exp. Med. 2002, 196, 9–22. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Vašák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta—Protein Struct. Mol. Enzym. 1985, 827, 36–44. [Google Scholar] [CrossRef]
- Kumari, M.R.; Hiramatsu, M.; Ebadi, M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic. Res. 1998, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.-I.; Takano, H.; Shimada, A.; Satoh, M. Metallothionein as an Anti-Inflammatory Mediator. Mediat. Inflamm. 2009, 2009, 101659. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Li, Y.; Sun, X.; Sun, X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothi-onein-overexpressing transgenic mice. Am. J. Pathol. 2003, 163, 1579–1586. [Google Scholar] [CrossRef]
- Duerr, G.D.; Dewald, D.; Schmitz, E.J.; Verfuerth, L.; Keppel, K.; Peigney, C.; Ghanem, A.; Welz, A.; Dewald, O. Metallothioneins 1 and 2 Modulate Inflammation and Support Remodeling in Ischemic Cardiomyopathy in Mice. Mediat. Inflamm. 2016, 2016, 7174127. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Q.; Han, X.Q.; Wang, Y.; Wang, X.; Zhu, Y.; Liu, N.F. Nepsilon-carboxymethyl-lysine promotes calcium deposition in VSMCs via intracellular oxidative stress-induced PDK4 activation and alters glucose metabolism. Oncotarget 2017, 8, 112841–112854. [Google Scholar] [CrossRef]
- Jost, T.; Henning, C.; Heymann, T.; Glomb, M.A. Comprehensive Analyses of Carbohydrates, 1,2-Dicarbonyl Compounds, and Advanced Glycation End Products in Industrial Bread Making. J. Agric. Food Chem. 2021, 69, 3720–3731. [Google Scholar] [CrossRef]
- Pötzsch, S.; Blankenhorn, A.; Navarrete Santos, A.; Silber, R.-E.; Somoza, V.; Simm, A. The effect of an AGE-rich dietary extract on the activation of NF-kappaB depends on the cell model used. Food. Funct. 2013, 4, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatog-raphy-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Treibmann, S.; Hellwig, A.; Hellwig, M.; Henle, T. Lysine-Derived Protein-Bound Heyns Compounds in Bakery Products. J. Agric. Food Chem. 2017, 65, 10562–10570. [Google Scholar] [CrossRef] [PubMed]
- Van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Oppenhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gas-trointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.; Liddell, J.R. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wirth, A.-K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, T.; Fukuda, T.; Miki, T.; Miura, O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemother-apeutic reagents in B-cell lymphoma cells. Oncogene 2003, 22, 4459–4468. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, M.; Li, Y.; Su, Z.; Wang, Y.; Chen, X.; Zhang, S.; Sun, W.; Kong, X. Bcl6 Knockdown aggravates hypoxia injury in cardiomyocytes via the P38 pathway. Cell Biol. Int. 2019, 43, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Zhang, M.S.; Tsang, F.H.C.; Bao, M.H.-R.; Xu, I.M.-J.; Lai, R.K.-H.; Chiu, D.K.-C.; Tse, A.P.-W.; Law, C.-T.; Chen, C.Y.-K.; et al. Adaptive and Constitutive Activations of Malic Enzymes Confer Liver Cancer Multilayered Protection Against Re-active Oxygen Species. Hepatology 2021, 74, 776–796. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Ju, H.-Q.; Liu, Z.X.; Chen, D.-L.; Wang, Y.; Zhao, Q.; Wu, Q.-N.; Zeng, Z.-L.; Qiu, H.-B.; Hu, P.-S.; et al. ME1 Regulates NADPH Homeostasis to Promote Gastric Cancer Growth and Metastasis. Cancer Res. 2018, 78, 1972–1985. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, N.; Haseeb, M.; Kim, M.; Choi, S. Role of Thioredoxin-Interacting Protein in Diseases and Its Therapeutic Outlook. Int. J. Mol. Sci. 2021, 22, 2754. [Google Scholar] [CrossRef]
- Metzig, M.; Nickles, D.; Falschlehner, C.; Lehmann-Hoch, J.; Straub, B.K.; Roth, W.; Boutros, M. An RNAi screen identifies USP2 as a factor required for TNF-alpha-induced NF-kappaB signaling. Int. J. Cancer 2011, 129, 607–618. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wang, Y.-P.; Zhang, X.; Li, G.-X.; Zheng, K.; Duan, C.-Z. lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after intracerebral hemorrhage by targeting miR-709 in mice. Brain Res. Bull. 2020, 162, 20–29. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Z.; Wang, X.-J.; Jiang, T.; Huang, Z.; Fang, D.; Zhang, D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell 2009, 34, 663–673. [Google Scholar] [CrossRef]
- Nakano-Kobayashi, A.; Fukumoto, A.; Morizane, A.; Nguyen, D.T.; Le, T.M.; Hashida, K.; Hosoya, T.; Takahashi, R.; Takahashi, J.; Hori, O. Therapeutics potentiating microglial p21-Nrf2 axis can rescue neurodegeneration caused by neu-roinflammation. Sci. Adv. 2020, 6, abc1428. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hou, F.; Liang, M.; Wang, G.; Zhang, X.; Li, H.; Xie, D.; Tian, J.; Liu, Z. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007, 71, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; He, C.; Hattori, M.; McEvoy, R.; Zheng, F.; Vlassara, H. Fetal or Neonatal Low-Glycotoxin Environment Prevents Autoimmune Diabetes in NOD Mice. Diabetes 2003, 52, 1441–1448. [Google Scholar] [CrossRef]
- Pouillart, P.; Mauprivez, H.; Ait-Ameur, L.; Cayzeele, A.; Lecerf, J.-M.; Tessier, F.J.; Birlouez-Aragon, I. Strategy for the study of the health impact of dietary Maillard products in clinical studies: The example of the ICARE clinical study on healthy adults. Ann. N. Y. Acad. Sci. 2008, 1126, 173–176. [Google Scholar] [CrossRef] [PubMed]



| Grain | Baking Time | Top Heat | Bottom Heat |
|---|---|---|---|
| rye | 108 min | 250 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wächter, K.; Gohde, B.; Szabó, G.; Simm, A. Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo. Nutrients 2022, 14, 4790. https://doi.org/10.3390/nu14224790
Wächter K, Gohde B, Szabó G, Simm A. Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo. Nutrients. 2022; 14(22):4790. https://doi.org/10.3390/nu14224790
Chicago/Turabian StyleWächter, Kristin, Birte Gohde, Gábor Szabó, and Andreas Simm. 2022. "Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo" Nutrients 14, no. 22: 4790. https://doi.org/10.3390/nu14224790
APA StyleWächter, K., Gohde, B., Szabó, G., & Simm, A. (2022). Rye Bread Crust as an Inducer of Antioxidant Genes and Suppressor of NF-κB Pathway In Vivo. Nutrients, 14(22), 4790. https://doi.org/10.3390/nu14224790





