Iron Supplementation in Pregnancy and Risk of Gestational Diabetes: A Narrative Review
Abstract
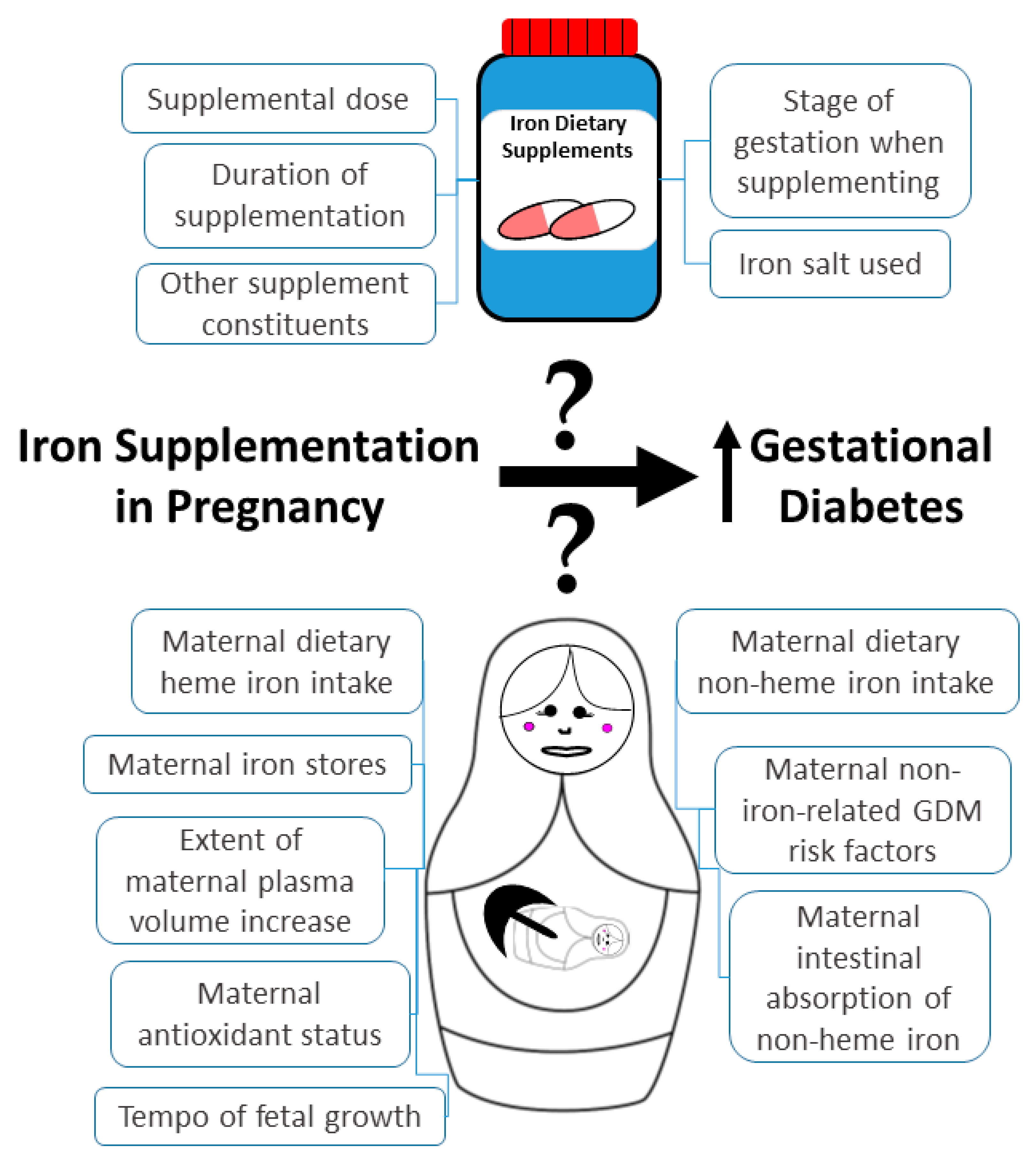
1. Introduction
2. Methods
3. Discussion
3.1. Studies Seeking Associations between Iron Supplementation in Pregnancy and GDM
3.1.1. Randomized Controlled Trials
3.1.2. Case–Control Studies
3.1.3. Cohort Studies
3.1.4. Systematic Reviews with Meta-Analyses
3.2. Potential Mechanisms of How Iron Supplementation in Pregnancy Could Affect GDM Risk
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Milman, N. Iron and pregnancy--a delicate balance. Ann. Hematol. 2006, 85, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Jin, L. Iron metabolism and ferroptosis in physiological and pathological pregnancy. Int. J. Mol. Sci. 2022, 23, 9395. [Google Scholar] [CrossRef]
- Scholl, T.O. Iron status during pregnancy: Setting the stage for mother and infant. Am. J. Clin. Nutr. 2005, 81, 1218S–1222S. [Google Scholar] [CrossRef]
- Brannon, P.M.; Taylor, C.L. Iron supplementation during pregnancy and infancy: Uncertainties and implications for research and policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Yan, H.; Wang, Z. Impact of iron supplementation on gestational diabetes mellitus: A literature review. Diabetes Obes. Metab. 2022, in press. [CrossRef]
- de Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; World Health Organization: Geneva, Switzerland, 2008; ISBN 9789241596657. [Google Scholar]
- Georgieff, M.K. Iron deficiency in pregnancy. AJOG 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Tiongco, R.E.; Arceo, E.; Clemente, B.; Pineda-Cortel, M.R. Association of maternal iron deficiency anemia with the risk of gestational diabetes mellitus: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 89–95. [Google Scholar] [CrossRef]
- Weinberg, E.D. Are iron supplements appropriate for iron replete pregnant women? Med. Hypotheses 2009, 73, 714–715. [Google Scholar] [CrossRef]
- Bencaiova, G.; Krafft, A.; Burkhardt, T.; Zimmermann, R. Hemoglobinopathies, body iron stores and gestational diabetes mellitus. Haematologica 2005, 90, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Cauza, E.; Hanusch-Enserer, U.; Bischof, M.; Spak, M.; Kostner, K.; Tammaa, A.; Dunky, A.; Ferenci, P. Increased C282Y heterozygosity in gestational diabetes. Fetal Diagn. Ther. 2005, 20, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.J.; Yao, A.; Chen, X.Y.; Liu, Y.; Ma, L.; Hou, Y.X. Associations of TMPRSS6 polymorphisms with gestational diabetes mellitus in Chinese Han pregnant women: A preliminary cohort study. Biol. Trace Elem. Res. 2021, 199, 473–481. [Google Scholar] [CrossRef]
- Mustafa, S.; Vukovich, T.; Prikoszovich, T.; Winzer, C.; Schneider, B.; Esterbauer, H.; Wagner, O.; Kautzky-Willer, A. Haptoglobin phenotype and gestational diabetes. Diabetes Care 2004, 27, 2103–2107. [Google Scholar] [CrossRef][Green Version]
- Khambalia, A.Z.; Aimone, A.; Nagubandi, P.; Roberts, C.L.; McElduff, A.; Morris, J.M.; Powell, K.L.; Tasevski, V.; Nassar, N. High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: A prospective study and systematic review. Diabetic Med. 2016, 33, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, F.; Zhou, J.; Liu, Z. The relationship between body iron status, iron intake and gestational diabetes: A systematic review and meta-analysis. Medicine 2016, 95, e2383. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cao, J.C.; Aranda, N.; Ribot, B.; Tous, M.; Arija, V. Elevated iron status and risk of gestational diabetes mellitus: A systematic review and meta-analysis. Matern. Child Nutr. 2017, 13, e12400. [Google Scholar] [CrossRef]
- Zhao, L.; Lian, J.; Tian, J.; Shen, Y.; Ping, Z.; Fang, X.; Min, J.; Wang, F. Dietary intake of heme iron and body iron status are associated with the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Asia Pac. J. Clin. Nutr. 2017, 26, 1092–1106. [Google Scholar] [CrossRef]
- Iqbal, S.; Ekmekcioglu, C. Maternal and neonatal outcomes related to iron supplementation or iron status: A summary of meta-analyses. J. Matern. Fetal Neonatal Med. 2019, 32, 1528–1540. [Google Scholar] [CrossRef]
- Durrani, L.; Ejaz, S.; Tavares, L.B.; Mohyeldin, M.; Abureesh, D.; Boorenie, M.; Khan, S. Correlation between high serum ferritin level and gestational diabetes: A systematic review. Cureus 2021, 13, e18990. [Google Scholar] [CrossRef]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef]
- Kataria, Y.; Wu, Y.; Horskjær, P.H.; Mandrup-Poulsen, T.; Ellervik, C. Iron status and gestational diabetes-a meta-analysis. Nutrients 2018, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The Role of Energy, Nutrients, Foods, and Dietary Patterns in the Development of Gestational Diabetes Mellitus: A Systematic Review of Observational Studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, C.; Gelaye, B.; Enquobahrie, D.A.; Frederick, I.O.; Williams, M.A. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 2011, 34, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Yeung, E.; Williams, M.A.; Qi, L.; Tobias, D.K.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 2011, 34, 1557–1563. [Google Scholar] [CrossRef]
- Chan, K.; Chan, B.; Lam, K.; Tam, S.; Lao, T. Iron supplement in pregnancy and development of gestational diabetes—a randomised placebo-controlled trial. BJOG 2009, 116, 789–798. [Google Scholar] [CrossRef]
- Ouladsahebmadarek, E.; Sayyah-Melli, M.; Taghavi, S.; Abbasalizadeh, S.; Seyedhejazie, M. The effect of supplemental iron elimination on pregnancy outcome. Pak. J. Med. Sci. 2011, 27, 641–645. [Google Scholar]
- Kinnunen, T.I.; Luoto, R.; Helin, A.; Hemminki, E. Supplemental iron intake and the risk of glucose intolerance in pregnancy: Re-analysis of a randomised controlled trial in Finland. Matern. Child Nutr. 2016, 12, 74–84. [Google Scholar] [CrossRef]
- Palma, S.; Perez-Iglesias, R.; Prieto, D.; Pardo, R.; Llorca, J.; Delgado-Rodriguez, M. Iron but not folic acid supplementation reduces the risk of low birthweight in pregnant women without anaemia: A case-control study. J. Epidemiol. Community Health 2008, 62, 120–124. [Google Scholar] [CrossRef]
- Bo, S.; Menato, G.; Villois, P.; Gambino, R.; Cassader, M.; Cotrino, I.; Cavallo-Perin, P. Iron supplementation and gestational diabetes in midpregnancy. AJOG 2009, 201, 158.e1–158.e6. [Google Scholar] [CrossRef]
- Jirakittidul, P.; Sirichotiyakul, S.; Ruengorn, C.; Siripenpong, S.; Imruetaicharoenchok, A.; Wiriyasirivaj, B. Iron supplementation in non-anemic pregnancy and risk of developing gestational diabetes mellitus. J. Endocrinol. Metab. 2018, 8, 139–143. [Google Scholar] [CrossRef]
- Liu, X.N.; Pang, J. A retrospective study of supplemental iron intake in singleton pregnancy women with risk of developing gestational diabetes mellitus. Medicine 2018, 97, e10819. [Google Scholar] [CrossRef] [PubMed]
- Javadian, P.; Alimohamadi, S.; Gharedaghi, M.H.; Hantoushzadeh, S. Gestational diabetes mellitus and iron supplement; effects on pregnancy outcome. Acta Med. Iran. 2014, 52, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Gungor, E.S.; Danisman, N.; Mollamahmutoglu, L. Maternal serum ferritin and hemoglobin values in patients with gestational diabetes mellitus. Saudi Med. J. 2007, 28, 478–480. [Google Scholar] [PubMed]
- Zhu, B.; Liang, C.; Xia, X.; Huang, K.; Yan, S.; Hao, J.; Zhu, P.; Gao, H.; Tao, F. Iron-related factors in early pregnancy and subsequent risk of gestational diabetes mellitus: The Ma’anshan Birth Cohort (MABC) Study. Biol. Trace Elem. Res. 2019, 191, 45–53. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Lan, X.; Zhang, J.; Wu, C.; Dong, H.; Yang, L.; Gao, Y.; Zhang, H.; Zeng, G. Effects of iron supplement intake on gestational diabetes mellitus in early and middle pregnancy in Chengdu City in 2017. Wei Sheng Yan Jiu 2020, 49, 227–232. [Google Scholar] [CrossRef]
- Si, S.; Shen, Y.; Xin, X.; Mo, M.; Shao, B.; Wang, S.; Luo, W.; Chen, Z.; Liu, H.; Chen, D.; et al. Hemoglobin concentration and iron supplement during pregnancy were associated with an increased risk of gestational diabetes mellitus. J. Diabetes 2021, 13, 211–221. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Zhong, C.; Huang, L.; Zhang, Y.; Chen, R.; Zhou, X.; Xu, S.; Li, Q.; Cui, W.; et al. Association between maternal plasma ferritin concentration, iron supplement use, and the risk of gestational diabetes: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 1100–1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Zhong, C.; Li, Q.; Wu, M.; Zhang, G.; Chen, R.; Liu, C.; Wu, J.; Huang, L.; et al. Periconceptional iron supplementation and risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Res. Clin. Pract. 2021, 176, 108853. [Google Scholar] [CrossRef]
- Petry, C.J.; Ong, K.K.; Hughes, I.A.; Dunger, D.B. Associations between maternal iron supplementation in pregnancy and changes in offspring size at birth reflect those of multiple micronutrient supplementation. Nutrients 2021, 13, 2480. [Google Scholar] [CrossRef]
- Moradi, M.; Noormohammadi, Z.; Daneshvar, M.; Basirat, V.; Petry, C.J.; Daneshzad, E. Iron supplementation during pregnancy and risk of gestational diabetes mellitus: A systematic review and meta-analysis. Front. Glob. Womens Health 2022, submitted.
- Zein, S.; Rachidi, S.; Hininger-Favier, I. Is oxidative stress induced by iron status associated with gestational diabetes mellitus? J. Trace Elem. Med. Biol. 2014, 28, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Han, H.; Yang, Z. Iron, oxidative stress and gestational diabetes. Nutrients 2014, 6, 3968–3980. [Google Scholar] [CrossRef] [PubMed]
- Lachili, B.; Hininger, I.; Faure, H.; Arnaud, J.; Richard, M.J.; Favier, A.; Roussel, A.M. Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol. Trace Elem. Res. 2001, 83, 103–110. [Google Scholar] [CrossRef]
- Marku, A.; Galli, A.; Marciani, P.; Dule, N.; Perego, C.; Castagna, M. Iron metabolism in pancreatic beta-cell function and dysfunction. Cells 2021, 10, 2841. [Google Scholar] [CrossRef]
- Rahier, J.; Loozen, S.; Goebbels, R.M.; Abrahem, M. The haemochromatotic human pancreas: A quantitative immunohistochemical and ultrastructural study. Diabetologia 1987, 30, 5–12. [Google Scholar] [CrossRef]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Q.; Lv, Y.; Song, X.; Qu, H.; Chen, Y. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr. Diabetes 2020, 10, 17. [Google Scholar] [CrossRef]
- Taylor, D.J.; Mallen, C.; McDougall, N.; Lind, T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. Br. J. Obstet. Gynaecol. 1982, 89, 1011–1017. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S. Dietary iron intake, iron status, and gestational diabetes. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1672S–1680S. [Google Scholar] [CrossRef] [PubMed]
| Date of Search | Database | Years Searched | Search Terms | Strings of Terms | # Hits |
|---|---|---|---|---|---|
| 1 September 2022 | Pubmed | 1966-08/2022 | iron, gestational diabetes | iron AND gestational diabetes | 238 |
| 1 September 2022 | Pubmed | 1966-08/2022 | iron supplementation, gestational diabetes | iron supplement* AND gestational diabetes | 85 |
| 1 September 2022 | Scopus | 1960-08/2022 | iron, gestational diabetes | iron AND gestational AND diabetes | 452 |
| 1 September 2022 | Scopus | 1960-08/2022 | iron supplementation, gestational diabetes | iron AND supplement* AND gestational AND diabetes | 163 |
| 1 September 2022 | Web of Science | 1900-08/2022 | iron, gestational diabetes | iron gestational diabetes | 352 |
| 1 September 2022 | Web of Science | 1900-08/2022 | iron supplementation, gestational diabetes | iron supplement* gestational diabetes | 133 |
| First Author, Year of Publication | Details | Key Results | Comments |
|---|---|---|---|
| Chan, 2009 [27] | Women with baseline hemoglobin concentrations of between 8 and 14 g/dL attending Hong Kong University Queen Mary Hospital, Hong Kong were randomly allocated to receive 60 mg of elemental iron supplement (as 300 mg ferrous sulphate) daily (n = 565) or placebo (n = 599) from their pregnancy hospital booking visit. There was no difference in baseline hemoglobin or ferritin levels, or between the ratio of ferritin to transferrin between the groups. Iron supplementation (or placebo) was administered for a further 16 weeks. | In the comparison of those that supplemented with iron against those that took the placebo, there was no significant difference in the incidence of GDM at 28 weeks’ gestation (~11% in both groups; odds ratio for GDM 1.04 (0.7–1.53), p = 0.9). | There was a relatively low general compliance and involvement in the follow-up. ~40% were lost to follow-up. Maternal ferritin (p = 0.003) and hemoglobin (p < 0.001) concentrations were higher in the iron supplement group at delivery. Offspring birth weight was increased ~100 g in iron-supplemented pregnancies (p = 0.001). |
| Ouladsahebmadarek, 2011 [28] | A double-blind-randomized clinical trial of 960 women attending Al Zahra Hospital in Isfahan, Iran, with singleton pregnancies in the first trimester who had not supplemented with iron in the preceding month and who had hemoglobin concentrations >12 g/dL. The supplemental group (n = 480) took 30 mg elemental iron/d plus an unspecified multivitamin preparation from 13 weeks of pregnancy until delivery. The control group (n = 480) took a placebo plus the multivitamin preparation. | In the comparison of those that supplemented with iron against those that took placebo, there was no significant difference in the incidence of GDM (0.5% vs. 0.8%, respectively; relative risk 0.67 (0.11–3.97), p = 0.7). | 85% of those that supplemented with iron and 78% of those who took placebo completed the course. GDM was recorded by a questionnaire, not from hospital notes or by performing oral glucose tolerance tests. |
| Kinnunen, 2016 [29] | Women from Tampere and five neighboring municipalities in Southern Finland were randomly allocated to receive 100 mg iron supplement daily (n = 1336; “routine” group) throughout pregnancy (regardless of their hemoglobin level) or no supplementation (n = 1358) (unless they were diagnosed with anemia (“selective” group), in which case they were supplemented with 100 mg/d (as two doses of 50 mg iron) just until their hemoglobin level increased to 11 g/dL). | In the comparison of those that routinely supplemented with iron against those that either did not or only did so selectively, there was no difference in the incidence of a composite variable related to glucose intolerance (GDM, glycosuria, and large-for-gestational-age baby): 11.0% v. 13.0% (p = 0.1), respectively. | This was a reanalysis of an original trial where GDM was not a planned outcome and therefore was not assessed systematically in all participants. Instead, GDM, glycosuria, and/or large-for-gestational-age records were abstracted from hospital records and combined into a composite variable. Few participants were overweight or obese. |
| First Author, Year of Publication | Details | Key Results | Comments |
|---|---|---|---|
| Palma, 2008 [30] | A retrospective study of 1256 pregnant women without anemia (322 who delivered a low-birth-weight baby; 934 who delivered non-low birth-weight-baby at term), attending the Marqués de Valdecilla University Hospital, Santander, Spain. Information about iron and other supplements was obtained from personal interviews and prenatal care records. A total of 91.0% of the women supplemented with iron, mainly with a daily dose of 80 mg ferrous sulfate as a single supplement. No details of baseline iron statuses were presented, although none of the women were anemic. Three sources of data for GDM were used: personal interviews (carried out within three days after delivery), clinical charts, and prenatal care records. | In the comparison of those that supplemented with iron against those that did not, no association was found between iron supplementation and GDM (4.6% of women who supplemented with iron developed GDM vs. 7.1% of women who did not supplement with iron developed GDM; risk ratio 0.65 (0.32–1.34), p = 0.2). | A woman was considered to have supplemented with iron if they took it for at least one week in pregnancy. No account of iron supplemental dose or duration (other than the above) was taken. Maternal iron supplementation was associated with a lower risk of low birth weight (odds ratio 0.58 (0.34–0.98), p < 0.05). |
| Bo, 2009 [31] | A study of 500 consecutive pregnant women with GDM and 500 normoglycemic women attending the Unit of Obstetrician and Gynecology of the University of Turin, Italy. Iron supplementation data were collected by interviewing. A woman was considered to have supplemented with iron if she did so for at least 2 weeks. Most (95.5%) of the 212 women that supplemented with iron (and no other micronutrients) in mid-pregnancy did so with a daily dose of 525 mg ferrous sulfate (equivalent to 105 mg elemental iron). No details were presented about baseline iron statuses or anemia. | In the comparison of those that supplemented with iron against those that did not, self-reported iron supplementation in pregnancy was associated with a higher prevalence of GDM (70.8% v. 44.4% (p < 0.001), respectively; unadjusted odds ratio 3.03 (2.18–4.20), p < 0.001; odds ratio adjusted for confounders 3.36 (1.50–7.53), p = 0.003). The duration of iron supplementation ranged from 2 to 10 weeks (with a median of 5 weeks). Only one participant was supplementing with iron prior to conception (and sensitivity analyses removing them showed no significant difference in results). | Iron supplementation in pregnancy was also significantly positively associated with increased insulin resistance and hyperglycemia, and the prevalence of the metabolic syndrome. |
| Jirakittidul, 2018 [32] | A retrospective study of pregnant women attending Vajira Hospital, Bangkok, Thailand for routine antenatal care. The early iron supplementation group (n = 966) was non-anemic women who started taking oral iron supplements (200 mg of ferrous fumarate; classified from hospital records) prior to 16 completed weeks of gestation, while the “control” group (n = 969) was those non-anemic women who started supplementing with iron later on in pregnancy. There was no difference in baseline hemoglobin levels between the groups. | In the comparison of those that supplemented with iron early against those that supplemented late, those that supplemented early had a higher prevalence of GDM (9.7% vs. 5.6%, respectively; risk ratio 1.83 (1.29–2.59), p < 0.001). | In this study, the control group consisted of women who supplemented their diets with iron, but only after 16 weeks’ gestation. There was no group where the women did not supplement their diets with iron. |
| Liu, 2018 [33] | A retrospective study of 259 women with singleton pregnancies and hemoglobin levels between 8–14 g/dL attending The People’s Hospital of Yan’an, and Affiliated Hospital of Yan’an University, Yan’an, China. The supplementary group (n = 135) took 300 mg iron/d from prior to 16 weeks’ gestation until delivery. The control group did not supplement their diets with iron. | In the comparison of those that supplemented with iron against those that did not, there was no significant difference in the prevalence of GDM (7.4% vs. 7.3%, respectively; risk ratio 1.02 (0.43–2.43), p = 1.0). | There were no reports of either the method of participant recruitment or how iron supplemental data were captured. Iron supplementation was associated with increased maternal hemoglobin levels at delivery and increased offspring birth weight. |
| First Author, Year of Publication | Details | Key Results | Comments |
|---|---|---|---|
| Bowers, 2011 [26] | 13,475 pregnancies (which took place from 1991 to 2001) from the Nurses’ Health Study II (a prospective study of female nurses in the U.S.A., aged 22–44 at recruitment in 1989). No information was presented about baseline iron statuses. | In the comparison of no iron supplementation in pregnancy against other quintiles of iron supplementation dose in pregnancy, no significant associations were observed with GDM risk (age-adjusted risk ratios 0.80~0.99 (0.62~1.04), p_trend = 0.6) (multivariate-adjusted risk ratios 0.86~1.04 (0.68~1.28), p_trend = 0.97). | For those women that supplemented their diets with iron, no restriction was placed on when iron supplementation started, or on its duration. It was not recorded whether the supplemental iron was part of a multiple micronutrient preparation or not. Analysis was of supplemental iron groups (0, ~5.1, ~15, ~30, and ~60 mg iron/d). GDM was self-reported. |
| Zhu, 2019 [37] | 3289 pregnant women from the Ma’anshan Birth Cohort, conducted in Ma’anshan city of Anhui province in China. No information was presented about baseline iron statuses. | Pre-pregnancy iron supplementation was associated with an increased risk of GDM (supplemented 18.4% vs. non-supplemented 12.6%; risk ratio 1.45 (1.06–1.97), p = 0.02). No significant associations between iron supplement use and risk of GDM in either first trimester (14.5% vs. 12.6%, respectively; risk ratio 1.16 (0.95–1.41), p = 0.1) or second trimester (14.5% vs. 13.0%, respectively; risk ratio 1.12 (0.87–1.43), p = 0.4) were observed, nor was there an interaction with iron supplementation in the apparently U-shaped association between hemoglobin level and GDM incidence. | Iron supplementation was self-reported by questionnaire. Both sole iron supplements and multivitamin/mineral supplements that contain iron were included as “iron supplement use.” No account was taken of dose or total duration of iron supplementation. Iron supplements were consumed by 5.9% before pregnancy, 23.6% in the first trimester, and 13.2% in the second trimester. |
| Hao, 2020 [38] | Purposive sampling from 807 pregnancies from Chengdu City hospital in China. No information was presented about baseline iron statuses. | There was a positive association between the iron supplement dose and the occurrence of GDM in 739 women during the second trimester of pregnancy (odds ratio 1. 06 (1. 02–1.10), p < 0.05). Compared with the lower iron supplemental intake (<60 mg iron/d) women in mid-pregnancy, the odds ratio for GDM was 1.41 (1.02–1. 94) (p < 0.05) in the higher iron supplemental intake (≥60 mg iron/d) women. | There was no group analyzed where none of the women supplemented with iron. A total of 5% of the women supplemented with iron in the first trimester of pregnancy and 67.9% in the second trimester (~32% supplementing with ≥60 mg iron/d). |
| Si, 2021 [39] | 1118 pregnancies from the Zhoushan Pregnant Women Cohort based in Zhoushan Maternal and Child Care Hospital, China. Iron was supplemented in the form of iron polysaccharide complex capsules or ferrous succinate tablets of unreported dose. No information was presented about baseline iron statuses. | 223 of the women developed GDM, whereas 905 did not. First trimester iron supplementation was not associated with GDM risk (risk ratio 1.21 (0.93–1.55), p = 0.2). However, iron supplementation in the second trimester in non-anemic women was associated with increased GDM risk (risk ratio 1.34 (1.06–1.70), p = 0.01). | Although an account was taken of the trimester in which iron was supplemented, no account was taken of the dose or the total duration of supplementation. |
| Zhang, 2021 [40] | 2117 pregnancies from the Tongji Maternal and Child Health Cohort from Wuhan, China: the same cohort but different analysis as [41]. Iron supplements that were taken by the participants were most commonly in the form of ferrous fumarate (19.8 or 60 mg/d) or ferrous glycinate (5 mg/d). Iron statuses at baseline (~16 weeks gestation) were collected in terms of plasma ferritin concentrations. | Supplemental iron at a dose of ≥60 mg/d during the second trimester of pregnancy was associated with an increased risk of GDM compared with non-users (14.0% vs. 8.9%, respectively; unadjusted risk ratio 1.56 (1.16–2.11), p = 0.003; adjusted risk ratio 1.37 (1.02–1.84), p = 0.04). Participants with medium plasma ferritin concentrations and high supplemental iron intake (supplemental iron ≥60 mg/d) were associated with an increased risk of GDM (14.7% vs. 8.4% in women who did not supplement), similar to the participants within the highest quartile of plasma ferritin concentrations (≥90.0 ng/mL) in early pregnancy (14.3%). This was possibly due to higher intestinal iron absorption in those without the highest ferritin concentrations. Supplementing with lower doses of iron or for a shorter duration was not significantly associated with GDM risk (p > 0.05). | High-dose iron supplementation was defined as elemental iron ≥60 mg/d on ≥5 days/wk for at least 4 wk. Non-users were those who reported no intake of iron-containing supplements. |
| Zhang, 2021 [41] | 5101 pregnancies from the Tongji Maternal and Child Health Cohort from Wuhan, China: the same cohort but different analysis as [40]. Iron statuses at baseline (~16 weeks gestation) were collected in terms of hemoglobin concentrations. | The Incidence of GDM was significantly higher in women who supplemented with ≥30 mg iron/d for more than 3 months in pregnancy than in those that did not supplement with iron (15.2% vs. 8.9%, p < 0.001). The unadjusted risk ratio for GDM was 1.70 (1.40–2.07). After adjustment for confounders, the risk ratio was 1.55 (1.27–1.88). Supplementing with lower doses or for a shorter duration was not significantly associated with GDM risk (all p > 0.05). | Iron supplements included both iron-only supplements and multiple micronutrient preparations that contained iron. Those considered to supplement with iron were women who supplemented with iron more than 5 times per week on average. Iron supplementation (any dose) was associated with higher hemoglobin levels at delivery. |
| Petry, 2021 [42] | 677–868 pregnancies from the Cambridge Baby Growth Study recruited from the Rosie Maternity Hospital, Cambridge, U.K. | Around 61% of the pregnant women self-reported supplementing their diets with iron. In the comparison of those women that supplemented with iron against those that did not, maternal iron supplementation in pregnancy was associated with increased GDM risk (risk ratio 1.67 (1.01–2.77), p = 0.048, n = 677). | No details of baseline iron statuses are presented, although ~3% of the participants reported having anemia in pregnancy. For those women that supplemented their diets with iron, no restriction was placed on when iron supplementation started, or its dose or duration. The median duration of supplementation was ~34–36 weeks (starting when pregnancy was confirmed). Most women that supplemented (~90%) did so using multiple micronutrient preparations at a dose of ~28-34 mg iron/d. Associations with one of the secondary phenotypes (offspring subscapular skinfold thickness at birth) was still evident when data from women who used multiple micronutrient preparations were excluded. |
| First Author, Year of Publication | Details | Key Results | Comments |
|---|---|---|---|
| Khambalia, 2015 [16] | Included just two randomized controlled trials, neither of which found associations between iron supplementation and GDM. | There was no significant association between iron supplementation in pregnancy and risk of GDM. | Both trials assessed iron supplement use in early pregnancy and found no association with risk of GDM. The Chan et al. trial scored highly (92%) in the quality assessment [27] following careful design. The other trial [28] scored poorly in the quality assessment (58%). It collected GDM data from a questionnaire rather than from hospital notes or oral glucose tolerance tests, had considerable loss to follow-up, and did not report on the methods of randomization or blinding used. |
| Zhao, 2017 [19] | Included four published studies: one case–control study, one cohort study, and two randomized controlled trials. | There was no significant association between iron supplementation in pregnancy and GDM in the cohort studies (risk ratio 1.75 (0.56–5.47); I2 = 86.8%), nor was there one for the randomized control trials (risk ratio 0.88 (0.72–1.07); I2 = 0%). | No overall pooled result for associations between iron supplementation in pregnancy and risk of GDM was presented. |
| Kataria, 2018 [23] | Included four published studies: two case–control studies, one cohort study, and one randomized controlled trial. | Supplemental iron intake was not associated with risk of GDM (unadjusted odds ratio 1.20 (0.63–2.29), odds ratio adjusted for confounders 1.09 (0.73–1.63)) with significant heterogeneity (unadjusted I2 = 93.7%, p = 2.26 × 10−10; adjusted I2 = 82.7%, p = 0.003). | There was no evidence of publication bias. |
| Moradi, 2022 [43] | Included 10 published studies: 2 case–control, 1 retrospective, and 7 prospective cohort designs. | There was a significant positive association between iron supplementation in pregnancy and GDM (odds ratio 1.30 (1.10–1.55), p = 0.002) with significant heterogeneity (I2 = 87.6%, p < 0.0001). Subgroup analyses revealed a significant association between iron supplementation in pregnancy and risk of GDM in cohort studies (odds ratio 1.23 (1.06–1.43), p = 0.005) with significant heterogeneity (I2 = 82.6%, p < 0.0001). This association was not observed in case–control studies (odds ratio 1.45 (0.32–6.65), p = 0.6; heterogeneity I2 = 92.4%, p < 0.0001). | Randomized controlled trials were not included in the meta-analysis as they did not fit the inclusion criteria. Further subgroup analyses revealed a significant association in high-quality studies (odds ratio 1.32 (1.10–1.58), p = 0.002) (I2 = 89.2%, p < 0.0001), but not in fair-quality studies (odds ratio 1.08 (0.43–2.76), p = 0.9) (I2 = 75.7%, p = 0.04). There was no evidence of publication bias. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petry, C.J. Iron Supplementation in Pregnancy and Risk of Gestational Diabetes: A Narrative Review. Nutrients 2022, 14, 4791. https://doi.org/10.3390/nu14224791
Petry CJ. Iron Supplementation in Pregnancy and Risk of Gestational Diabetes: A Narrative Review. Nutrients. 2022; 14(22):4791. https://doi.org/10.3390/nu14224791
Chicago/Turabian StylePetry, Clive J. 2022. "Iron Supplementation in Pregnancy and Risk of Gestational Diabetes: A Narrative Review" Nutrients 14, no. 22: 4791. https://doi.org/10.3390/nu14224791
APA StylePetry, C. J. (2022). Iron Supplementation in Pregnancy and Risk of Gestational Diabetes: A Narrative Review. Nutrients, 14(22), 4791. https://doi.org/10.3390/nu14224791






