Novel Interactions of Myristic Acid and FADS3 Variants Predict Atopic Dermatitis among Indonesian Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. Sample Collection and Handling
2.2.2. GPL-FA Measurements
2.2.3. Genotyping
2.2.4. Statistical Analysis
3. Results
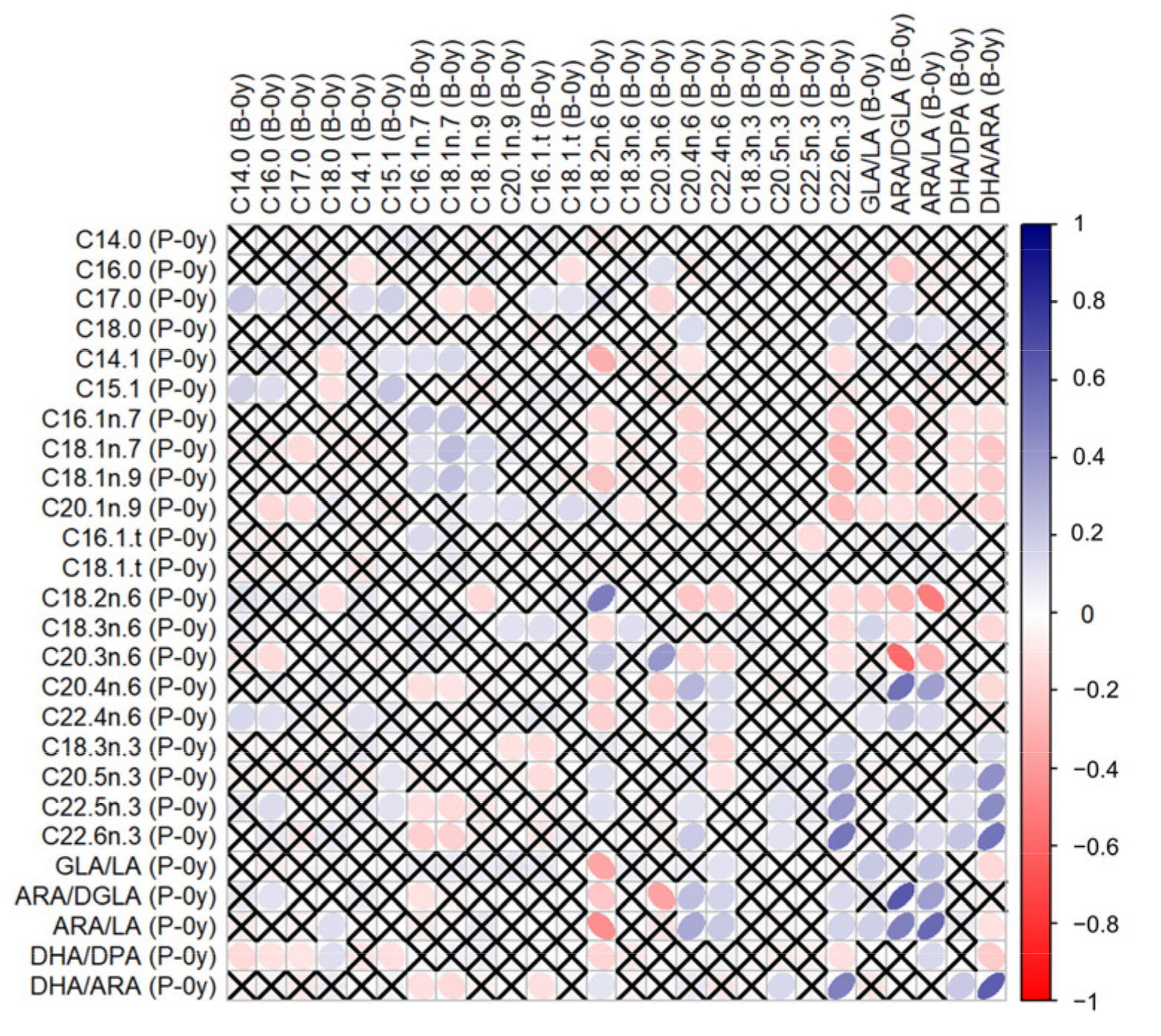
3.1. Correlations between GPL-FA
3.2. Association of GPL-FA with AD
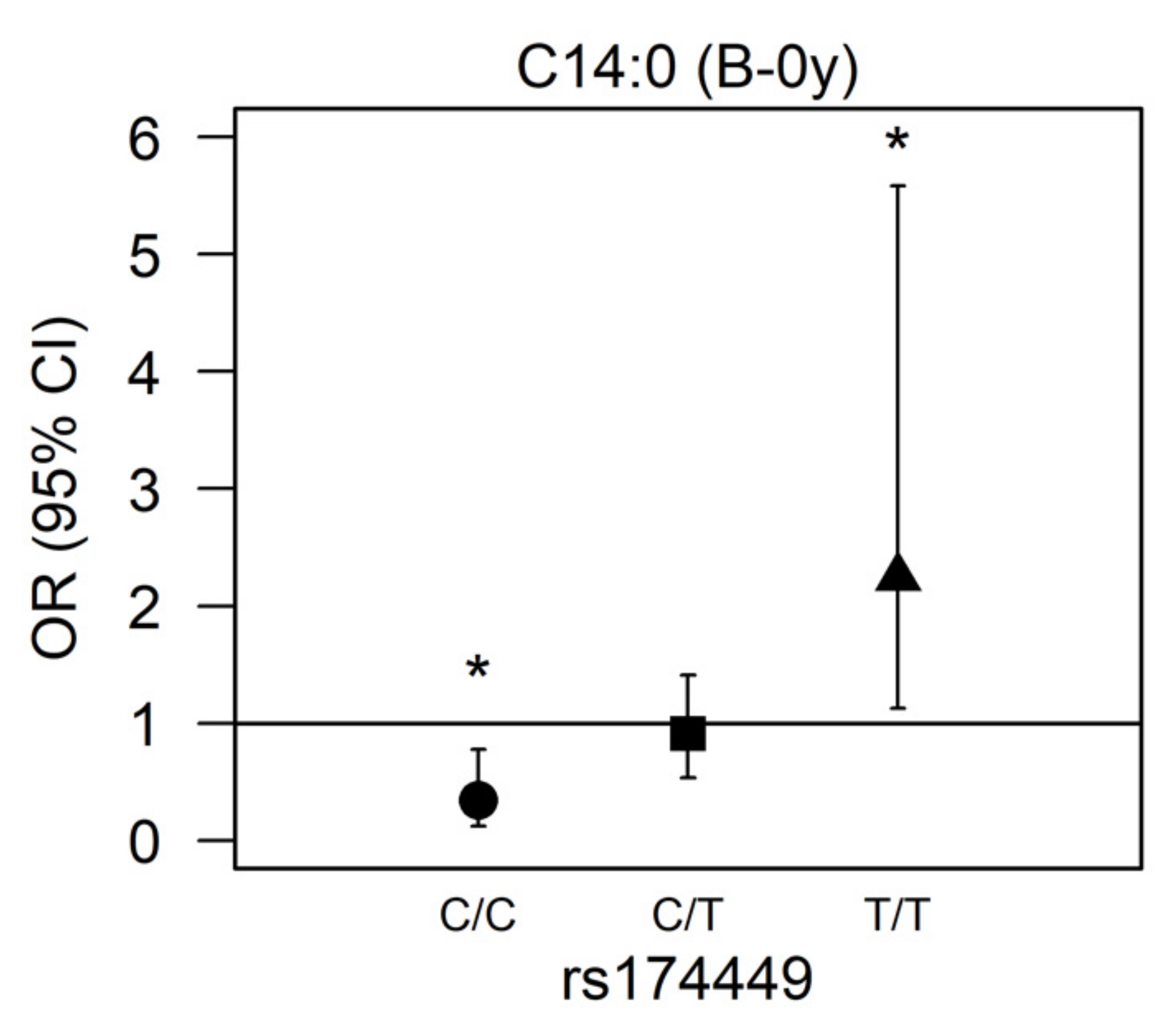
3.3. Effect Modification by FADS Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Mocanu, M.; Vâță, D.; Alexa, A.-I.; Trandafir, L.; Patrașcu, A.-I.; Hâncu, M.F.; Gheucă-Solovăstru, L. Atopic Dermatitis—Beyond the Skin. Diagnostics 2021, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Beattie, P.E.; Lewis-Jones, M.S. A Comparative Study of Impairment of Quality of Life in Children with Skin Disease and Children with Other Chronic Childhood Diseases. Br. J. Dermatol. 2006, 155, 145–151. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental Health Comorbidity in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Leung, D.Y.M. Bacterial and Viral Infections in Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Leasure, A.C.; Damsky, W.; Cohen, J.M. Association between Atopic Dermatitis and COVID-19 Infection: A Case-Control Study in the All of Us Research Program. JAAD Int. 2022, 6, 77–81. [Google Scholar] [CrossRef]
- COVID-19-Associated Surge of Atopic Dermatitis. eBioMedicine 2021, 64, 103268. [CrossRef]
- Maiello, N.; Comberiati, P.; Giannetti, A.; Ricci, G.; Carello, R.; Galli, E. New Directions in Understanding Atopic March Starting from Atopic Dermatitis. Children 2022, 9, 450. [Google Scholar] [CrossRef]
- Foisy, M.; Boyle, R.J.; Chalmers, J.R.; Simpson, E.L.; Williams, H.C. The Prevention of Eczema in Infants and Children: An Overview of Cochrane and Non-Cochrane Reviews. Evid.-Based Child Health Cochrane Rev. J. 2011, 6, 1322–1339. [Google Scholar] [CrossRef]
- Williams, H.C.; Chalmers, J. Prevention of Atopic Dermatitis. Acta Derm.-Venereol. 2020, 100, 380–388. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enteral Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef] [PubMed]
- Afghani, J.; Traidl-Hoffmann, C.; Schmitt-Kopplin, P.; Reiger, M.; Mueller, C. An Overview of the Latest Metabolomics Studies on Atopic Eczema with New Directions for Study. Int. J. Mol. Sci. 2022, 23, 8791. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy, and Atopic Dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common Genetic Variants of the FADS1 FADS2 Gene Cluster and Their Reconstructed Haplotypes Are Associated with the Fatty Acid Composition in Phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef]
- Standl, M.; Sausenthaler, S.; Lattka, E.; Koletzko, S.; Bauer, C.-P.; Wichmann, H.-E.; von Berg, A.; Berdel, D.; Krämer, U.; Schaaf, B.; et al. FADS Gene Variants Modulate the Effect of Dietary Fatty Acid Intake on Allergic Diseases in Children. Clin. Exp. Allergy 2011, 41, 1757–1766. [Google Scholar] [CrossRef]
- Standl, M.; Sausenthaler, S.; Lattka, E.; Koletzko, S.; Bauer, C.-P.; Wichmann, H.-E.; von Berg, A.; Berdel, D.; Krämer, U.; Schaaf, B.; et al. FADS Gene Cluster Modulates the Effect of Breastfeeding on Asthma. Results from the GINIplus and LISAplus Studies: FADS Genes, Breastfeeding and Asthma. Allergy 2012, 67, 83–90. [Google Scholar] [CrossRef]
- Tanjung, C.; Rzehak, P.; Sudoyo, H.; Mansyur, M.; Munasir, Z.; Immanuel, S.; Irawan, R.; Reischl, E.; Demmelmair, H.; Hadinegoro, S.R.; et al. The Association of Fatty Acid Desaturase Gene Polymorphisms on Long-Chain Polyunsaturated Fatty Acid Composition in Indonesian Infants. Am. J. Clin. Nutr. 2018, 108, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic Dermatitis Endotypes and Implications for Targeted Therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Demmelmair, H.; Koletzko, B.; Glaser, C. Fatty Acid Status Determination by Cheek Cell Sampling Combined with Methanol-Based Ultrasound Extraction of Glycerophospholipids. Lipids 2011, 46, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, C.; Rzehak, P.; Mansyur, M.; Munasir, Z.; Sudoyo, H.; Immanuel, S.; Irawan, R.; Reischl, E.; Demmelmair, H.; Koletzko, B.; et al. Study Protocol to Investigate the Environmental and Genetic Aetiology of Atopic Dermatitis: The Indonesian Prospective Study of Atopic Dermatitis in Infants (ISADI). BMJ Open 2017, 7, e012475. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Koletzko, B.; Zeilinger, S.; Hibbeln, J.R.; Klopp, N.; Ring, S.M.; Steer, C.D. Umbilical Cord PUFA Are Determined by Maternal and Child Fatty Acid Desaturase (FADS) Genetic Variants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2013, 109, 1196–1210. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-Project.org/ (accessed on 19 May 2021).
- Warnes, G.; Gorjanc, G.; Leisch, F.; Man, M. Genetics: Population Genetics; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Nyholt, D.R. A Simple Correction for Multiple Testing for Single-Nucleotide Polymorphisms in Linkage Disequilibrium with Each Other. Am. J. Hum. Genet. 2004, 74, 765–769. [Google Scholar] [CrossRef]
- Cinar, O.; Viechtbauer, W. Poolr: Methods for Pooling P-Values from (Dependent) Tests; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Silverberg, B.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic Dermatitis in the Pediatric Population: A Cross-Sectional, International Epidemiologic Study. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2021, 126, 417–428. [Google Scholar] [CrossRef]
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef]
- Ravn, N.H.; Halling, A.-S.; Berkowitz, A.G.; Rinnov, M.R.; Silverberg, J.I.; Egeberg, A.; Thyssen, J.P. How Does Parental History of Atopic Disease Predict the Risk of Atopic Dermatitis in a Child? A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2020, 145, 1182–1193. [Google Scholar] [CrossRef]
- Uphoff, E.; Cabieses, B.; Pinart, M.; Valdés, M.; Antó, J.M.; Wright, J. A Systematic Review of Socioeconomic Position in Relation to Asthma and Allergic Diseases. Eur. Respir. J. 2015, 46, 364–374. [Google Scholar] [CrossRef]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Marques dos Santos, M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T. A Biodiversity Hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Ofenloch, R.; Schuttelaar, M.; Svensson, A.; Bruze, M.; Naldi, L.; Cazzaniga, S.; Elsner, P.; Gonçalo, M.; Diepgen, T. Socioeconomic Status and the Prevalence of Skin and Atopic Diseases in Five European Countries. Acta Derm. Venerol. 2019, 99, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M. Early Immunologic Changes during the Onset of Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 152–157. [Google Scholar] [CrossRef]
- Martorano, L.M.; Grayson, M.H. Respiratory Viral Infections and Atopic Development: From Possible Mechanisms to Advances in Treatment. Eur. J. Immunol. 2018, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P.; Page, K.; Abramovich, D.R.; Ashton, J.; Brown, D. Long-Chain Polyunsaturated Fatty Acid Transport across the Perfused Human Placenta. Placenta 1997, 18, 635–642. [Google Scholar] [CrossRef]
- Montes, R.; Chisaguano, A.M.; Castellote, A.I.; Morales, E.; Sunyer, J.; López-Sabater, M.C. Fatty-Acid Composition of Maternal and Umbilical Cord Plasma and Early Childhood Atopic Eczema in a Spanish Cohort. Eur. J. Clin. Nutr. 2013, 67, 658–663. [Google Scholar] [CrossRef]
- Standl, M.; Demmelmair, H.; Koletzko, B.; Heinrich, J. Cord Blood LC-PUFA Composition and Allergic Diseases during the First 10 Yr. Results from the LISAplus Study. Pediatr. Allergy Immunol. 2014, 25, 344–350. [Google Scholar] [CrossRef]
- Honda, T.; Kabashima, K. Prostanoids and Leukotrienes in the Pathophysiology of Atopic Dermatitis and Psoriasis. Int. Immunol. 2019, 31, 589–595. [Google Scholar] [CrossRef]
- Nagel, G.; Nieters, A.; Becker, N.; Linseisen, J. The Influence of the Dietary Intake of Fatty Acids and Antioxidants on Hay Fever in Adults. Allergy 2003, 58, 1277–1284. [Google Scholar] [CrossRef]
- Trak-Fellermeier, M.A.; Brasche, S.; Winkler, G.; Koletzko, B.; Heinrich, J. Food and Fatty Acid Intake and Atopic Disease in Adults. Eur. Respir. J. 2004, 23, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; Björkstén, B.; Burr, M.; Pearce, N.; Robertson, C.F. Diet and Asthma, Allergic Rhinoconjunctivitis and Atopic Eczema Symptom Prevalence: An Ecological Analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) Data. Eur. Respir. J. 2001, 17, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- Mack Correa, M.C.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular Interactions of Plant Oil Components with Stratum Corneum Lipids Correlate with Clinical Measures of Skin Barrier Function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Dillard, C.J. Saturated Fats: A Perspective from Lactation and Milk Composition. Lipids 2010, 45, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.F.; Fielding, B.A.; Frayn, K.N. Metabolic Interaction of Dietary Sugars and Plasma Lipids with a Focus on Mechanisms and de Novo Lipogenesis. Proc. Nutr. Soc. 2007, 66, 52–59. [Google Scholar] [CrossRef]
- Rioux, V.; Pédrono, F.; Legrand, P. Regulation of Mammalian Desaturases by Myristic Acid: N-Terminal Myristoylation and Other Modulations. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 1–8. [Google Scholar] [CrossRef]
- Legrand, P.; Rioux, V. The Complex and Important Cellular and Metabolic Functions of Saturated Fatty Acids. Lipids 2010, 45, 941–946. [Google Scholar] [CrossRef]
- Beauchamp, E.; Goenaga, D.; Le Bloc’h, J.; Catheline, D.; Legrand, P.; Rioux, V. Myristic Acid Increases the Activity of Dihydroceramide Δ4-Desaturase 1 through Its N-Terminal Myristoylation. Biochimie 2007, 89, 1553–1561. [Google Scholar] [CrossRef]
- Jojima, K.; Edagawa, M.; Sawai, M.; Ohno, Y.; Kihara, A. Biosynthesis of the Anti-Lipid-Microdomain Sphingoid Base 4,14-Sphingadiene by the Ceramide Desaturase FADS3. FASEB J. 2020, 34, 3318–3335. [Google Scholar] [CrossRef]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative Profiling and Comprehensive Quantification of Stratum Corneum Ceramides in Humans and Mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, N.; Obata, Y.; Moradian, S.; Sano, H.; Yoshino, S.; Aburai, K.; Takayama, K.; Sakamoto, K.; Holleran, W.M.; Elias, P.M.; et al. Altered Sphingoid Base Profiles Predict Compromised Membrane Structure and Permeability in Atopic Dermatitis. J. Dermatol. Sci. 2013, 72, 296–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, M. The Pathogenic and Therapeutic Implications of Ceramide Abnormalities in Atopic Dermatitis. Cells 2021, 10, 2386. [Google Scholar] [CrossRef]
- Damiani, G.; Eggenhöffner, R.; Pigatto, P.D.M.; Bragazzi, N.L. Nanotechnology Meets Atopic Dermatitis: Current Solutions, Challenges and Future Prospects. Insights and Implications from a Systematic Review of the Literature. Bioact. Mater. 2019, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 390) | AD = Yes (N = 60) | AD = No (N = 330) | p-Value | |
|---|---|---|---|---|
| Sex (females) | 191 (49) | 29 (48) | 162 (49) | 1.000 |
| Sex (males) | 199 (51) | 31 (52) | 168 (51) | |
| Parental education (low) | 79 (20) | 6 (10) | 73 (22) | 0.016 |
| Parental education (medium) | 280 (72) | 45 (75) | 235 (71) | |
| Parental education (high) | 31 (8) | 9 (15) | 22 (7) | |
| Breastfed (yes) | 234 (60) | 33 (55) | 201 (61) | 0.474 |
| Breastfed (no) | 156 (40) | 27 (45) | 129 (39) | |
| Paternal smoking (yes) | 297 (76) | 44 (73) | 253 (77) | 0.695 |
| Paternal smoking (no) | 93 (24) | 16 (27) | 77 (23) | |
| Atopic history (yes) | 162 (42) | 46 (77) | 116 (35) | <0.001 |
| Atopic history (no) | 228 (58) | 14 (23) | 214 (65) | |
| Siblings (0) | 121 (31) | 20 (33) | 101 (31) | 0.916 |
| Siblings (1) | 141 (36) | 21 (35) | 120 (36) | |
| Siblings (2+) | 128 (33) | 19 (32) | 109 (33) | |
| Frequency of illness (days) | 11 (23) | 23.5 (19) | 9 (20) | <0.001 |
| Umbilical Plasma (P-0y) | Buccal Cells Birth (B-0y) | Buccal Cells AD/1 Year (B-1y) | ||||
|---|---|---|---|---|---|---|
| Fatty Acids | n | wt% | n | wt% | n | wt% |
| C14:0 | 390 | 0.53 (0.12) | 387 | 1.66 (0.91) | 297 | 2.86 (2.06) |
| C16:0 | 390 | 29.8 (1.19) | 386 | 17.6 (2.56) | 299 | 21.3 (5.41) |
| C17:0 | 390 | 0.18 (0.06) | 387 | 0.71 (0.26) | 299 | 0.94 (0.50) |
| C18:0 | 390 | 14.8 (1.19) | 387 | 18.1 (1.92) | 299 | 12.8 (3.12) |
| C20:0 | 390 | 0.06 (0.05) | - | - | ||
| C14:1 | 390 | 0.03 (0.01) | 386 | 0.07 (0.08) | 294 | 0.07 (0.12) |
| C15:1 | 390 | 0.05 (0.02) | 387 | 0.34 (0.18) | 299 | 0.26 (0.23) |
| C16:1n-7 | 390 | 1.74 (0.50) | 387 | 7.70 (1.94) | 299 | 3.95 (1.87) |
| C18:1n-7 | 390 | 2.84 (0.48) | 387 | 5.08 (0.92) | 298 | 3.50 (1.20) |
| C18:1n-9 | 390 | 10.6 (1.36) | 387 | 32.5 (3.29) | 299 | 29.8 (3.93) |
| C20:1n-9 | 390 | 0.07 (0.02) | 387 | 0.21 (0.16) | 298 | 0.25 (0.18) |
| C20:3n-9 | 390 | 0.66 (0.44) | - | - | ||
| C16:1t | 390 | 0.03 (0.03) | 387 | 0.14 (0.10) | 298 | 0.12 (0.10) |
| C18:1t | 390 | 0.26 (0.16) | 387 | 0.56 (0.66) | 295 | 0.11 (0.11) |
| C18:2tt | 390 | 0.08 (0.03) | - | - | ||
| C18:2n-6 | 388 | 8.61 (1.71) | 387 | 5.96 (1.21) | 299 | 17.2 (3.40) |
| C18:3n-6 | 390 | 0.15 (0.05) | 387 | 0.22 (0.09) | 298 | 0.11 (0.14) |
| C20:2n-6 | 390 | 0.48 (0.15) | - | - | ||
| C20:3n-6 | 390 | 5.64 (1.20) | 387 | 1.69 (0.43) | 297 | 0.69 (0.36) |
| C20:4n-6 | 390 | 15.5 (2.20) | 387 | 3.91 (1.16) | 299 | 1.72 (0.95) |
| C22:4n-6 | 390 | 0.63 (0.15) | 387 | 0.40 (0.20) | 298 | 0.25 (0.19) |
| C22:5n-6 | 390 | 1.25 (0.44) | - | - | ||
| C18:3n-3 | 388 | 0.05 (0.02) | 387 | 0.32 (0.32) | 299 | 0.60 (0.50) |
| C20:3n-3 | 390 | 0.13 (0.03) | - | - | ||
| C20:5n-3 | 389 | 0.10 (0.04) | 386 | 0.12 (0.09) | 299 | 0.13 (0.16) |
| C22:5n-3 | 390 | 0.21 (0.11) | 387 | 0.27 (0.20) | 299 | 0.30 (0.25) |
| C22:6n-3 | 390 | 5.03 (1.48) | 387 | 1.25 (0.48) | 299 | 0.82 (0.50) |
| Ratios | n | wt%/wt% | n | wt%/wt% | n | wt%/wt% |
| GLA/LA | 388 | 0.02 (0.01) | 387 | 0.04 (0.02) | 298 | 0.01 (0.01) |
| ARA/DGLA | 390 | 2.70 (0.91) | 387 | 2.34 (0.65) | 297 | 2.56 (1.43) |
| ARA/LA | 388 | 1.79 (0.46) | 387 | 0.66 (0.22) | 299 | 0.10 (0.06) |
| DHA/DPA | 390 | 23.2 (8.15) | 383 | 4.76 (4.47) | 299 | 2.71 (2.42) |
| DHA/ARA | 390 | 0.32 (0.09) | 387 | 0.32 (0.09) | 299 | 0.48 (0.19) |
| Umbilical Plasma (P-0y) | Buccal Cells Birth (B-0y) | Buccal cells AD/1 Year (B-1y) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| SFA | C14:0 | 1.220 | 0.872; 1.674 | 0.2283 | 0.971 | 0.704; 1.295 | 0.8480 | 0.929 | 0.598; 1.389 | 0.7292 |
| C16:0 | 0.775 | 0.522; 1.132 | 0.1954 | 0.898 | 0.620; 1.261 | 0.5517 | 0.533 | 0.314; 0.863 | 0.0141 | |
| C17:0 | 1.217 | 0.779; 1.863 | 0.3743 | 1.062 | 0.786; 1.402 | 0.6784 | 0.652 | 0.399; 1.038 | 0.0784 | |
| C18:0 | 1.083 | 0.731; 1.619 | 0.6920 | 0.871 | 0.639; 1.205 | 0.3889 | 1.138 | 0.777; 1.676 | 0.5088 | |
| C20:0 | 1.051 | 0.870; 1.234 | 0.5729 | - | - | - | - | - | - | |
| MUFA | C14:1 | 1.228 | 0.874; 1.726 | 0.2356 | 0.839 | 0.590; 1.120 | 0.2800 | 0.667 | 0.438; 0.912 | 0.0295 |
| C15:1 | 1.079 | 0.869; 1.309 | 0.4608 | 1.223 | 0.918; 1.594 | 0.1493 | 0.875 | 0.671; 1.095 | 0.2801 | |
| Omega-7 | C16:1n-7 | 1.251 | 0.831; 1.875 | 0.2777 | 1.031 | 0.693; 1.548 | 0.8806 | 1.058 | 0.691; 1.607 | 0.7917 |
| C18:1n-7 | 1.011 | 0.661; 1.534 | 0.9589 | 1.540 | 1.048; 2.295 | 0.0305 | 1.004 | 0.638; 1.589 | 0.9851 | |
| Omega-9 | C18:1n-9 | 1.095 | 0.753; 1.582 | 0.6289 | 0.979 | 0.670; 1.452 | 0.9146 | 3.539 | 2.193; 5.988 | <0.001 |
| C20:1n-9 | 0.923 | 0.608; 1.287 | 0.6651 | 0.93 | 0.729; 1.116 | 0.4957 | 1.297 | 0.945; 1.762 | 0.0989 | |
| C20:3n-9 | 0.899 | 0.598; 1.303 | 0.5892 | - | - | - | - | - | - | |
| Trans | C16:1 t | 0.984 | 0.594; 1.596 | 0.9486 | 0.966 | 0.763; 1.165 | 0.7393 | 0.761 | 0.507; 1.081 | 0.1563 |
| C18:1 t | 0.714 | 0.461; 1.079 | 0.1192 | 1.041 | 0.841; 1.247 | 0.6782 | 1.229 | 0.925; 1.593 | 0.1289 | |
| C18:2 tt | 1.045 | 0.790; 1.355 | 0.7482 | - | - | - | - | - | - | |
| Omega-6 | C18:2n-6 | 1.118 | 0.712; 1.760 | 0.6280 | 0.798 | 0.538; 1.145 | 0.2420 | 0.567 | 0.397; 0.800 | 0.0015 |
| C18:3n-6 | 1.282 | 0.849; 1.934 | 0.2345 | 0.941 | 0.742; 1.138 | 0.5680 | 0.884 | 0.648; 1.138 | 0.3849 | |
| C20:2n-6 | 1.062 | 0.729; 1.525 | 0.7465 | - | - | - | - | - | - | |
| C20:3n-6 | 0.992 | 0.657; 1.503 | 0.9701 | 1.015 | 0.675; 1.522 | 0.9427 | 1.072 | 0.910; 1.251 | 0.3765 | |
| C20:4n-6 | 0.829 | 0.567; 1.217 | 0.3360 | 0.822 | 0.553; 1.213 | 0.3279 | 2.021 | 1.358; 3.072 | <0.001 | |
| C22:4n-6 | 1.298 | 0.889; 1.885 | 0.1723 | 0.922 | 0.729; 1.104 | 0.4172 | 1.275 | 0.945; 1.699 | 0.0998 | |
| C22:5n-6 | 0.962 | 0.638; 1.434 | 0.8498 | - | - | - | - | - | - | |
| Omega-3 | C18:3n-3 | 0.912 | 0.679; 1.175 | 0.5067 | 0.891 | 0.637; 1.155 | 0.4409 | 0.927 | 0.676; 1.226 | 0.6160 |
| C20:3n-3 | 1.063 | 0.702; 1.588 | 0.7686 | - | - | - | - | - | - | |
| C20:5n-3 | 1.081 | 0.869; 1.321 | 0.4635 | 0.875 | 0.661; 1.096 | 0.2958 | 0.934 | 0.666; 1.243 | 0.6666 | |
| C22:5n-3 | 1.452 | 1.030; 2.050 | 0.0326 | 0.954 | 0.669; 1.307 | 0.7789 | 0.905 | 0.633; 1.227 | 0.5516 | |
| C22:6n-3 | 1.181 | 0.803; 1.734 | 0.3941 | 1.073 | 0.725; 1.582 | 0.7238 | 2.032 | 1.338; 3.131 | 0.0010 | |
| Ratios | GLA/LA | 1.169 | 0.796; 1.691 | 0.4145 | 0.967 | 0.759; 1.184 | 0.7608 | 1.000 | 0.817; 1.152 | 0.9960 |
| ARA/DGLA | 0.945 | 0.630; 1.387 | 0.7776 | 0.803 | 0.516; 1.229 | 0.3191 | 1.106 | 0.692; 1.763 | 0.6715 | |
| ARA/LA | 0.840 | 0.559; 1.233 | 0.3864 | 0.912 | 0.618; 1.337 | 0.6409 | 1.832 | 1.351; 2.595 | <0.001 | |
| DHA/DPA | 0.591 | 0.371; 0.912 | 0.0215 | 1.085 | 0.768; 1.515 | 0.6368 | 1.472 | 0.975; 2.224 | 0.0646 | |
| DHA/ARA | 1.255 | 0.863; 1.820 | 0.2307 | 1.222 | 0.848; 1.760 | 0.2800 | 0.940 | 0.621; 1.356 | 0.7560 | |
| SNP | Major/Minor Allele | MAF (%) | HWE p-Value |
|---|---|---|---|
| rs174448 | T/C | 47.7 | 0.684 |
| rs174449 | C/T | 47.4 | 0.154 |
| rs174455 | C/T | 45.5 | 0.414 |
| rs174548 | C/G | 26.7 | 0.698 |
| rs174556 | A/G | 26.7 | 0.698 |
| rs174561 | G/A | 26.7 | 0.698 |
| rs174570 | T/C | 23.2 | 0.568 |
| rs174574 | A/C | 21.6 | 0.552 |
| rs174575 | C/G | 29.3 | 0.902 |
| rs174576 | A/C | 21.6 | 0.373 |
| rs174578 | A/T | 21.6 | 0.373 |
| rs174579 | C/T | 27.6 | 0.899 |
| rs174602 | C/T | 41.2 | 0.531 |
| rs2727271 | T/A | 49.5 | 0.684 |
| rs3834458 | DEL/T | 21.9 | 0.460 |
| rs498793 | C/T | 14.4 | 0.537 |
| rs526126 | C/G | 22.5 | 0.080 |
| rs968567 | C/T | 0.4 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanjung, C.; Harris, C.P.; Demmelmair, H.; Dwitya, S.; Munasir, Z.; Sudoyo, H.; Standl, M.; Sjarif, D.R.; Koletzko, B. Novel Interactions of Myristic Acid and FADS3 Variants Predict Atopic Dermatitis among Indonesian Infants. Nutrients 2022, 14, 4676. https://doi.org/10.3390/nu14214676
Tanjung C, Harris CP, Demmelmair H, Dwitya S, Munasir Z, Sudoyo H, Standl M, Sjarif DR, Koletzko B. Novel Interactions of Myristic Acid and FADS3 Variants Predict Atopic Dermatitis among Indonesian Infants. Nutrients. 2022; 14(21):4676. https://doi.org/10.3390/nu14214676
Chicago/Turabian StyleTanjung, Conny, Carla P. Harris, Hans Demmelmair, Sarah Dwitya, Zakiudin Munasir, Herawati Sudoyo, Marie Standl, Damayanti Rusli Sjarif, and Berthold Koletzko. 2022. "Novel Interactions of Myristic Acid and FADS3 Variants Predict Atopic Dermatitis among Indonesian Infants" Nutrients 14, no. 21: 4676. https://doi.org/10.3390/nu14214676
APA StyleTanjung, C., Harris, C. P., Demmelmair, H., Dwitya, S., Munasir, Z., Sudoyo, H., Standl, M., Sjarif, D. R., & Koletzko, B. (2022). Novel Interactions of Myristic Acid and FADS3 Variants Predict Atopic Dermatitis among Indonesian Infants. Nutrients, 14(21), 4676. https://doi.org/10.3390/nu14214676






