Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study
Abstract
1. Background
2. Methods
2.1. Study Design, Institution, and Ethics
2.2. Screening for Eligibility of Study Participants
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Study Protocol
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Population (n = 48)
3.2. Effects on Anthropometric and Glucometabolic Parameters
3.3. Liver Enzymes, Scores and US Appearance of NAFLD
3.4. Body Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | NonAlcoholic Fatty Liver Disease |
| T2D | Type 2 Diabetes |
| IR | Insulin Resistance |
| HDL | High Density Lipoproteins |
| NASH | NonAlcoholic Steatohepatitis |
| MAFLD | Metabolic Associated Fatty Liver Disease |
| CV | Cardiovascular |
| SMM | Skeletal Muscle Mass |
| VAT | Visceral Adipose Tissue |
| GLP-1 RAs | Glucagon-Like Peptide-1 Receptor Agonists |
| SGLT2i | Sodium Glucose Transporter type 2 Inhibitors |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| BW | Body Weight |
| WC | Waist Circumference |
| BMI | Body Mass Index |
| HbA1c | Glycated Hemoglobin |
| eGFR | Estimated Glomerular Filtration Rate |
| AST | ASpartate aminoTransferase |
| ALT | ALanine aminoTransferase |
| γGT | Gamma-Glutamyl Transferase |
| US | Ultrasound |
| qw | Once (every or quaque form latin)-weekly |
| APRI | AST to Platelet Ratio Index |
| HSI | Hepatic Steatosis Index |
| FLI | Fatty Liver Index |
| SMF-BIA | Segmental Multi Frequency-Bioelectrical Impedance Analysis |
| TBW | Total Body Water |
| ECW | Extracellular Water |
| SMI | Skeletal Muscle Index |
| FMI | Fat Mass Index |
| FFMI | Fat-Free Mass Index |
| HG | Hand Grip |
| MQI | Muscle Quality Index |
| US-VAT | Ultrasonographic VAT |
| US-LSS | Ultrasound Liver Steatosis Score |
| BIA-VAT | Bioelectrical Impedance Analysis of Visceral Adipose Tissue |
| R | Responders |
| NR | Non-Responders |
References
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of Non-Alcoholic and Alcoholic Fatty Liver Diseases. Transl. Gastroenterol. Hepatol. 2020, 5. [Google Scholar] [CrossRef]
- Oligschlaeger, Y.; Shiri-Sverdlov, R. NAFLD Preclinical Models: More than a Handful, Less of a Concern? Biomedicines 2020, 8, 28. [Google Scholar] [CrossRef]
- De Nucci, S.; Castellana, F.; Zupo, R.; Lampignano, L.; Di Chito, M.; Rinaldi, R.; Giannuzzi, V.; Cozzolongo, R.; Piazzolla, G.; Giannelli, G.; et al. Associations between serum biomarkers and Nonalcoholic Liver Disease: Results of a Clinical Study of Mediterranean Patients with Obesity. Front Nutr. 2022, 8, 1002669. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Panza, F.; Castellana, M.; Lampignano, L.; Cincione, R.I.; Triggiani, V.; Giannelli, G.; Dibello, V.; Sardone, R.; et al. Non Alcoholic Fatty Liver Disease is positively associated with increased glycated haemoglobin levels in subjects without diabetes. J. Clin. Med. 2021, 10, 1695. [Google Scholar] [CrossRef]
- De Pergola, G.; Castellana, F.; Zupo, R.; De Nucci, S.; Panza, F.; Castellana, M.; Lampignano, L.; Di Chito, M.; Triggiani, V.; Sardone, R.; et al. A family history of type 2 diabetes as a predictor of fatty liver disease in diabetes-free individuals with excessive body weight. Sci. Rep. 2021, 11, 24084. [Google Scholar] [CrossRef]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Jarvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. The Role of Insulin Resistance in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Gruben, N.; Shiri-Sverdlov, R.; Koonen, D.P.Y.; Hofker, M.H. Nonalcoholic Fatty Liver Disease: A Main Driver of Insulin Resistance or a Dangerous Liaison? Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Quek, J.; Ng, C.H.; Tang, A.S.P.; Chew, N.; Chan, M.; Khoo, C.M.; Wei, C.P.; Chin, Y.H.; Tay, P.; Lim, G.; et al. Metabolic Associated Fatty Liver Disease Increases the Risk of Systemic Complications and Mortality. A Meta-Analysis and Systematic Review of 12 620 736 Individuals. Endocr. Pract. 2022, 28, 667–672. [Google Scholar] [CrossRef]
- De Fré, C.H.; De Fré, M.A.; Kwanten, W.J.; Op de Beeck, B.J.; Van Gaal, L.F.; Francque, S.M. Sarcopenia in patients with non-alcoholic fatty liver disease: Is it a clinically significant entity? Obes. Rev. 2019, 20, 353–363. [Google Scholar] [CrossRef]
- Seo, D.H.; Lee, Y.H.; Park, S.W.; Choi, Y.J.; Huh, B.W.; Lee, E.; Huh, K.B.; Kim, S.H.; Cha, B.S. Sarcopenia is associated with non-alcoholic fatty liver disease in men with type 2 diabetes. Diabetes Metab. 2020, 46, 362–369. [Google Scholar] [CrossRef]
- Shida, T.; Akiyama, K.; Oh, S.; Sawai, A.; Isobe, T.; Okamoto, Y.; Ishige, K.; Mizokami, Y.; Yamagata, K.; Onizawa, K.; et al. Skeletal muscle mass to visceral fat area ratio is an important determinant affecting hepatic conditions of non-alcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 535–547. [Google Scholar] [CrossRef]
- Shi, Y.X.; Chen, X.Y.; Qiu, H.N.; Jiang, W.R.; Zhang, M.Y.; Huang, Y.P.; Ji, Y.P.; Zhang, S.; Li, C.J.; Lin, J.N. Visceral fat area to appendicular muscle mass ratio as a predictor for nonalcoholic fatty liver disease independent of obesity. Scand. J. Gastroenterol. 2021, 56, 312–320. [Google Scholar] [CrossRef]
- Nseir, W.; Hellou, E.; Assy, N. Role of Diet and Lifestyle Changes in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 9338–9344. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and Future Pharmacological Therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging Therapeutic Approaches for the Treatment of NAFLD and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Ahmann, A.; Cariou, B.; Chow, F.; Davies, M.J.; Jódar, E.; Mehta, R.; Woo, V.; Lingvay, I. Comparative Efficacy, Safety, and Cardiovascular Outcomes with Once-Weekly Subcutaneous Semaglutide in the Treatment of Type 2 Diabetes: Insights from the SUSTAIN 1-7 Trials. Diabetes Metab. 2019, 45, 409–418. [Google Scholar] [CrossRef]
- Capehorn, M.S.; Catarig, A.M.; Furberg, J.K.; Janez, A.; Price, H.C.; Tadayon, S.; Vergès, B.; Marre, M. Efficacy and Safety of Once-Weekly Semaglutide 1.0 mg vs Once-Daily Liraglutide 1.2 mg as Add-on to 1-3 Oral Antidiabetic Drugs in Subjects with Type 2 Diabetes (SUSTAIN 10). Diabetes Metab. 2020, 46, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Husain, M.; Bain, S.C.; Jeppesen, O.K.; Lingvay, I.; Sørrig, R.; Treppendahl, M.B.; Vilsbøll, T. Semaglutide (SUSTAIN and PIONEER) Reduces Cardiovascular Events in Type 2 Diabetes across Varying Cardiovascular Risk. Diabetes. Obes. Metab. 2020, 22, 442–451. [Google Scholar] [CrossRef]
- Volpe, S.; Lisco, G.; Racaniello, D.; Fanelli, M.; Colaianni, V.; Vozza, A.; Triggiani, V.; Sabbà, C.; Tortorella, C.; De Pergola, G.; et al. Once-Weekly Semaglutide Induces an Early Improvement in Body Composition in Patients with Type 2 Diabetes: A 26-Week Prospective Real-Life Study. Nutrients 2022, 14, 2414. [Google Scholar] [CrossRef]
- De Matteis, C.; Cariello, M.; Graziano, G.; Battaglia, S.; Suppressa, P.; Piazzolla, G.; Sabbà, C.; Moschetta, A. AST to Platelet Ratio Index (APRI) Is an Easy-to-Use Predictor Score for Cardiovascular Risk in Metabolic Subjects. Sci. Rep. 2021, 11, 14834. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Jensen, B.; Braun, W.; Pourhassan, M.; Gallagher, D.; Müller, M.J. Quantification of Whole-Body and Segmental Skeletal Muscle Mass Using Phase-Sensitive 8-Electrode Medical Bioelectrical Impedance Devices. Eur. J. Clin. Nutr. 2017, 71, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Hatziioannou, A.; Perelas, A.; Perrea, D.N. Sonographic Assessment of Regional Adiposity. AJR Am. J. Roentgenol. 2007, 189, 1545–1553. [Google Scholar] [CrossRef]
- Ferraioli, G.; Monteiro, L.B.S. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of Semaglutide on Liver Enzymes and Markers of Inflammation in Subjects with Type 2 Diabetes and/or Obesity. Aliment. Pharmacol. Ther. 2019, 50, 193–203. [Google Scholar] [CrossRef]
- Piazzolla, G.; Vozza, A.; Volpe, S.; Bergamasco, A.; Triggiani, V.; Lisco, G.; Falconieri, M.; Tortorella, C.; Solfrizzi, V.; Sabbà, C. Effectiveness and Clinical Benefits of New Anti-Diabetic Drugs: A Real-Life Experience. Open Med. 2022, 17, 1203–1215. [Google Scholar] [CrossRef]
- Jennison, E.; Patel, J.; Scorletti, E.; Byrne, C.D. Diagnosis and management of non-alcoholic fatty liver disease. Postgrad. Med. J. 2019, 95, 314–322. [Google Scholar] [CrossRef]
- Pownall, H.J.; Rosales, C.; Gillard, B.K.; Gotto, A.M., Jr. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat. Rev. Cardiol. 2021, 18, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Leerapu, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021, 15, 827–840. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Sundby Palle, M.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Ono, H.; Kawano, T.; Yoshida, Y.; Okubo, T.; Hayama, K.; Nakagawa-Iwashita, A.; Itokawa, N.; et al. Efficacy and safety of oral semaglutide in patients with non-alcoholic fatty liver disease complicated by type 2 diabetes mellitus: A pilot study. JGH Open 2022, 6, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, M.; Zhang, C.; Feng, X.; Chen, J.; Li, Q.; Zhang, Y.; Xu, W.; Dong, Y.; Jiang, Y.; et al. Reproducibility of Ultrasound-Guided Attenuation Parameter (UGAP) to the Noninvasive Evaluation of Hepatic Steatosis. Sci. Rep. 2022, 12, 2876. [Google Scholar] [CrossRef]
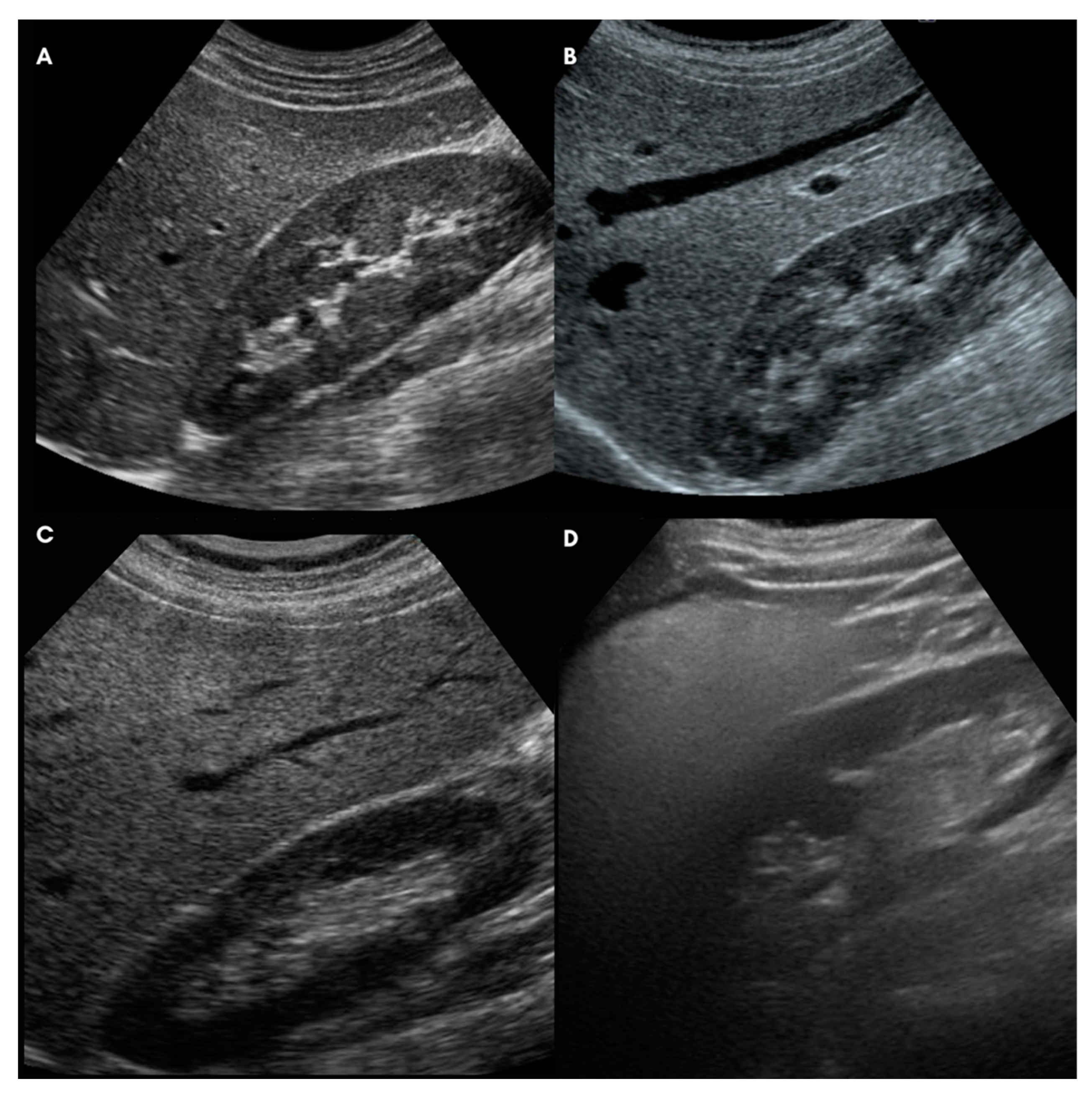







| Ultrasound-Liver Steatosis Score (modified from Hamaguchi et al. 2007) [36] |
| A. Bright liver and hepatorenal echo contrast |
| 0: Bright liver and hepatorenal US contrast are negative |
| 1: Bright liver and/or hepatorenal US contrast are slightly positive |
| 2: Bright liver and/or hepatorenal US contrast are mildly positive |
| 3: Bright liver and/or hepatorenal US contrast are strongly positive |
| B. Deep attenuation |
| 0: Deep attenuation is completely negative |
| 1: Deep attenuation is mild (the deep parts of the liver are visualized but appear hypoechoic) |
| 2: Deep attenuation is severe (the diaphragm, but not the deep liver parenchyma, can be distinguished) |
| 3: Total barrage of the ultrasound beam (neither deep liver parenchyma nor diaphragm can be distinguished) |
| C: Vessel blurring |
| 0: Vessel blurring is negative |
| 1: The borders of intrahepatic vessels are not perfectly defined |
| 2: The border of intrahepatic vessels is mildly unclear or the lumen of intrahepatic vessels is narrowed |
| 3: Intrahepatic vessels are not visible (“featureless liver”) |
| D: Parenchymal US-pattern |
| 0: Parenchymal US-pattern is homogeneous |
| 1: Parenchymal US-pattern is slightly inhomogeneous |
| 2: Parenchymal US-pattern is mildly inhomogeneous |
| 3: Parenchymal US-pattern is severely inhomogeneous. |
| Ultrasound Steatosis Score (0–12) = A + B + C + D |
| Baseline Characteristics | Mean (S.D.) | Median (Min; Max) |
|---|---|---|
| Age (years) | 57.7 (8.4) | 59 (42;73) |
| Diabetes duration (years) | 6 (6) | 3.5 (0; 20) |
| Body weight (kg) | 104.7 (22.5) | 99 (70.5; 170) |
| Body mass index (kg/m2) | 38.8 (8.3) | 36.9 (24.7; 61) |
| Waist circumference (cm) | 123.2 (15.3) | 120 (97; 161) |
| Fasting glycemia (mg/dL) | 129.5 (34.7) | 122 (87; 231) |
| Glycated hemoglobin (mmol/mol) | 53.2 (20.2) | 44.5 (33; 128) |
| Fasting serum C-peptide (ng/mL) | 3.7 (1.5) | 3.5 (0.3; 8.1) |
| Fasting serum insulin (mUI/L) | 22.6 (15.4) | 19.9 (0.4; 75) |
| HOMA-IR index | 6.8 (4.6) | 5.5 (0.1; 21.8) |
| Total Cholesterol (mg/dL) | 166.5 (37.8) | 165 (102; 247) |
| LDL Cholesterol (mg/dL) | 90.3 (31.8) | 90 (36; 164) |
| HDL Cholesterol (mg/dL) | 47.9 (13.9) | 46 (30; 116) |
| Triglycerides (mg/dL) | 140.7 (63.3) | 144 (44; 313) |
| Serum creatinine (mg/dL) | 0.9 (0.2) | 0.85 (0.40; 1.65) |
| Glomerular filtration rate (mL/min/1.73 m2) | 88.9 (21.6) | 89.5 (41; 153) |
| AST (IU/L) | 28.2 (16.7) | 23 (10; 90) |
| ALT (IU/L) | 43.7 (32.6) | 36 (15; 156) |
| γGT (IU/L) | 58.2 (75.6) | 39 (12; 430) |
| APRI score | 0.3 (0.2) | 0.3 (0.1; 1) |
| HIS | 47.9 (8.8) | 45.7 (28.5; 69.6) |
| FLI | 91.7 (7.7) | 93.6 (73.4; 100) |
| US-Subcutaneous Adipose Tissue (cm) | 2.2 (0.8) | 2 (0.9; 4) |
| US-Visceral Adipose Tissue (cm) | 7.2 (2.8) | 6.6 (3; 15.7) |
| US-LSS | 7.8 (2.5) | 8 (2; 12) |
| BMI | WC | BIA-VAT | US-VAT | APRI | HSI | FLI | US-LSS | US-SAT | FMI | FFMI | SMI | HG | MQI | HOMA IR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 0.92 | 0.71 | 0.65 | 0.01 | 0.96 | 0.72 | 0.46 | 0.31 | 0.88 | 0.73 | 0.56 | −0.18 | −0.23 | 0.22 |
| ** | ** | ** | n.s. | ** | ** | * | n.s. | ** | ** | ** | n.s. | n.s. | n.s. | |
| WC | 0.75 | 0.71 | 0.06 | 0.87 | 0.79 | 0.56 | 0.36 | 0.79 | 0.69 | 0.51 | −0.04 | −0.17 | 0.30 | |
| ** | ** | n.s. | ** | ** | ** | * | ** | ** | ** | n.s. | n.s. | * | ||
| BIA-VAT | 0.74 | 0.16 | 0.59 | 0.53 | 0.44 | 0.13 | 0.50 | 0.82 | 0.69 | 0.28 | −0.21 | 0.18 | ||
| ** | n.s. | ** | ** | ** | n.s. | ** | ** | ** | n.s. | n.s. | n.s. | |||
| US-VAT | 0.01 | 0.50 | 0.59 | 0.57 | 0.22 | 0.45 | 0.62 | 0.51 | 0.15 | −0.16 | 0.31 | |||
| n.s. | ** | ** | ** | n.s. | ** | ** | ** | n.s. | n.s. | n.s. | ||||
| APRI | 0.02 | 0.09 | −0.05 | 0.16 | −0.05 | 0.14 | 0.09 | 0.10 | −0.09 | 0.16 | ||||
| n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| HSI | 0.65 | 0.35 | 0.34 | 0.88 | 0.62 | 0.44 | −0.30 | −0.19 | 0.16 | |||||
| ** | * | * | ** | ** | ** | n.s. | n.s. | n.s. | ||||||
| FLI | 0.53 | 0.44 | 0.57 | 0.51 | 0.43 | −0.10 | −0.27 | 0.41 | ||||||
| ** | ** | ** | ** | ** | n.s. | n.s. | ** | |||||||
| US-LSS | 0.37 | 0.32 | 0.41 | 0.35 | 0.17 | −0.17 | 0.41 | |||||||
| * | * | ** | * | n.s. | n.s. | ** | ||||||||
| US-SAT | 0.38 | 0.05 | 0.18 | −0.11 | −0.25 | 0.48 | ||||||||
| * | n.s. | n.s. | n.s. | n.s. | ** | |||||||||
| FMI | 0.41 | 0.29 | −0.25 | −0.17 | 0.12 | |||||||||
| ** | n.s. | n.s. | n.s. | n.s. | ||||||||||
| FFMI | 0.84 | 0.14 | −0.26 | 0.21 | ||||||||||
| ** | n.s. | n.s. | n.s. | |||||||||||
| SMI | 0.11 | −0.54 | 0.33 | |||||||||||
| n.s. | ** | * | ||||||||||||
| HG | 0.65 | 0.11 | ||||||||||||
| ** | n.s. | |||||||||||||
| MQI | −0.31 | |||||||||||||
| * | ||||||||||||||
| HOMA IR | ||||||||||||||
| Parameters | Variation over Time | |||
|---|---|---|---|---|
| T0 | T3 | T6 | T12 | |
| Visceral Adipose Tissue (L) | 6.2 (0.5) | −0.8 (0.2) ** | −1.1 (0.2) **,# | −1.6 (0.2) **,## |
| Fat Mass Index (kg/m2) | 17.1 (6.1) | −2.3 (0.4) ** | −3.2 (0.4) **,## | −3.3 (0.4) ** |
| Fat-Free Mass Index (kg/m2) | 21.4 (3.1) | −0.7 (0.2) ** | −0.8 (0.2) ** | −1.3 (0.2) **,## |
| Skeletal Muscle Index (kg/m2) | 10.6 (2) | −0.4 (0.2) * | −0.3 (0.2) | −1.3 (0.3) **,## |
| Skeletal Muscle Mass (kg) to VAT (L) ratio | 5.4 (0.3) | 0.5 (0.3) | 0.9 (0.6) | 1.8 (0.8) **,# |
| HG (kg) | 33.6 (1.6) | 0.04 (1.5) * | 0.6 (1.2) ** | −0.3 (1.5) |
| MQI (kg/kg) | 1 (0.1) | 0.2 (0.1) * | 0.3 (0.1) ** | 0.1 (0.1) |
| Total Body Water (L) | 42.8 (9) | −0.2 (0.9) | −0.1 (1) | −0.1 (1.3) |
| Extracellular Body Water (L) | 19.4 (0.6) | −0.1 (0.4) | −0.01 (0.4) | −0.01 (0.6) |
| ECW to TBW ratio | 45.6 (0.4) | −0.1 (0.3) | −0.01 (0.3) | −0.2 (0.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, S.; Lisco, G.; Fanelli, M.; Racaniello, D.; Colaianni, V.; Triggiani, D.; Donghia, R.; Crudele, L.; Rinaldi, R.; Sabbà, C.; et al. Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study. Nutrients 2022, 14, 4673. https://doi.org/10.3390/nu14214673
Volpe S, Lisco G, Fanelli M, Racaniello D, Colaianni V, Triggiani D, Donghia R, Crudele L, Rinaldi R, Sabbà C, et al. Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study. Nutrients. 2022; 14(21):4673. https://doi.org/10.3390/nu14214673
Chicago/Turabian StyleVolpe, Sara, Giuseppe Lisco, Margherita Fanelli, Davide Racaniello, Valentina Colaianni, Domenico Triggiani, Rossella Donghia, Lucilla Crudele, Roberta Rinaldi, Carlo Sabbà, and et al. 2022. "Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study" Nutrients 14, no. 21: 4673. https://doi.org/10.3390/nu14214673
APA StyleVolpe, S., Lisco, G., Fanelli, M., Racaniello, D., Colaianni, V., Triggiani, D., Donghia, R., Crudele, L., Rinaldi, R., Sabbà, C., Triggiani, V., De Pergola, G., & Piazzolla, G. (2022). Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study. Nutrients, 14(21), 4673. https://doi.org/10.3390/nu14214673










