Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approval, Registration, and Patient Consent
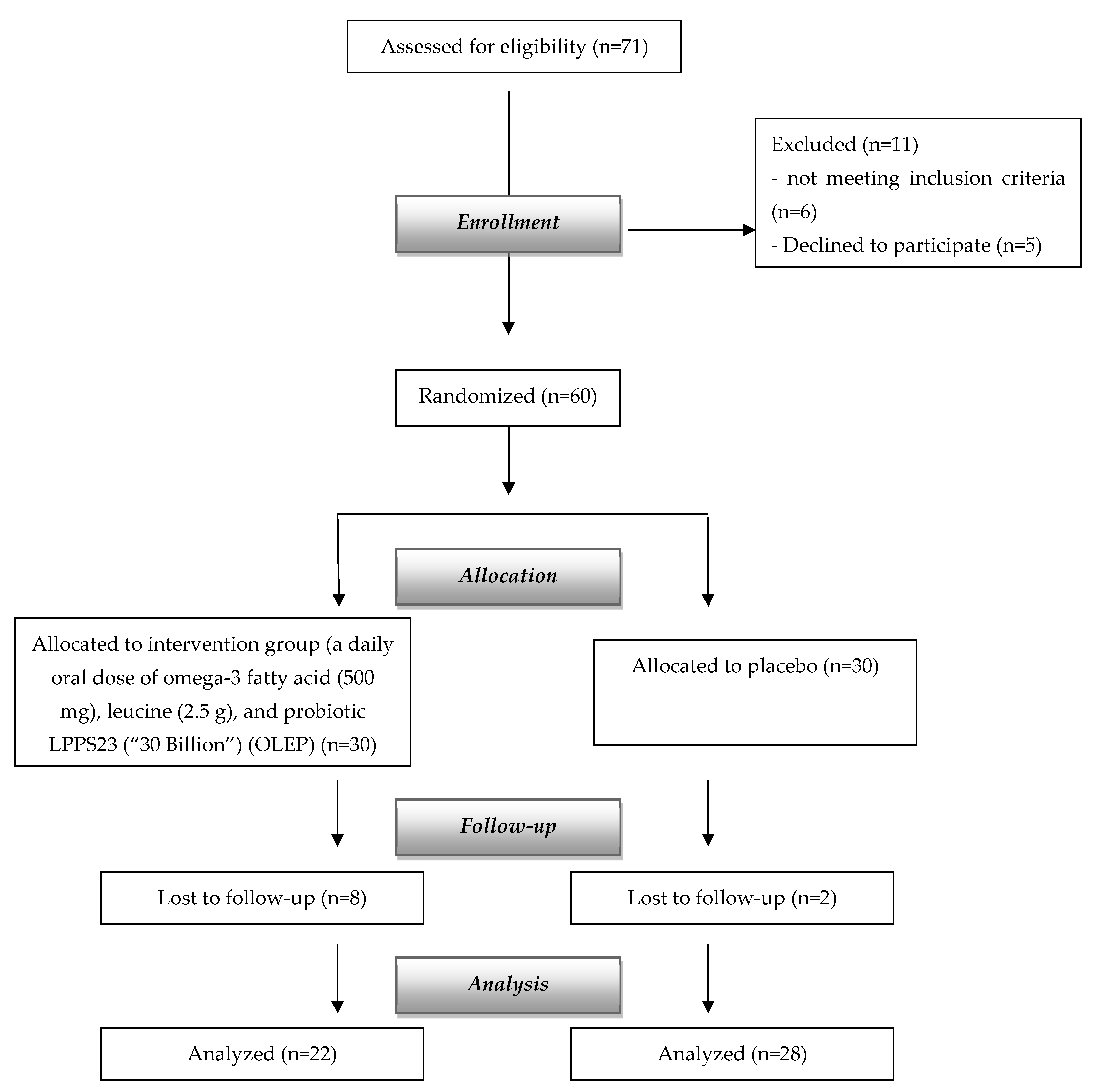
2.2. Study Design and Sample Size
2.3. Participants
2.4. Nutritional Assessment and Nutritional Interventions
2.5. Anthropometric Measurements
2.6. Body Composition Assessment
2.7. Muscle Strength Evaluation
2.8. Functional Status Evaluation
2.9. Physical Performance Assessment
2.10. Quality of Life Assessment
2.11. Mood Assessment
2.12. Evaluation of Blood Pressure
2.13. Biochemical Parameters
2.14. Safety Assessment and Monitoring of Adverse Events
2.15. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; van der Harst, P. Biological ageing and cardiovascular disease. Heart 2008, 94, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Dhillon, R.J.S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.P.; Syddall, H.E.; Jameson, K.; Robinson, S.; Denison, H.; Roberts, H.C.; Edwards, M.; Dennison, E.; Cooper, C.; Aihie Sayer, A. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013, 42, 378–384. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef]
- Dodds, R.M.; Roberts, H.C.; Cooper, C.; Sayer, A.A. The Epidemiology of Sarcopenia. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2015, 18, 461–466. [Google Scholar] [CrossRef]
- Vitale, G.; Cesari, M.; Mari, D. Aging of the endocrine system and its potential impact on sarcopenia. Eur. J. Intern. Med. 2016, 35, 10–15. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Musci, R.V.; Hinkley, J.M.; Miller, B.F. Mitochondria as a Target for Mitigating Sarcopenia. Front. Physiol. 2018, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.-J.; Chevalier, S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients 2018, 10, 1099. [Google Scholar] [CrossRef]
- Dardevet, D.; Rémond, D.; Peyron, M.-A.; Papet, I.; Savary-Auzeloux, I.; Mosoni, L. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci. World J. 2012, 2012, 269531. [Google Scholar] [CrossRef]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef]
- Wall, B.T.; van Loon, L.J.C. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Casperson, S.L.; Sheffield-Moore, M.; Hewlings, S.J.; Paddon-Jones, D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin. Nutr. 2012, 31, 512–519. [Google Scholar] [CrossRef]
- Balage, M.; Dardevet, D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 265–270. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Mackenzie, I.; Doherty, M.K.; Whitfield, P.D.; Bell, G.; Dick, J.; Shaw, A.; Rao, F.V.; Ashcroft, S.P.; Philp, A.; et al. Lipid remodeling and an altered membrane-associated proteome may drive the differential effects of EPA and DHA treatment on skeletal muscle glucose uptake and protein accretion. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E605–E619. [Google Scholar] [CrossRef]
- Kamolrat, T.; Gray, S.R. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 2013, 432, 593–598. [Google Scholar] [CrossRef]
- Gray, S.R.; Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 104–109. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef]
- Di Girolamo, F.G.; Situlin, R.; Mazzucco, S.; Valentini, R.; Toigo, G.; Biolo, G. Omega-3 fatty acids and protein metabolism: Enhancement of anabolic interventions for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 145–150. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Huang, S.-Y.; Huang, K.-C.; Hsu, C.-C.; Yang, K.-C.; Li, L.-A.; Chan, C.-H.; Huang, H.-Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging (Albany. NY). 2019, 11, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Chen, L.-H.; Wang, M.-F.; Hsu, C.-C.; Chan, C.-H.; Li, J.-X.; Huang, H.-Y. Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2018, 10, 894. [Google Scholar] [CrossRef]
- Logan, S.L.; Spriet, L.L. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community- dwelling older females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef]
- Walowski, C.O.; Braun, W.; Maisch, M.J.; Jensen, B.; Peine, S.; Norman, K.; Müller, M.J.; Bosy-Westphal, A. Reference values for skeletal muscle mass—Current concepts and methodological considerations. Nutrients 2020, 12, 755. [Google Scholar] [CrossRef]
- Moon, J.J.; Park, S.-G.; Ryu, S.M.; Park, C.-H. New Skeletal Muscle Mass Index in Diagnosis of Sarcopenia. J. Bone Metab. 2018, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 56–61. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed Up Go: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand. J. Work. Environ. Health 1990, 16, 55–58. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of leucine administration in sarcopenia: A randomized and placebo-controlled clinical trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Boonla, C.; Dissayabutra, T.; Kaewwilai, L.; Muensri, S.; Chotipanich, C.; Joutsa, J.; Rinne, J.; Bhidayasiri, R. Biochemical and clinical effects of Whey protein supplementation in Parkinson’s disease: A pilot study. J. Neurol. Sci. 2016, 367, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Riva, A.; Petrangolini, G.; Di Paolo, E.; Gasparri, C. Effects of n-3 EPA and DHA supplementation on fat free mass and physical performance in elderly. A systematic review and meta-analysis of randomized clinical trial. Mech. Ageing Dev. 2021, 196, 111476. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Dawson Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic Administration Increases Amino Acid Absorption from Plant Protein: A Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef]
- Chapman, I.M. Obesity Paradox during Aging. Interdiscip. Top. Gerontol. 2010, 37, 20–36. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Foster, M. Detrimental and protective fat: Body fat distribution and its relation to metabolic disease. Horm. Mol. Biol. Clin. Investig. 2014, 17, 13–27. [Google Scholar] [CrossRef]
- Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; O’Donnel, C.J.; Fox, C.S. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care 2009, 32, 1068–1075. [Google Scholar] [CrossRef]
- Malafarina, V.; Úriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 2012, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]


| Variable | Intervention Group (n = 22) | Placebo Group (n = 28) | Total (n = 50) | p-Value |
|---|---|---|---|---|
| Age (years) | 78.84 ± 5.80 | 80.50 ± 3.74 | 79.71 ± 4.84 | 0.231 |
| Weight (kg) | 57.53 ± 11.41 | 52.32 ± 8.33 | 54.77 ± 10.14 | 0.072 |
| BMI (kg/m2) | 22.63 ± 2.74 | 21.96 ± 1.34 | 22.27 ± 2.12 | 0.271 |
| ALM (kg) | 15.646 ± 3.666 | 13.935 ± 2.020 | 14.773 ± 3.035 | 0.052 |
| ALM/h2 | 6.20 ± 1.23 | 5.80 ± 0.83 | 6.00 ± 1.04 | 0.270 |
| SMI | 27.40 ± 4.98 | 26.61 ± 4.18 | 26.99 ± 4.60 | 0.521 |
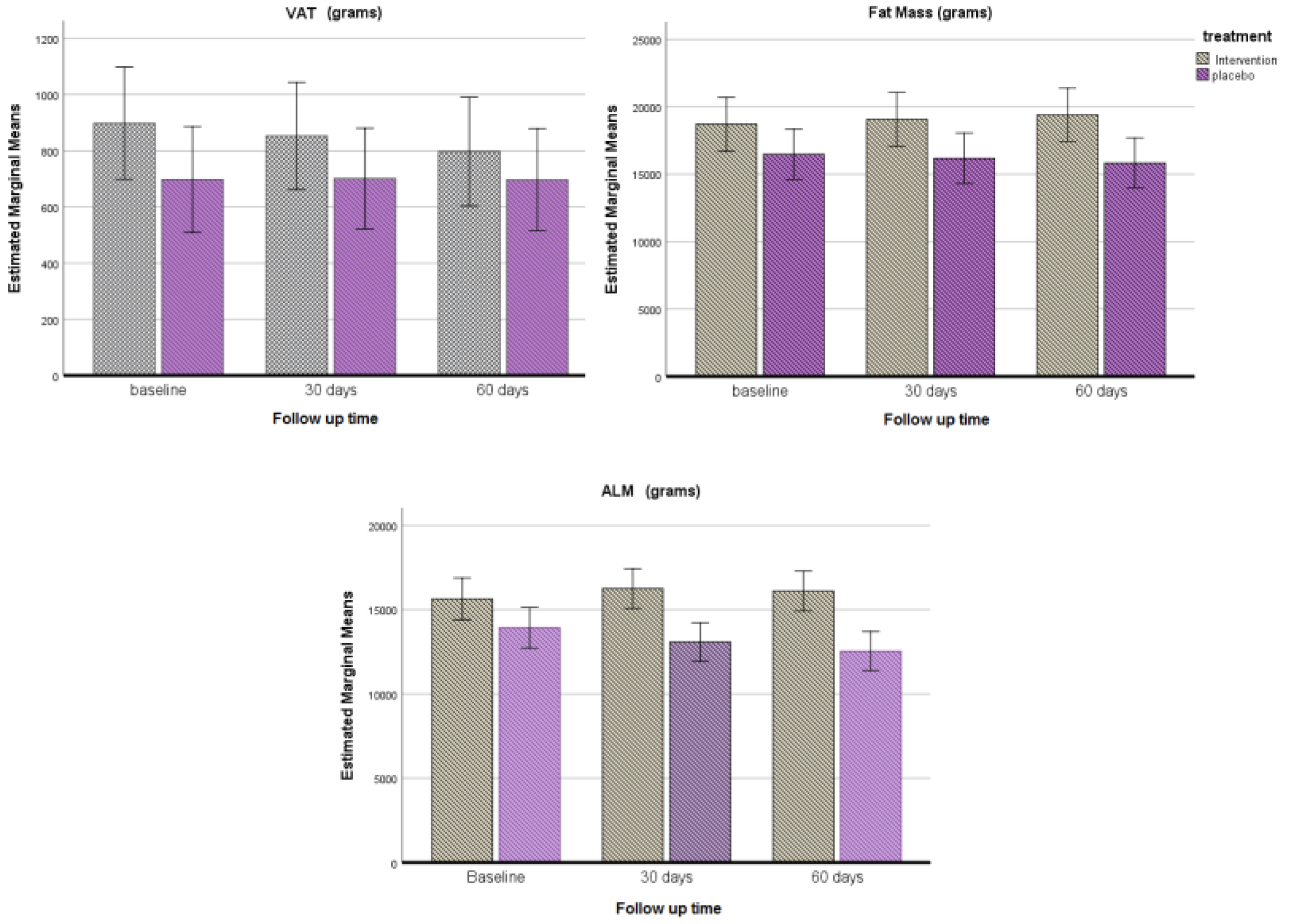
| FM (g) | 18,701.52 ± 4953.04 | 16,474.92 ± 4516.35 | 17,520.06 ± 4809.19 | 0.106 |
| VAT (g) | 898.48 ± 648.860 | 698.04 ± 238.10 | 792.12 ± 482.40 | 0.149 |
| WC (cm) | 81.22 ± 6.95 | 79.46 ± 8.14 | 80.29 ± 7.58 | 0.424 |
| DBP (mm Hg) | 77.30 ± 4.19 | 78.65 ± 5.08 | 78.02 ± 4.68 | 0.319 |
| SBP (mm Hg) | 126.96 ± 5.59 | 125.58 ± 5.89 | 126.22 ± 5.73 | 0.406 |
| Tinetti (score) | 17.83 ± 4.01 | 16.88 ± 3.70 | 17.33 ± 3.84 | 0.397 |
| SPPB total (score) | 4.22 ± 1.45 | 4.38 ± 0.64 | 4.31 ± 1.08 | 0.595 |
| Handgrip (kg) | 18.74 ± 4.33 | 17.64 ± 4.68 | 18.06 ± 4.52 | 0.328 |
| Barthel (score) | 60.43 ± 18.64 | 55.58 ± 19.30 | 57.86 ± 18.96 | 0.376 |
| ADL (score) | 3.22 ± 0.85 | 3.19 ± 1.50 | 3.20 ± 1.22 | 0.944 |
| Physical SF-12 (score) | 33.80 ± 6.83 | 37.29 ± 11.82 | 35.65 ± 9.86 | 0.220 |
| Mental SF-12 (score) | 40.78 ± 8.73 | 40.89 ± 12.40 | 40.83 ± 10.56 | 0.973 |
| GDS (score) | 14.13 ± 1.98 | 13.88 ± 1.77 | 14.00 ± 1.86 | 0.649 |
| Creatinine (mg/dL) | 0.86 ± 0.13 | 0.79 ± 0.21 | 0.82 ± 0.18 | 0.181 |
| AST (IU/L) | 19.26 ± 8.86 | 18.04 ± 8.81 | 18.61 ± 8.77 | 0.631 |
| ALT (IU/L) | 16.17 ± 7.99 | 16.38 ± 10.87 | 16.29 ± 9.53 | 0.939 |
| GGT (IU/L) | 15.90 ± 3.15 | 16.37 ± 2.39 | 16.15 ± 2.75 | 0.560 |
| FBG (mg/dL) | 86.09 ± 11.16 | 88.92 ± 9.43 | 87.59 ± 10.27 | 0.340 |
| CRP (mg/dL) | 1.20 ± 1.45 | 0.94 ± 0.59 | 1.06 ± 1.08 | 0.392 |
| Leucine | 105.30 ± 18.33 | 99.95 ± 24.19 | 102.46 ± 21.59 | 0.393 |
| Isoleucine | 88.25 ± 24.09 | 88.63 ± 25.47 | 88.45 ± 24.58 | 0.958 |
| Valine | 133.30 ± 36.23 | 143.98 ± 41.39 | 138.97 ± 39.02 | 0.344 |
| Total AA | 326.85 ± 38.43 | 332.56 ± 57.64 | 329.88 ± 49.15 | 0.689 |
| Variable | Intervention Group (N = 22) Mean Change from Baseline (95% CI) | Placebo Group (N = 28) Mean Change from Baseline (95% CI) | Difference between Groups (95% CI) | p-Value between Group |
|---|---|---|---|---|
| Weight (kg) | 1.378 (0.813, −1.943) | −1.442 (−1.972, −0.912) * | 2.820 (2.028, 3.611) | <0.001 |
| BMI (kg/m2) | 0.539 (0.308, 0.769) * | −0.600 (−0.816, −0.384) * | 1.139 (0.816, 1.462) | <0.001 |
| ALM (kg) | 0.350 (−0.607, 1.307) | −1.268 (−2205.44, −332.26) * | 1.618 (−255.75, 2982.00) | 0.245 |
| ALM/h2 | 0.516 (−0.114; 1.146) | −0.505 (−1.103; 0.097) | 1.021 (−1.910; −0.132) | <0.05 |
| SMI (%) | 0.282 (−1.098; 1.662) | −0.950 (−2.269; 0.368) | 1.232 (−0.719; 3.170) | 0.208 |
| FM (g) | 700.522 (360.688, 1040.356) * | −646.962 (−965.724, −328.200) * | 1347.484 (871.386, 1823.582) | <0.001 |
| VAT (g) | −113.171 (−188.061, −38.280) * | 9.151 (−61.096, 79.398) | −122.321 (−227.241, −17.402) | <0.001 |
| WC (cm) | 0.441 (−0.005, 0.887) | −0.582 (−1.000, −0.164) * | 1.023 (0.399, 1.648) | <0.001 |
| DBP (mm Hg) | 0.214 (−1.364; 1.764) | −0.574 (−2.055; 0.907) | 0.788 (−1.423; 3.000) | 0.477 |
| SBP (mm Hg) | −0.332 (−2.004, 1.341) | −0.399 (−1.968, 1.170) | 0.067 (−2.276, 2.411) | 0.954 |
| Tinetti (score) | 1.940 (0.989, 2.891) * | −0.447 (−1.339, 0.446) | 2.386 (1.054, 3.719) | <0.001 |
| SPPB total (score) | 2.667 (2.108, 3.226) * | 0.448 (−0.076, 0.973) | 2.219 (1.436, 3.002) | <0.001 |
| Handgrip (kg) | 3.332 (2.400, 4.264) * | −0.755 (−1.629, 0.119) | 4.087 (2.781, 5.392) | <0.001 |
| Barthel (score) | 4.222 (0.480, 7.964) * | 0.111 (−3.399, 3.621) | 4.111 (−1.132, 9.353) | 0.121 |
| ADL (score) | 0.506 (0.127, 0.885) * | −0.101 (−0.457, 0.254) | 0.607 (0.076, 1.138) | <0.001 |
| Physical SF-12 (score) | 2.747 (−0.472, 5.966) | 3.519 (0.499, 6.538) * | −0.772 (−5.281, 3.738) | 0.732 |
| Mental SF-12 (score) | 1.238 (−0.631, 3.107) | 3.789 (2.036, 5.543) * | −2.551 (−5.170, 0.067) | 0.056 |
| GDS (score) | −0.262 (−0.631, 0.106) | 0.155 (−0.190, 0.501) | −0.418 (−0.934, 0.099) | 0.110 |
| Creatinine (mg/dL) | −0.025 (−0.078, 0.027) | 0.043 (−0.007, 0.092) | −0.068 (−0.141, 0.005) | 0.069 |
| AST (IU/L) | 0.421 (−2.311, 3.154) | 0.435 (−2.128, 2.998) | −0.013 (−3.841, 3.815) | 0.994 |
| ALT (IU/L) | 3.150 (0.042, 6.258) * | 0.059 (−2.856, 2.974) | 3.091 (−1.263, 7.445) | 0.160 |
| GGT (IU/L) | 0.235 (−0.236, 0.705) | 0.069 (−0.372, 0.511) | 0.165 (−0.494, 0.824) | 0.617 |
| FBG (mg/dL) | −0.904 (−5.580, 3.773) | −2.470 (−6.856, 1.917) | 1.566 (−4.986, 8.118) | 0.633 |
| CRP (mg/dL) | −0.690 (−1.094, −0.286) * | 0.266 (−0.113, 0.645) | −0.956 (−1.522, −0.390) | <0.001 |
| Leucine | 17.832 (11.653, 24.010) * | 4.814 (−0.981, 10.610) | 13.017 (4.361, 21.674) | <0.001 |
| Isoleucine | 17.855 (13.369, 22.341) * | −2.304 (−6.512, 1.903) | 20.159 (13.875, 26.444) | <0.001 |
| Valine | 24.020 (16.177, 31.863) * | −3.116 (−10.472, 4.241) | 27.135 (16.148, 38.123) | <0.001 |
| Total AA | 59.706 (47.960, 71.452) * | −0.606 (−11.623, 10.412) | 60.312 (43.856, 76.768) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2022, 14, 4566. https://doi.org/10.3390/nu14214566
Rondanelli M, Gasparri C, Barrile GC, Battaglia S, Cavioni A, Giusti R, Mansueto F, Moroni A, Nannipieri F, Patelli Z, et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients. 2022; 14(21):4566. https://doi.org/10.3390/nu14214566
Chicago/Turabian StyleRondanelli, Mariangela, Clara Gasparri, Gaetan Claude Barrile, Santina Battaglia, Alessandro Cavioni, Riccardo Giusti, Francesca Mansueto, Alessia Moroni, Fabrizio Nannipieri, Zaira Patelli, and et al. 2022. "Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial" Nutrients 14, no. 21: 4566. https://doi.org/10.3390/nu14214566
APA StyleRondanelli, M., Gasparri, C., Barrile, G. C., Battaglia, S., Cavioni, A., Giusti, R., Mansueto, F., Moroni, A., Nannipieri, F., Patelli, Z., Razza, C., Tartara, A., & Perna, S. (2022). Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients, 14(21), 4566. https://doi.org/10.3390/nu14214566





