Postprandial Glycemic Response to Whole Fruit versus Blended Fruit in Healthy, Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Fruit Treatments
2.3. Experimental Trial Procedure
2.4. Statistical Analysis
3. Results
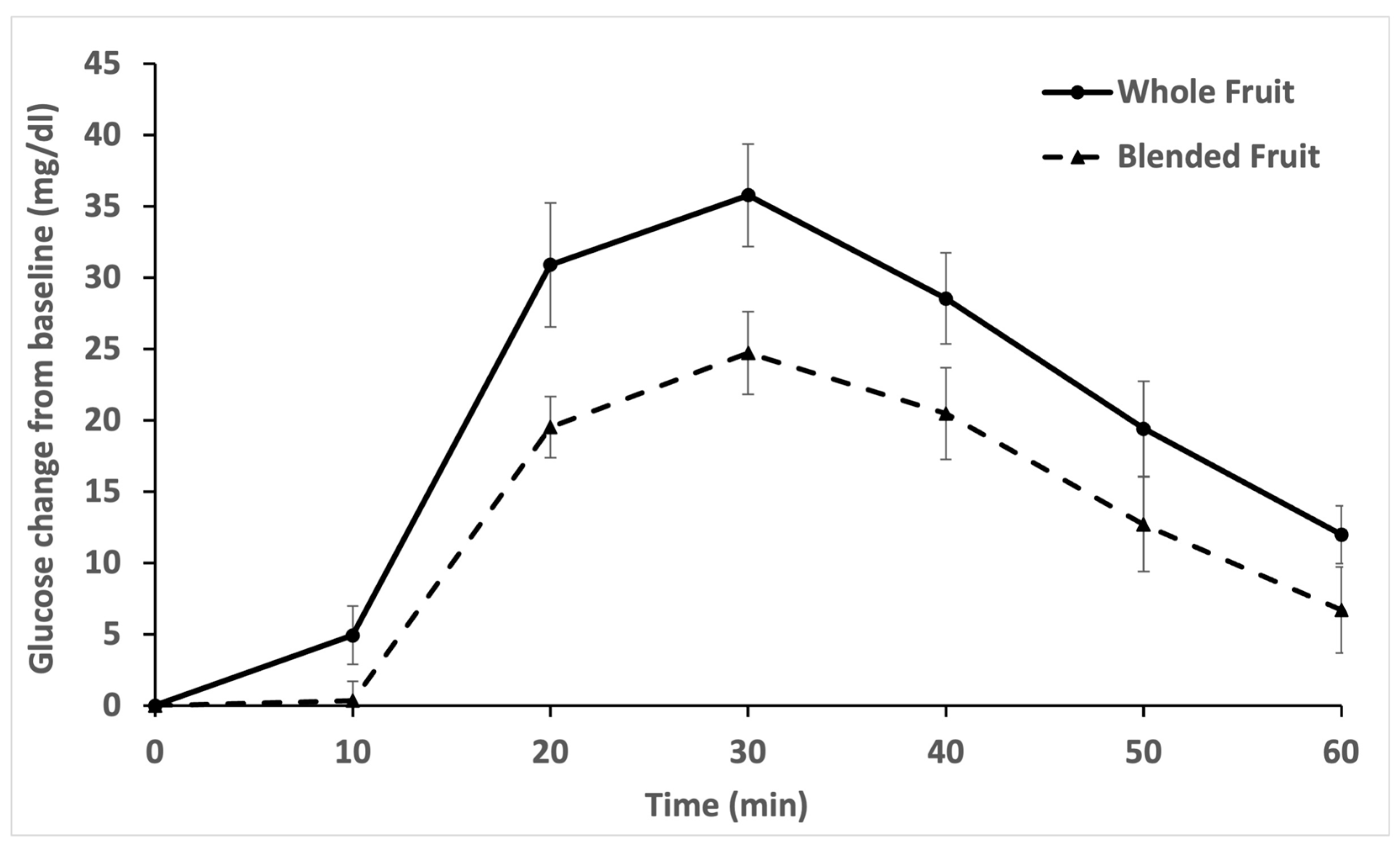
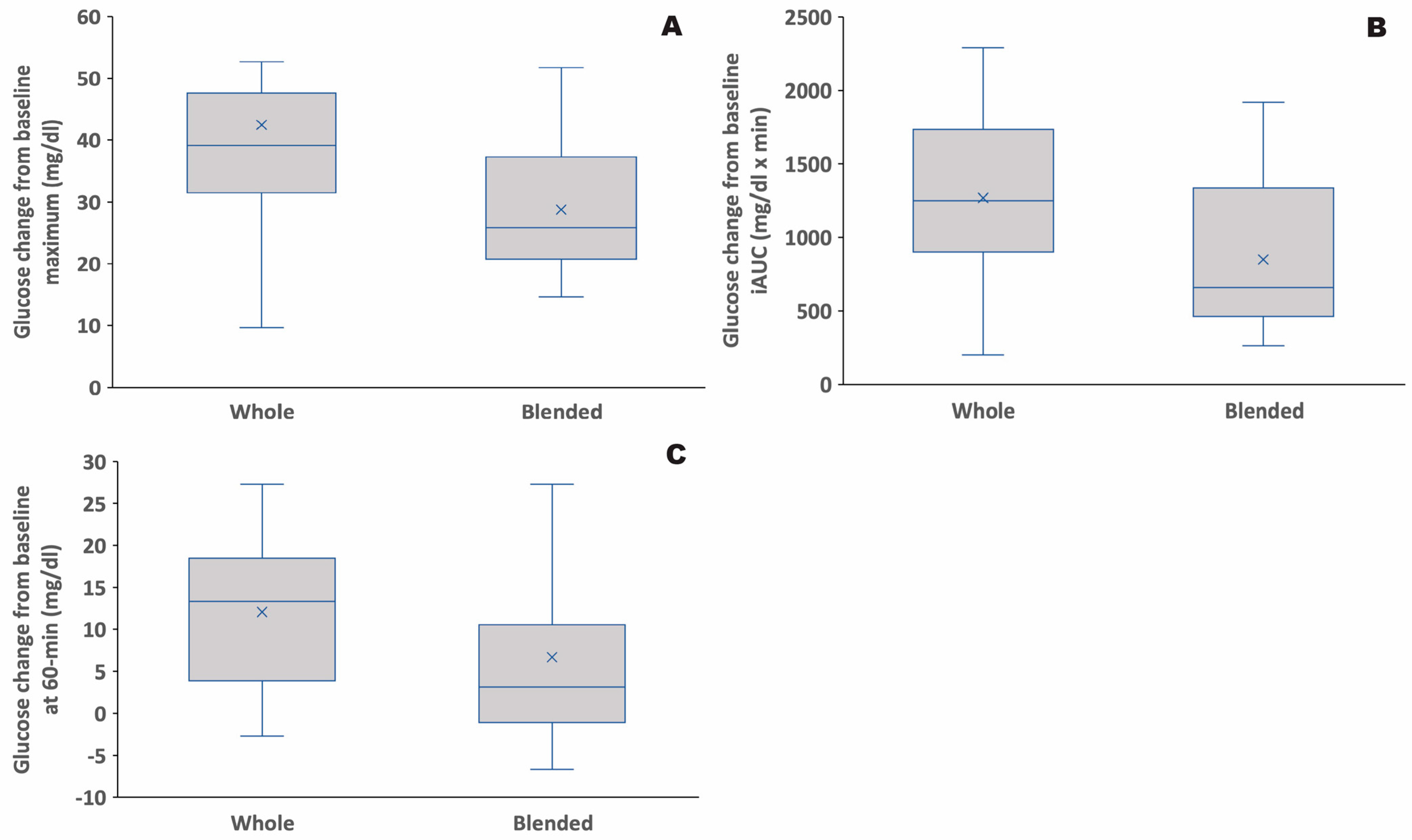
3.1. Glycemic Response to Whole vs. Blended Fruit
3.2. Sex as a Factor in Glycemic Response to Whole vs. Blended Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFronzo, R.A.; Ferrannini, E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef] [PubMed]
- Crofts, C.A.P.; Zinn, C.; Wheldon, M.; Schofield, G. Hyperinsulinemia: A unifying theory of chronic disease. Diabesity 2015, 1, 34–43. [Google Scholar] [CrossRef]
- Grooms, K.N.; Ommerborn, M.J.; Pham, D.Q.; Djoussé, L.; Clark, C.R. Dietary Fiber Intake and Cardiometabolic Risks among US Adults, NHANES 1999-2010. Am. J. Med. 2013, 126, 1059–1067.e4. [Google Scholar] [CrossRef]
- Hagander, B.; Asp, N.-G.; Efendić, S.; Nilsson-Ehle, P.; Scherstén, B. Dietary fiber decreases fasting blood glucose levels and plasma LDL concentration in noninsulin-dependent diabetes mellitus patients. Am. J. Clin. Nutr. 1988, 47, 852–858. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs Jr, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: A systematic review and meta-analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses II: In vivo digestion process. Food Hydrocoll. 2020, 107, 105908. [Google Scholar] [CrossRef]
- Villemejane, C.; Wahl, R.; Aymard, P.; Denis, S.; Michon, C. In vitro digestion of short-dough biscuits enriched in proteins and/or fibres, using a multi-compartmental and dynamic system (1): Viscosity measurement and prediction. Food Chem. 2015, 182, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Haber, G.B.; Heaton, K.W.; Murphy, D.; Burroughs, L.F. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet 1977, 2, 679–682. [Google Scholar] [CrossRef]
- Redfern, K.; Cammack, V.; Sweet, N.; Preston, L.; Jarvis, M.; Rees, G. Nutrient-extraction blender preparation reduces postprandial glucose responses from fruit juice consumption. Nutr. Diabetes 2017, 7, e288. [Google Scholar] [CrossRef]
- Alkutbe, R.; Redfern, K.; Jarvis, M.; Rees, G. Nutrient Extraction Lowers Postprandial Glucose Response of Fruit in Adults with Obesity as well as Healthy Weight Adults. Nutrients 2020, 12, 766. [Google Scholar] [CrossRef]
- González-Rodríguez, M.; Pazos-Couselo, M.; García-López, J.M.; Rodríguez-Segade, S.; Rodríguez-García, J.; Túñez-Bastida, C.; Gude, F. Postprandial glycemic response in a non-diabetic adult population: The effect of nutrients is different between men and women. Nutr. Metab. 2019, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Gannon, M.C.; Nuttall, F.Q. Postprandial plasma glucose, insulin, glucagon and triglyceride responses to a standard diet in normal subjects. Diabetologia 1976, 12, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Niclis, C.; Prado, D.; Diaz, M.D.P.; Soria, E.A.; Albrecht, C. Postprandial glycemic response in different bakery formulations with adequate palatability: Sex-related effects. Nutr. Food Sci. 2021. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- USDA. Agricultural Research Service: FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 11 October 2022).
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Hirsch, G.E.; Facco, E.M.P.; Rodrigues, D.B.; Vizzotto, M.; Emanuelli, T. Physicochemical characterization of blackberry from the Southern Region of Brazil/Caracterizacao fisico-quimica de variedades de amora-preta da regiao sul do Brasil. Ciência Rural 2012, 42, 942–948. [Google Scholar] [CrossRef][Green Version]
- Siriwoharn, T. Wrolstad, RPolyphenolic Composition of Marion and Evergreen Blackberries. J. Food Sci. 2004, 69, FCT233–FCT240. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.A.; Stewart, D. Different Polyphenolic Components of Soft Fruits Inhibit α-Amylase and α-Glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Majewski, M.; Kucharczyk, E.; Kaliszan, R.; Markuszewski, M.; Fotschki, B.; Juśkiewicz, J.; Borkowska-Sztachańska, M.; Ognik, K. The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats. Nutrients 2020, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Rastogi, N.K.; Raghavarao, K.; Tharanathan, R. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, F.; Pan, S. Effect of grinding methods on structural, physicochemical, and functional properties of insoluble dietary fiber from orange peel. Int. J. Polym. Sci. 2016, 2016, 6269302. [Google Scholar] [CrossRef]
- Spiller, G.A.; Jensen, C.D.; Pattison, T.S.; Chuck, C.S.; Whittam, J.H.; Scala, J. Effect of protein dose on serum glucose and insulin response to sugars. Am. J. Clin. Nutr. 1987, 46, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.; Krezowski, P. Effect of Protein Ingestion on the Glucose and Insulin Response to a Standardized Oral Glucose Load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef]
- Dimitreli, G.; Thomareis, A.S. Effect of temperature and chemical composition on processed cheese apparent viscosity. J. Food Eng. 2004, 64, 265–271. [Google Scholar] [CrossRef]
- Holt, S.H.; Miller, J.C.; Petocz, P. An insulin index of foods: The insulin demand generated by 1000-kJ portions of common foods. Am. J. Clin. Nutr. 1997, 66, 1264–1276. [Google Scholar] [CrossRef]
- Bolton, R.P.; Heaton, K.W.; Burroughs, L.F. The role of dietary fiber in satiety, glucose, and insulin: Studies with fruit and fruit juice. Am. J. Clin. Nutr. 1981, 34, 211–217. [Google Scholar] [CrossRef]
- Dahlqvist, A. Assay of intestinal disaccharidases. Anal. Biochem. 1968, 22, 99–107. [Google Scholar]
- German, D.P.; Bittong, R.A. Digestive enzyme activities and gastrointestinal fermentation in wood-eating catfishes. J. Comp. Physiol. B 2009, 179, 1025–1042. [Google Scholar]

| Males (n = 8) | Females (n = 12) | Sex Effect | All (n = 20) | Treatment Effect | Treat * Sex Effect | ||||
|---|---|---|---|---|---|---|---|---|---|
| Whole | Blended | Whole | Blended | p Value | Whole | Blended | p Value | p Value | |
| Max | 48.9 ± 10.8 | 28.2 ± 3.6 | 38.2 ± 2.5 | 29.1 ± 3.3 | 0.64 | 42.5 ± 4.6 | 28.8 ± 2.4 | 0.004 | 0.42 |
| iAUC | 1314 ± 269 | 721 ± 142 | 1239 ± 116 | 935 ± 155 | 0.47 | 1269 ± 124 | 850 ± 109 | 0.005 | 0.73 |
| 60-min | 11.3 ± 3.5 | 0.3 ± 3.5 | 12.6 ± 2.6 | 11.0 ± 4.2 | 0.17 | 12.1 ± 2.0 | 6.7 ± 3.0 | 0.057 * | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crummett, L.T.; Grosso, R.J. Postprandial Glycemic Response to Whole Fruit versus Blended Fruit in Healthy, Young Adults. Nutrients 2022, 14, 4565. https://doi.org/10.3390/nu14214565
Crummett LT, Grosso RJ. Postprandial Glycemic Response to Whole Fruit versus Blended Fruit in Healthy, Young Adults. Nutrients. 2022; 14(21):4565. https://doi.org/10.3390/nu14214565
Chicago/Turabian StyleCrummett, Lisa T., and Riley J. Grosso. 2022. "Postprandial Glycemic Response to Whole Fruit versus Blended Fruit in Healthy, Young Adults" Nutrients 14, no. 21: 4565. https://doi.org/10.3390/nu14214565
APA StyleCrummett, L. T., & Grosso, R. J. (2022). Postprandial Glycemic Response to Whole Fruit versus Blended Fruit in Healthy, Young Adults. Nutrients, 14(21), 4565. https://doi.org/10.3390/nu14214565






