The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Searches
2.4. Study Selection
2.5. Data Collection Process
2.6. Intra-Study Risk of Bias Assessment
Summary Measures
2.7. Synthesis of Results
2.8. Risk of Bias across Studies
2.9. Additional Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Results of Individual Studies
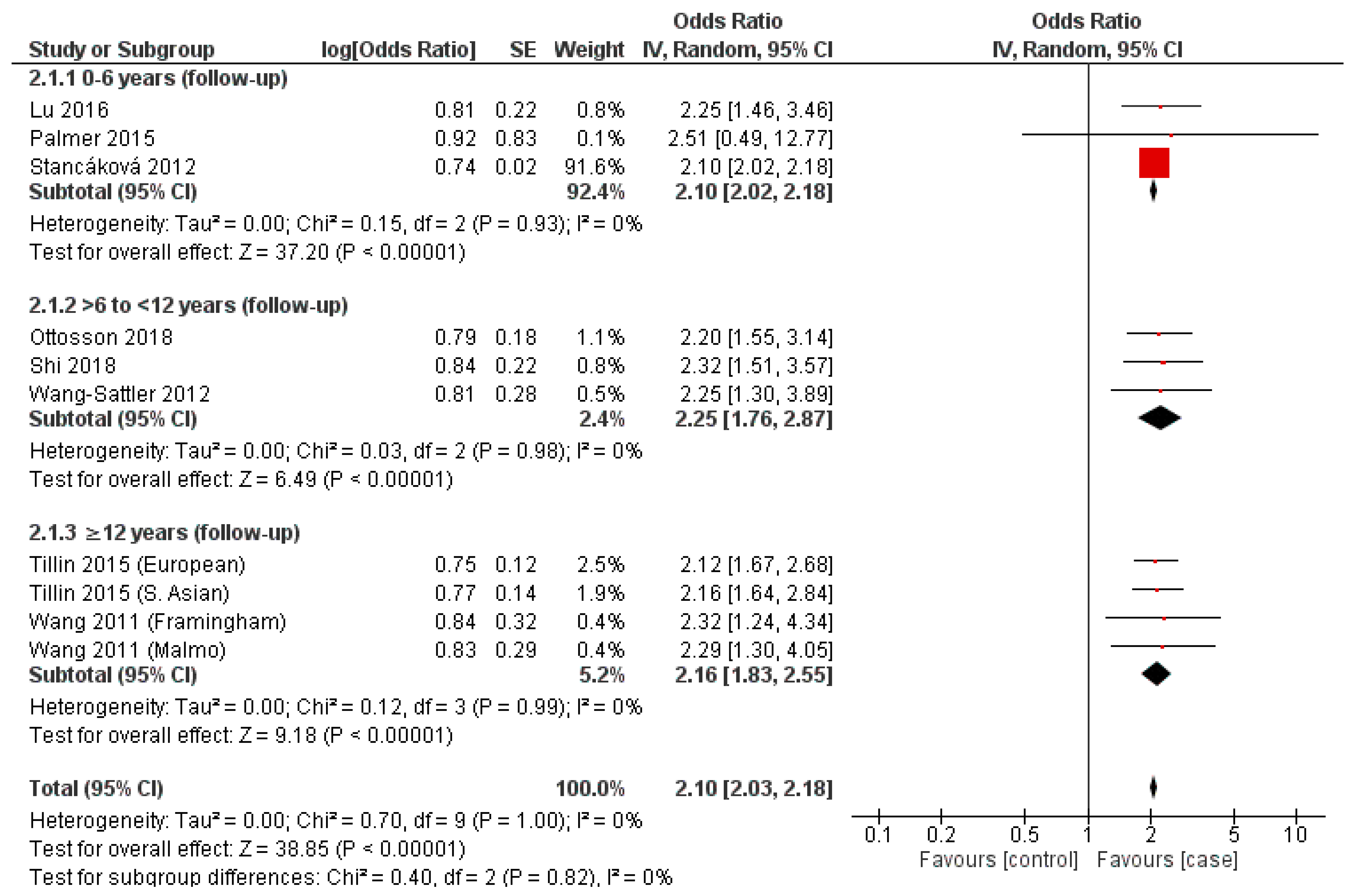
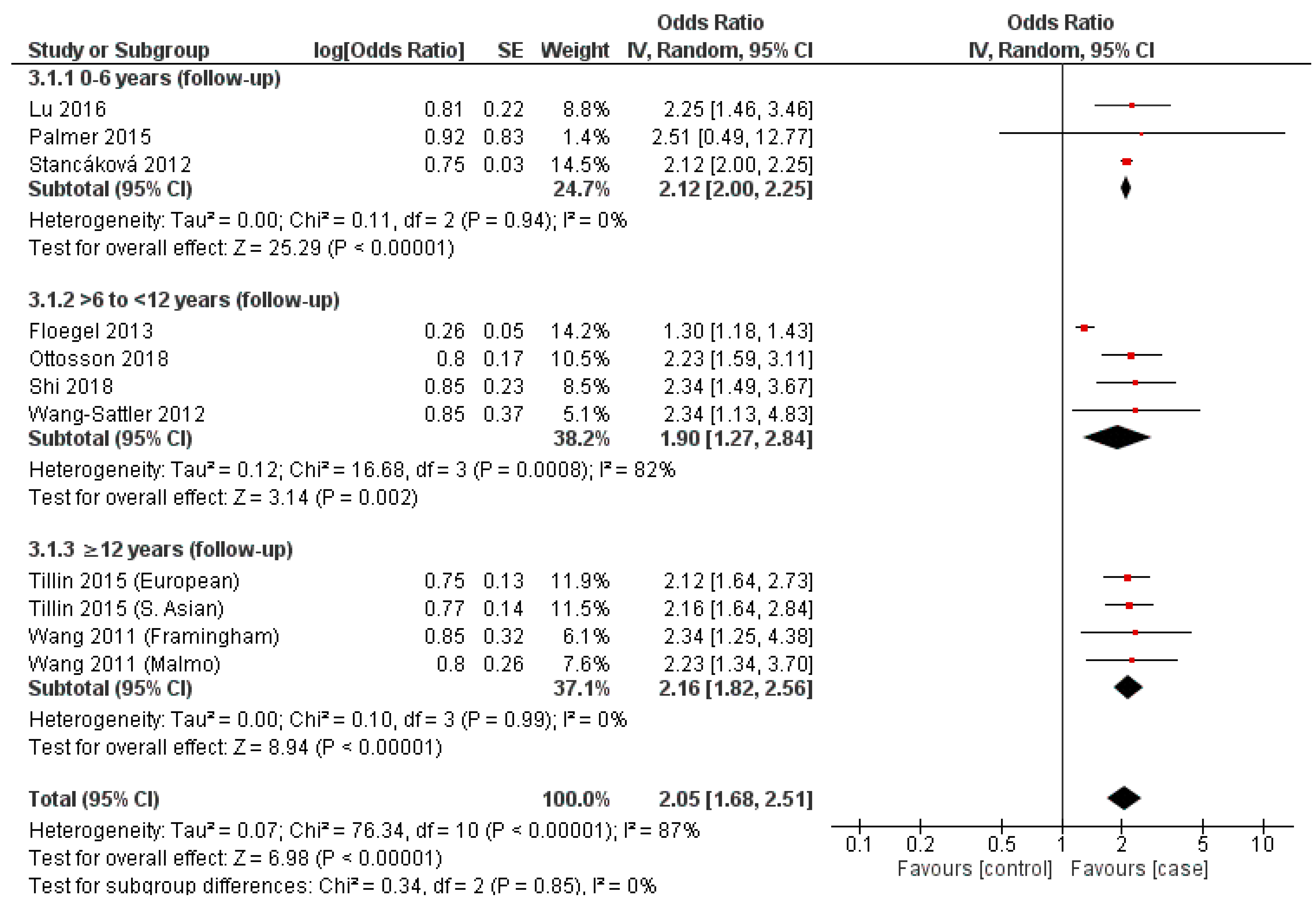
3.5. Valine
3.6. Leucine
3.7. Isoleucine
3.8. Synthesis of Results
Risk of Bias across Studies
3.9. Sensitivity
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | Branch Chain Amino Acid |
| BCKA | Branched-chain α-ketoacids |
| mTORc1 | Mammalian Target Rapamycin Complex 1 |
| NICE | National Institute for Clinical Excellence |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PICOS | Population, Intervention, Comparison and Studies |
| PROSPERO | International Prospective Register of Systematic Reviews |
| T2DM | Type 2 Diabetes Mellitus |
| BMI | Body Mass Index |
| WHO | World Health Organisation |
| ID | Identification |
| IR | Insulin Resistance |
| SD | Standard Deviation |
| C2 | Aceylcarnitine |
| CI | Confidence Interval |
| TSA | Trial Sequential Analysis |
| FBC | Fasting Blood Glucose |
| BP | Blood Pressure |
| IV | Inverse Variance |
| ORs | Odds Ratios |
| RoB | Risk of Bias |
| SMD | Standardised Mean Difference |
References
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight Facts: How Do We Define Obesity and Global Strategy on World Health. 2003. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 September 2022).
- Herman, W.H.; Ye, W.; Griffin, S.J.; Simmons, R.K.; Davies, M.J.; Khunti, K.; Rutten, G.E.; Sandbaek, A.; Lauritzen, T.; Borch-Johnsen, K.; et al. Early Detection and Treatment of Type 2 Diabetes Reduce Cardiovascular Morbidity and Mortality: A Simulation of the Results of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe). Diabetes Care 2015, 38, 1449–1455. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Byun, J.; Pennathur, S. Analytical Approaches to Metabolomics and Applications to Systems Biology. Semin. Nephrol. 2010, 30, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Pallares-Méndez, R.; Aguilar-Salinas, C.A.; Cruz-Bautista, I.; del Bosque-Plata, L. Metabolomics in diabetes, a review. Ann. Med. 2016, 8, 89–102. Available online: http://search.ebscohost.com/login.aspx?direct=true&scope=site&site=ehost-live&db=mdc&AN=26883715 (accessed on 1 September 2022). [CrossRef]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of Branched-Chain Amino Acid Metabolism in Type 2 Diabetes, Obesity, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Merz, B.; Frommherz, L.; Rist, M.J.; Kulling, S.E.; Bub, A.; Watzl, B. Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women—Results from the KarMeN Study. Nutrients 2018, 10, 623. [Google Scholar] [CrossRef]
- Rousseau, M.; Guénard, F.; Garneau, V.; Allam-Ndoul, B.; Lemieux, S.; Pérusse, L.; Vohl, M.-C. Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Williams, M. Dietary Supplements and Sports Performance: Amino Acids. J. Int. Soc. Sports Nutr. 2005, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; LaCroix, A.Z.; Thomson, C.A.; Tinker, L.; Larson, J.C.; Qi, Q.; Qi, L.; Cooper-DeHoff, R.M.; Phillips, L.S.; Prentice, R.L.; et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br. J. Nutr. 2017, 117, 1523–1530. Available online: http://gateway.proquest.com/openurl?ctx_ver=Z39.88-2004&res_id=xri:pqm&req_dat=xri:pqil:pq_clntid=145298&rft_val_fmt=ori/fmt:kev:mtx:journal&genre=article&issn=0007-1145&volume=117&issue=11&spage=1523 (accessed on 1 September 2022). [CrossRef] [PubMed]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-Chain and Aromatic Amino Acids Are Predictors of Insulin Resistance in Young Adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Ramzan, I.; Taylor, M.; Phillips, B.; Wilkinson, D.; Smith, K.; Hession, K.; Idris, I.; Atherton, P. A Novel Dietary Intervention Reduces Circulatory Branched-Chain Amino Acids by 50%: A Pilot Study of Relevance for Obesity and Diabetes. Nutrients 2020, 13, 95. [Google Scholar] [CrossRef]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.-C.; Pesta, D.; Zaharia, O.-P.; Bódis, K.; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Kramer, N.B.; Evers, S.S.; Shin, J.H.; Silverwood, S.; Wang, Y.; Burant, C.F.; Sandoval, D.A.; Seeley, R.J. The Role of Elevated Branched-Chain Amino Acids in the Effects of Vertical Sleeve Gastrectomy to Reduce Weight and Improve Glucose Regulation. Cell Rep. 2020, 33, 108239. [Google Scholar] [CrossRef]
- Long, J.; Yang, Z.; Wang, L.; Han, Y.; Peng, C.; Yan, C.; Yan, D. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 174. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Osté, M.C.J.; Kieneker, L.M.; Gruppen, E.G.; Wolak-Dinsmore, J.; Otvos, J.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. J. Clin. Med. 2018, 7, 513. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, H.-Y.; Fan, Z.-Y.; He, Y.; Yan, Y.-X. Metabolomics Signatures in Type 2 Diabetes: A Systematic Review and Integrative Analysis. J. Clin. Endocrinol. Metab. 2019, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Esfandyari, S.; Azizi, F. Dietary Protein and Amino Acid Profiles in Relation to Risk of Dysglycemia: Findings from a Prospective Population-Based Study. Nutrients 2017, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High dietary intake of branched-chain amino acids is associated with an increased risk of insulin resistance in adults. J. Diabetes 2018, 10, 357–364. [Google Scholar] [CrossRef]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’Yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Geidenstam, N.; Al-Majdoub, M.; Ekman, M.; Spégel, P.; Ridderstråle, M. Metabolite profiling of obese individuals before and after a one year weight loss program. Int. J. Obes. 2017, 41, 1369–1378. [Google Scholar] [CrossRef]
- Palmer, N.D.; Stevens, R.D.; Antinozzi, P.A.; Anderson, A.; Bergman, R.N.; Wagenknecht, L.E.; Newgard, C.B.; Bowden, D.W. Metabolomic Profile Associated With Insulin Resistance and Conversion to Diabetes in the Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2015, 100, E463–E468. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Ong, C.-N.; Subramaniam, T.; Choi, H.W.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: An untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016, 59, 2349–2359. [Google Scholar] [CrossRef]
- Ottosson, F.; Smith, E.; Melander, O.; Fernandez, C. Altered Asparagine and Glutamate Homeostasis Precede Coronary Artery Disease and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3060–3069. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Gulseth, H.L.; Langleite, T.M.; Norheim, F.; Olsen, T.; Refsum, H.; Jensen, J.; Birkeland, K.I.; Drevon, C.A. Branched-chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia 2020, 64, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Wada, E.; Kobayashi, M.; Kohno, D.; Kikuchi, O.; Suga, T.; Matsui, S.; Yokota-Hashimoto, H.; Honzawa, N.; Ikeuchi, Y.; Tsuneoka, H.; et al. Disordered branched chain amino acid catabolism in pancreatic islets is associated with postprandial hypersecretion of glucagon in diabetic mice. J. Nutr. Biochem. 2021, 97, 108811. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Branch Chain Amino Acid Time Progression-Search Results-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=diabetesbranchchainaminoacidtimeprogression&page=4 (accessed on 25 July 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses-ScienceOpen. Available online: https://www.scienceopen.com/document?vid=54b48470-4655-4081-b5d4-e8ebe8d1792e (accessed on 2 December 2020).
- What Is Heterogeneity? Available online: https://handbook-5-1.cochrane.org/chapter_9/9_5_1_what_is_heterogeneity.htm (accessed on 2 December 2020).
- Identifying Publication Bias in Meta-Analyses of Continuous Outcomes|Cochrane Training. Available online: https://training.cochrane.org/resource/identifying-publication-bias-meta-analyses-continuous-outcomes (accessed on 26 January 2021).
- Chapter 10: Analysing Data and Undertaking Meta-Analyses|Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 27 January 2021).
- Shi, L.; Brunius, C.; Lehtonen, M.; Auriola, S.; Bergdahl, I.A.; Rolandsson, O.; Hanhineva, K.; Landberg, R. Plasma metabolites associated with type 2 diabetes in a Swedish population: A case-control study nested in a prospective cohort. Diabetologia 2018, 61, 849–861. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; De Angelis, M.H.; Peters, A.; et al. Identification of Serum Metabolites Associated With Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Stancáková, A.; Civelek, M.; Saleem, N.K.; Soininen, P.; Kangas, A.J.; Cederberg, H.; Paananen, J.; Pihlajamäki, J.; Bonnycastle, L.L.; Morken, M.A.; et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 2012, 61, 1895–1902. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3379649 (accessed on 1 September 2022). [CrossRef]
- Tillin, T.; Hughes, A.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.; Godsland, I.F.; Eastwood, S.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef]
- Chen, S.; Akter, S.; Kuwahara, K.; Matsushita, Y.; Nakagawa, T.; Konishi, M.; Honda, T.; Yamamoto, S.; Hayashi, T.; Noda, M.; et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci. Rep. 2019, 9, 7010. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Zoncu, R.; Sabatini, D.M. Amino acids and mTORC1: From lysosomes to disease. Trends Mol. Med. 2012, 18, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Del Coco, L.; Vergara, D.; de Matteis, S.; Mensà, E.; Sabbatinelli, J.; Prattichizzo, F.; Bonfigli, A.R.; Storci, G.; Bravaccini, S.; Pirini, F.; et al. NMR-Based Metabolomic Approach Tracks Potential Serum Biomarkers of Disease Progression in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 720. [Google Scholar] [CrossRef]

| Lead Author | Publication Date | Study Design | Cases (n) | Control (n) | Patient Demographics (Intervention(s)) | Follow-Up Period (Y) | Covariates | Cases Group Status | Control Group Status | Individual BCAAs | BCAA Metabolites |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F Ottosson [31] | 2018 | Case-control | 204 | 496 | Mean of 69.5 years, predominantly male (69%), Swedish nationality | 6.3 | Age and Sex. | T2DM | Non-diabetic | Leucine, Isoleucine, Valine | C5, C4 and C3 |
| Y Lu [30] | 2016 | Case-control | 197 | 197 | 55.15 ± 2.8 years, predominantly female (59.4%), Chinese nationality | 6 | BMI, smoking, history of hypertension | T2DM | Non-diabetic, | Leucine, Isoleucine, Valine | C10 |
| L Shi [41] | 2018 | Case-control | 503 | 503 | 50.1 ± 8 years, predominantly female (55.5%), Swedish nationality | 10 | BMI, FBC, PA, education, smoking, consumption of alcohol, dietary fibre, red and processed meat, and coffee, plasma total cholesterol, triglycerols, and systolic and diastolic BP. | T2DM (no medication | Non-diabetic, | Leucine, Isoleucine, Valine | C3 and KMV |
| T Wang (Framingham) [42] | 2011 | Case-control | 189 | 189 | 56.5 ± 8.5 years, predominantly male (58%), USA nationality | 12 | Age, sex, BMI, fasting glucose, and parental history. | T2DM | Non-diabetic | Leucine, Isoleucine, Valine | - |
| T Wang (Malmo) [42] | 2011 | Case-control | 163 | 163 | Mean of 58 years, predominantly female (55%), Swedish nationality | 12.6 | Age, sex, BMI, and fasting glucose. | T2DM | Non-diabetic | Leucine, Isoleucine, Valine | - |
| A Floegel [43] | 2013 | Case-control | 800 | 2282 | 52.1 ± 8.1 years, predominantly women (52.1%), German nationality | 7 | age, sex, alcohol intake, smoking, physical activity, education, coffee intake, red meat intake, prevalent hypertension, BMI, and waist circumference (cm) | T2DM | Non-diabetic | Isoleucine, Valine | - |
| R Wang-Sattler [44] | 2012 | Case-control | 91 | 866 | 64.7 ± 5.45 years, predominantly male (53%), German nationality | 7 | Age, sex, BMI, physical activity, alcohol intake, smoking, systolic BP, HDL cholesterol, HbA1c, fasting glucose and fasting insulin | T2DM(no medication) | Non-diabetic | Leucine, Isoleucine, Valine | C2 |
| A Stancáková [45] | 2012 | Case-control | 646 | 3026 | 57 ± 7 years, all male, Finnish nationality | 4.7 | Age and BMI | T2DM(no medication) | Non-diabetic | Leucine, Isoleucine, Valine | - |
| T Tillin (European) [46] | 2015 | Case-control | 643 | 1007 | 50.6 ± 7.0 years, all male, South Asian origin | 19 | Age, WHR, truncal skinfold thickness, Matsuda-IR, HDL cholesterol level, current smoking, and alcohol consumption. | T2DM(no medication) | Non-diabetic | Leucine, Isoleucine, Valine | - |
| T Tillin (South Asian) [46] | 2015 | Case-control | 801 | 1279 | 52.75 ± 7.25 years, all male, European origin | 19 | Age, WHR, truncal skinfold thickness, Matsuda-IR, HDL cholesterol level, current smoking, and alcohol consumption. | T2DM(no medication) | Non-diabetic | Leucine, Isoleucine, Valine | - |
| ND Palmer [29] | 2015 | Case-control | 76 | 70 | 56 ± 8 years, predominantly female (63%), European-American, Hispanic, and African American ethnicity | 5 | Age, sex, and BMI | T2DM(no medication) | Non-diabetic | Leucine or isoleucine, valine | C2, C5 and C10 |
| Lead Author | Publication Date | Valine Outcome Measure | Leucine Outcome Measure | Isoleucine Outcome Measure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log(OR) | SE | p-Value | Log(OR) | SE | p-Value | Log(OR) | SE | p-Value | ||
| F Ottosson [31] | 2018 | 0.73 | 0.01 | 0.94 | 0.74 | 0.02 | 0.197 | 0.75 | 0.03 | 0.064 |
| Y Lu [30] | 2016 | 0.94 | 0.84 | 0.0003 * | 0.92 | 0.83 | 0.0023 | 0.92 | 0.83 | 0.4 * |
| L Shi [41] | 2018 | 0.84 | 0.27 | <0.001 * | 0.81 | 0.22 | 0.002 | 0.81 | 0.22 | 0.002 * |
| T Wang [42] | 2011 | 0.75 | 0.14 | 0.34 | 0.79 | 0.18 | 0.034 | 0.80 | 0.17 | 0.01 * |
| T Wang [42] | 2011 | 0.24 | 0.05 | 5.89 × 10−5 * | - | - | - | 0.26 | 0.05 | 3.04 × 10−5 * |
| A Floegel [43] | 2013 | 0.81 | 0.28 | 0.03 * | 0.81 | 0.28 | 0.06 | 0.85 | 0.37 | 0.008 * |
| R Wang-Sattler [44] | 2012 | 0.79 | 0.15 | 0.016 * | 0.84 | 0.22 | 0.006 | 0.85 | 0.23 | 0.001 * |
| A Stancáková [45] | 2012 | 0.82 | 0.29 | 0.02 * | 0.84 | 0.32 | 0.006 | 0.85 | 0.32 | 0.004 * |
| T Tillin [46] | 2015 | 0.88 | 0.57 | 0.01 * | 0.83 | 0.29 | 0.009 | 0.80 | 0.26 | 0.09 |
| T Tillin [46] | 2015 | 0.78 | 0.14 | 0.044 * | 0.77 | 0.14 | 0.074 | 0.77 | 0.14 | 0.13 |
| ND Palmer [29] | 2015 | 0.73 | 0.12 | 0.9 | 0.75 | 0.12 | 0.4 | 0.75 | 0.13 | 0.4 |
| Temporal Subgroup (Years Follow-Up) | Data Sources (Lead Author; Year of Publication; BCAAs) | Covariates (Lead Author; Variables) | OR | 95% CI (Lower–Upper) | p-Value |
|---|---|---|---|---|---|
| 0 to 6 | Palmer, 2015 [29] (iso, leu, val) Lu, 2016 [30] (iso, leu, val) | Palmer; Age, sex, BMI, and AIR Lu; BMI, smoking, history of hypertension | 2.28 | 1.77–2.94 | p < 0.00001 |
| >6 to <12 | Ottosson, 2018 [31] (iso, leu, val) | Ottosson; age and sex | 2.17 | 1.81–2.60 | p < 0.00001 |
| ≥12 | Wang (Malmo dataset), 2011 [42] (iso, leu, val) | Wang [Malmo dataset]; age, sex, BMI, and fasting glucose | 2.27 | 1.59–3.25 | p < 0.00001 |
| Lead Author | Valine | Leucine | Isoleucine | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | HOMA-IR | HbA1c | BMI | HOMA-IR | HbA1c | BMI | HOMA-IR | HbA1c | ||||||||||
| Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | Outcome | p-Value | |
| F Ottosson [31] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Y Lu [30] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| L Shi (S) [41] | 0.24 | - | 0.2 | 0.01 | - | - | 0.26 | - | 0.25 | <0.001 | - | - | 0.26 | - | 0.29 | <0.001 | - | - |
| T Wang (P) (Framingham) [42] | - | - | 0.24 | 0.0008 | - | - | - | - | 0.24 | 0.0009 | - | - | - | - | 0.24 | 0.0007 | - | - |
| T Wang (Malmo) [42] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A Floegel [43] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| R Wang-Sattler (P) [44] | 0.27 | - | - | - | 0.08 | - | 0.19 | - | - | - | 0.09 | - | 0.19 | - | - | - | 0.09 | - |
| A Stancáková [45] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| T Tillin (S) (European) [46] | 0.22 | <0.05 | 0.27 | <0.05 | - | - | 0.18 | <0.05 | 0.26 | <0.05 | - | - | 0.23 | <0.05 | 0.34 | <0.05 | - | - |
| T Tillin (S) (South.Asian) [46] | 0.29 | <0.05 | 0.33 | <0.05 | - | - | 0.27 | <0.05 | 0.31 | <0.05 | - | - | 0.31 | <0.05 | 0.35 | <0.05 | - | - |
| ND Palmer [29] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramzan, I.; Ardavani, A.; Vanweert, F.; Mellett, A.; Atherton, P.J.; Idris, I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients 2022, 14, 4411. https://doi.org/10.3390/nu14204411
Ramzan I, Ardavani A, Vanweert F, Mellett A, Atherton PJ, Idris I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients. 2022; 14(20):4411. https://doi.org/10.3390/nu14204411
Chicago/Turabian StyleRamzan, Imran, Arash Ardavani, Froukje Vanweert, Aisling Mellett, Philip J. Atherton, and Iskandar Idris. 2022. "The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis" Nutrients 14, no. 20: 4411. https://doi.org/10.3390/nu14204411
APA StyleRamzan, I., Ardavani, A., Vanweert, F., Mellett, A., Atherton, P. J., & Idris, I. (2022). The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients, 14(20), 4411. https://doi.org/10.3390/nu14204411







