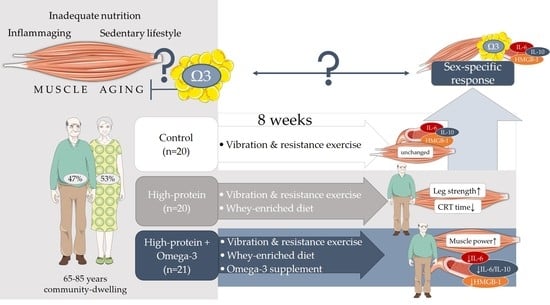
Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population Sample
2.2. Dietary Intervention
2.3. Vibration and Home-Based Resistance Exercise
2.4. Anthropometric Measurements
2.5. Muscle Power
2.6. Muscle Strength and Function
2.7. Laboratory Assessments
2.8. Physical Activity
2.9. Dietary Assessment
2.10. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Exercise Adherence, Dietary Compliance and Nutrient Intake
3.3. Higher Protein Intake Improved Leg Strength, CRT Time, and Fat-Free Mass
3.4. Effects of Omega-3 Supplementation on Muscle Power, Inflammation, and IGF-1
3.5. Sex-Specific Differences in Muscle Parameters and Inflammation
4. Discussion
4.1. Different Effects on Muscle Strength and Power
4.2. Impact on Growth Factors
4.3. No Significant Changes in the Control Group
4.4. Sex-Specific Differences
4.5. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Ferrucci, L.; Ragno, E.; Corsi, A.; Bandinelli, S.; Bonafè, M.; Olivieri, F.; Giovagnetti, S.; Franceschi, C.; Guralnik, J.M.; et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E481–E487. [Google Scholar] [CrossRef] [PubMed]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 2016, 594, 1965–1978. [Google Scholar] [CrossRef]
- Wiegmann, S.; Felsenberg, D.; Armbrecht, G.; Dietzel, R. Longitudinal changes in muscle power compared to muscle strength and mass. J. Musculoskelet. Neuronal Interact. 2021, 21, 13–25. [Google Scholar] [PubMed]
- Simpkins, C.; Yang, F. Muscle power is more important than strength in preventing falls in community-dwelling older adults. J. Biomech. 2022, 134, 111018. [Google Scholar] [CrossRef]
- Bean, J.F.; Leveille, S.G.; Kiely, D.K.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, 728–733. [Google Scholar] [CrossRef]
- Runge, M.; Hunter, G. Determinants of musculoskeletal frailty and the risk of falls in old age. J. Musculoskelet. Neuronal Interact. 2006, 6, 167–173. [Google Scholar]
- Lusardi, M.M.; Fritz, S.; Middleton, A.; Allison, L.; Wingood, M.; Phillips, E.; Criss, M.; Verma, S.; Osborne, J.; Chui, K.K. Determining Risk of Falls in Community Dwelling Older Adults: A Systematic Review and Meta-analysis Using Posttest Probability. J. Geriatr. Phys. Ther. 2017, 40, 1–36. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Luiking, Y.C.; Halfens, R.J.G.; Evers, S.; Lenaerts, E.L.A.; Verlaan, S.; Wallace, M.; Schols, J.; Meijers, J.M.M. Muscle, Health and Costs: A Glance at their Relationship. J. Nutr. Health Aging 2018, 22, 766–773. [Google Scholar] [CrossRef]
- Marín, P.J.; Rhea, M.R. Effects of vibration training on muscle power: A meta-analysis. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2010, 24, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Herpich, C.; Norman, K. Anti-Inflammatory Diets and Fatigue. Nutrients 2019, 11, 2315. [Google Scholar] [CrossRef]
- Haß, U.; Herpich, C.; Kochlik, B.; Weber, D.; Grune, T.; Norman, K. Dietary Inflammatory Index and Cross-Sectional Associations with Inflammation, Muscle Mass and Function in Healthy Old Adults. J. Nutr. Health Aging 2022, 26, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Cordingley, D.M.; Shaw, K.A.; Forbes, S.C.; Leonhardt, T.; Bristol, A.; Candow, D.G.; Chilibeck, P.D. Effects of Omega-3 Supplementation Alone and Combined with Resistance Exercise on Skeletal Muscle in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2221. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Bird, J.K.; Troesch, B.; Warnke, I.; Calder, P.C. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: A scoping systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 46, 73–86. [Google Scholar] [CrossRef]
- Macaluso, A.; De Vito, G. Muscle strength, power and adaptations to resistance training in older people. Eur. J. Appl. Physiol. 2004, 91, 450–472. [Google Scholar] [CrossRef]
- Osawa, Y.; Oguma, Y. Effects of whole-body vibration on resistance training for untrained adults. J. Sport. Sci. Med. 2011, 10, 328–337. [Google Scholar]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F.; Bedu-Addo, G.; Schulze, M.B.; Mockenhaupt, F.P.; Danquah, I. Serum phospholipid fatty acids, dietary patterns and type 2 diabetes among urban Ghanaians. Nutr. J. 2017, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.; Connor Gorber, S.; Tremblay, M.S. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. 2010, 21, 63–69. [Google Scholar]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sport. Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Mikhail, A.; Lan, L.; Di Carlo, A.; Hamilton, B.; Barnard, K.; Hettinga, B.P.; Hatcher, E.; Tarnopolsky, M.G.; Nederveen, J.P.; et al. A Five-Ingredient Nutritional Supplement and Home-Based Resistance Exercise Improve Lean Mass and Strength in Free-Living Elderly. Nutrients 2020, 12, 2391. [Google Scholar] [CrossRef]
- Bell, K.E.; Snijders, T.; Zulyniak, M.; Kumbhare, D.; Parise, G.; Chabowski, A.; Phillips, S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS ONE 2017, 12, e0181387. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef]
- Gholamhosseini, S.; Nematipour, E.; Djazayery, A.; Javanbakht, M.H.; Koohdani, F.; Zareei, M.; Djalali, M. ω-3 fatty acid differentially modulated serum levels of IGF1 and IGFBP3 in men with CVD: A randomized, double-blind placebo-controlled study. Nutrition 2015, 31, 480–484. [Google Scholar] [CrossRef]
- Bagheri, A.; Soltani, S.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr. J. 2020, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Prieske, O.; Herz, M.; Moran, J.; Höhne, J.; Kliegl, R.; Ramirez-Campillo, R.; Behm, D.G.; Hortobágyi, T.; Granacher, U. Home-based exercise programmes improve physical fitness of healthy older adults: A PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res. Rev. 2021, 67, 101265. [Google Scholar] [CrossRef] [PubMed]
- Landen, S.; Hiam, D.; Voisin, S.; Jacques, M.; Lamon, S.; Eynon, N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J. Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Song, J.; Singer, K. Age and Sex: Impact on adipose tissue metabolism and inflammation. Mech. Ageing Dev. 2021, 199, 111563. [Google Scholar] [CrossRef]
| Control (n = 20) | p-Value ¥ | Protein (n = 20) | p-Value ¥ | Omega-3 (n = 21) | p-Value ¥ | p-Value † | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | (V1 vs. V2) | V1 | V2 | (V1 vs. V2) | V1 | V2 | (V1 vs. V2) | (V1) | |
| Sex (women/men) | 10/10 | 11/9 | 11/10 | 0.895 | ||||||
| Age (years) | 69.9 ± 4.5 | 71.5 ± 4.6 | 70.4 ± 5.1 | 0.561 | ||||||
| Waist/height ratio | 0.58 ± 0.06 | 0.57 ± 0.06 | 0.287 | 0.60 ± 0.05 | 0.60 ± 0.06 | 0.110 | 0.59 ± 0.06 | 0.59 ± 0.05 | 0.653 | 0.355 |
| BMI (kg/m2) | 26.9 ± 2.7 | 26.9 ± 2.7 | 0.918 | 28.2 ± 2.3 | 28.4 ± 2.4 | 0.284 | 27.8 ± 2.7 | 28.0 ± 2.7 | 0.011 | 0.249 |
| FFMI (kg/m2) | 18.1 ± 2.1 | 18.0 ± 2.2 | 0.101 | 18.8 ± 2.1 | 19.2 ± 2.2 | 0.144 | 18.4 ± 2.4 | 18.8 ± 2.5 | 0.146 | 0.628 |
| Grip strength (kg/BMI) | 1.21 (0.59) | 1.20 (0.59) | 0.627 | 1.00 (0.43) | 0.95 (0.46) | 0.260 | 1.15 (0.57) | 1.13 (0.56) | 0.794 | 0.272 |
| Gait speed (m/s) | 1.33 (0.25) | 1.30 (0.25) | 0.204 | 1.37 (0.29) | 1.29 (0.30) | 0.351 | 1.31 (0.22) | 1.32 (0.22) | 0.024 | 0.817 |
| Muscle power (watt/m2) | 308 ± 68 | 311 ± 66 | 0.625 | 280 ± 68 | 289 ± 74 | 0.292 | 297 ± 62 | 317 ± 69 | 0.001 | 0.929 |
| CRT time (s) | 4.68 (1.55) | 5.46 (1.60) | 0.184 | 5.13 (1.29) | 4.39 (1.27) | 0.057 | 4.60 (1.13) | 4.51 (0.76) | 0.823 | 0.300 |
| Leg strength (kg/m2) * | 22.2 ± 6.6 | 22.5 ± 6.3 | 0.716 | 20.6 ± 6.6 | 23.9 ± 7.4 | 0.004 | 22.4 ± 4.0 | 24.4 ± 6.0 | 0.029 | 0.566 |
| Omega-3 plasma index (%) | 5.1 ± 1.2 | 4.5 ± 1.2 | 0.015 | 5.6 ± 1.7 | 4.7 ± 1.1 | 0.006 | 4.9 ± 1.4 | 8.6 ± 1.9 | <0.001 | 0.286 |
| CRP (mg/mL) | 1.20 (1.63) | 1.00 (1.57) | 0.765 | 1.23 (2.10) | 1.07 (1.90) | 0.268 | 2.21 (3.12) | 1.52 (2.82) | 0.478 | 0.464 |
| IL-6 (pg/mL) | 2.97 (1.34) | 2.98 (1.32) | 0.911 | 2.62 (1.65) | 2.62 (1.08) | 0.911 | 3.01 (2.10) | 2.56 (1.72) | 0.004 | 0.625 |
| IL-10 (pg/mL) | 8.21 (4.28) | 8.58 (3.82) | 0.627 | 7.84 (3.55) | 8.12 (3.90) | 0.687 | 9.43 (4.21) | 8.02 (4.22) | 0.001 | 0.311 |
| IL-6/IL-10 ratio | 0.37 (0.38) | 0.35 (0.33) | 0.911 | 0.34 (0.25) | 0.28 (0.18) | 0.575 | 0.34 (0.23) | 0.31 (0.20) | 0.821 | 0.979 |
| HMGB-1 (ng/mL) | 0.38 (1.29) | 0.23 (0.80) | 0.286 | 0.29 (0.50) | 0.26 (0.36) | 0.723 | 0.25 (0.64) | 0.20 (0.26) | 0.006 | 0.797 |
| IGF-1 (ng/mL) | 204.7 ± 55.5 | 206.1 ± 48.8 | 0.814 | 205.5 ± 62.1 | 215.1 ± 70.9 | 0.492 | 230.4 ± 56.6 | 259.2 ± 63.7 | 0.004 | 0.277 |
| IGFBP-3 (mg/mL) | 4.35 (1.37) | 4.43 (1.86) | 0.478 | 3.91 (2.55) | 3.56 (4.44) | 0.370 | 4.92 (2.83) | 5.32 (3.36) | 0.085 | 0.272 |
| IGF-1/IGFBP-3 ratio | 47.0 ± 15.1 | 47.6 ± 16.1 | 0.840 | 55.3 ± 20.8 | 54.0 ± 21.4 | 0.741 | 48.8 ± 13.7 | 49.8 ± 15.4 | 0.744 | 0.272 |
| Myostatin (ng/mL) | 2.52 (0.93) | 2.85 (1.40) | 0.351 | 2.36 (1.60) | 2.50 (1.38) | 0.709 | 2.72 (1.08) | 2.90 (1.52) | 0.821 | 0.981 |
| Baseline Values | Mixed Models with Interaction Effects # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 20) | Protein (n = 20) | Omega-3 (n = 21) | Protein Effects (Protein vs. Control) | Combined Effects (Omega-3 vs. Control) | Omega-3 Additional Effects (Omega-3 vs. Protein) | |||||
| Outcome Variable | p-Value † | β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value | |||
| Muscle power (watt/m2) | 308 ± 68 | 280 ± 68 | 297 ± 62 | 0.929 | 4.688 (11.632) | 0.688 | 16.469 (10.452) | 0.118 | 12.572 (9.381) | 0.184 |
| CRT time (s) | 4.68 (1.55) | 5.13 (1.29) | 4.60 (1.13) | 0.300 | −0.939 (0.355) | 0.009 | −0.420 (0.352) | 0.236 | 0.431 (0.303) | 0.159 |
| Leg strength (kg/m2) | 22.2 ± 6.6 | 20.6 ± 6.6 | 22.4 ± 4.0 | 0.566 | 3.109 (1.388) | 0.027 | 1.965 (1.266) | 0.124 | −1.177 (1.503) | 0.436 |
| FFMI (kg/m2) | 18.1 ± 2.1 | 18.8 ± 2.1 | 18.4 ± 2.4 | 0.628 | 0.517 (0.252) | 0.042 | 0.581 (0.236) | 0.015 | 0.043 (0.279) | 0.877 |
| IL-6 (pg/mL) | 2.97 (1.34) | 2.62 (1.65) | 3.01 (2.10) | 0.625 | −0.030 (0.044) | 0.500 | −0.078 (0.040) | 0.056 | −0.048 (0.034) | 0.161 |
| IL-10 (pg/mL) | 8.21 (4.28) | 7.84 (3.55) | 9.43 (4.21) | 0.311 | −0.017 (0.030) | 0.563 | −0.046 (0.023) | 0.050 | −0.028 (0.025) | 0.252 |
| IL-6/IL-10 ratio | 0.37 (0.38) | 0.34 (0.25) | 0.34 (0.23) | 0.979 | −0.013 (0.047) | 0.792 | −0.032 (0.043) | 0.464 | −0.020 (0.035) | 0.579 |
| HMGB-1 (ng/mL) | 0.38 (1.29) | 0.29 (0.50) | 0.25 (0.64) | 0.797 | 0.076 (0.158) | 0.631 | −0.152 (0.143) | 0.287 | −0.227 (0.133) | 0.093 |
| IGF-1 (ng/mL) | 204.7 ± 55.5 | 205.5 ± 62.1 | 230.4 ± 56.6 | 0.277 | 0.006 (0.031) | 0.840 | 0.044 (0.020) | 0.029 | 0.037 (0.032) | 0.241 |
| IGFBP-3 (mg/mL) | 4.35 (1.37) | 3.91 (2.55) | 4.92 (2.83) | 0.272 | 0.025 (0.038) | 0.518 | 0.046 (0.035) | 0.183 | 0.022 (0.037) | 0.584 |
| IGF-1/IGFBP-3 ratio | 47.0 ± 15.1 | 55.3 ± 20.8 | 48.8 ± 13.7 | 0.272 | −0.018 (0.044) | 0.687 | −0.003 (0.039) | 0.945 | 0.015 (0.041) | 0.716 |
| Myostatin (ng/mL) | 2.52 (0.93) | 2.36 (1.60) | 2.72 (1.08) | 0.981 | −0.030 (0.035) | 0.390 | −0.011 (0.035) | 0.757 | 0.019 (0.031) | 0.538 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haß, U.; Kochlik, B.; Herpich, C.; Rudloff, S.; Norman, K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 4274. https://doi.org/10.3390/nu14204274
Haß U, Kochlik B, Herpich C, Rudloff S, Norman K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients. 2022; 14(20):4274. https://doi.org/10.3390/nu14204274
Chicago/Turabian StyleHaß, Ulrike, Bastian Kochlik, Catrin Herpich, Stefan Rudloff, and Kristina Norman. 2022. "Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial" Nutrients 14, no. 20: 4274. https://doi.org/10.3390/nu14204274
APA StyleHaß, U., Kochlik, B., Herpich, C., Rudloff, S., & Norman, K. (2022). Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients, 14(20), 4274. https://doi.org/10.3390/nu14204274