Efficacy and Safety of 6-Month High Dietary Protein Intake in Hospitalized Adults Aged 75 or Older at Nutritional Risk: An Exploratory, Randomized, Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- (a)
- Dementia (Mini-Cog score below 3 [14]) or psychiatric disorder.
- (b)
- Current steroid use.
- (c)
- Moderate or severe liver disease (Child-Pugh classification above 7) or moderate to severe renal dysfunction (serum creatinine above 2.0 mg/dL).
- (d)
- Heart failure that restricted physical activity (New York Heart Association functional classification of II or greater).
- (e)
- Severe, chronic complications of diabetes such as overt proteinuria, proliferative retinopathy or severe neuropathy.
- (f)
- Infectious disease of the systemic inflammatory reaction group or trauma (4 or higher on the abbreviated injury scale).
- (g)
- Gastrointestinal reconstruction surgery.
- (h)
- Untreated cancer.
- (i)
- Cardiac pacemaker or implanted defibrillator.
- (j)
- Limb motor paralysis due to central nervous system disease.
- (k)
- Participation in this clinical study deemed not appropriate.
2.3. Target Sample Size and Rationale
2.4. Randomization and Masking
2.5. Procedures
2.6. Outcomes
2.7. Statistical Analysis
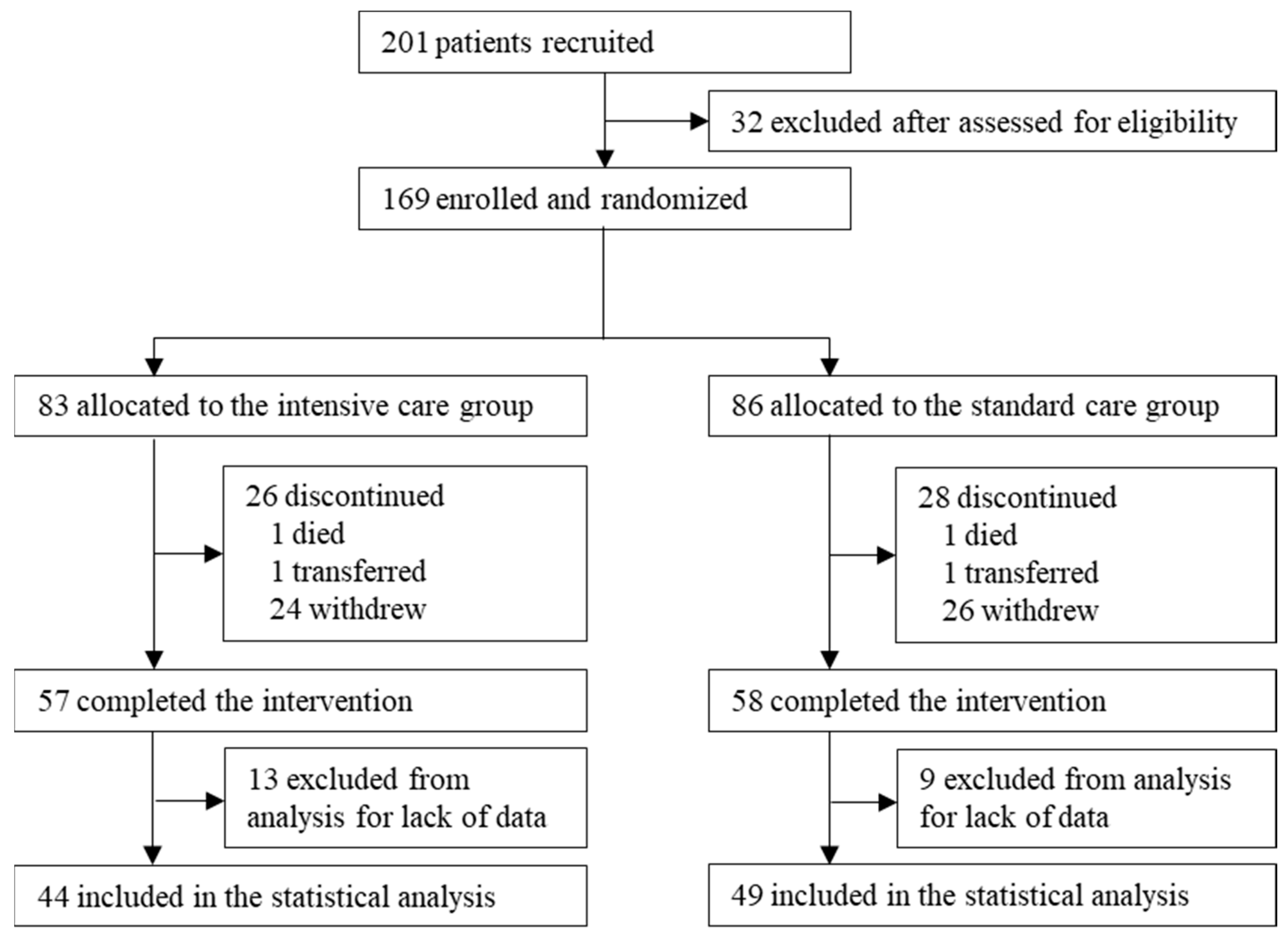
3. Results
3.1. Characteristics of the Participants
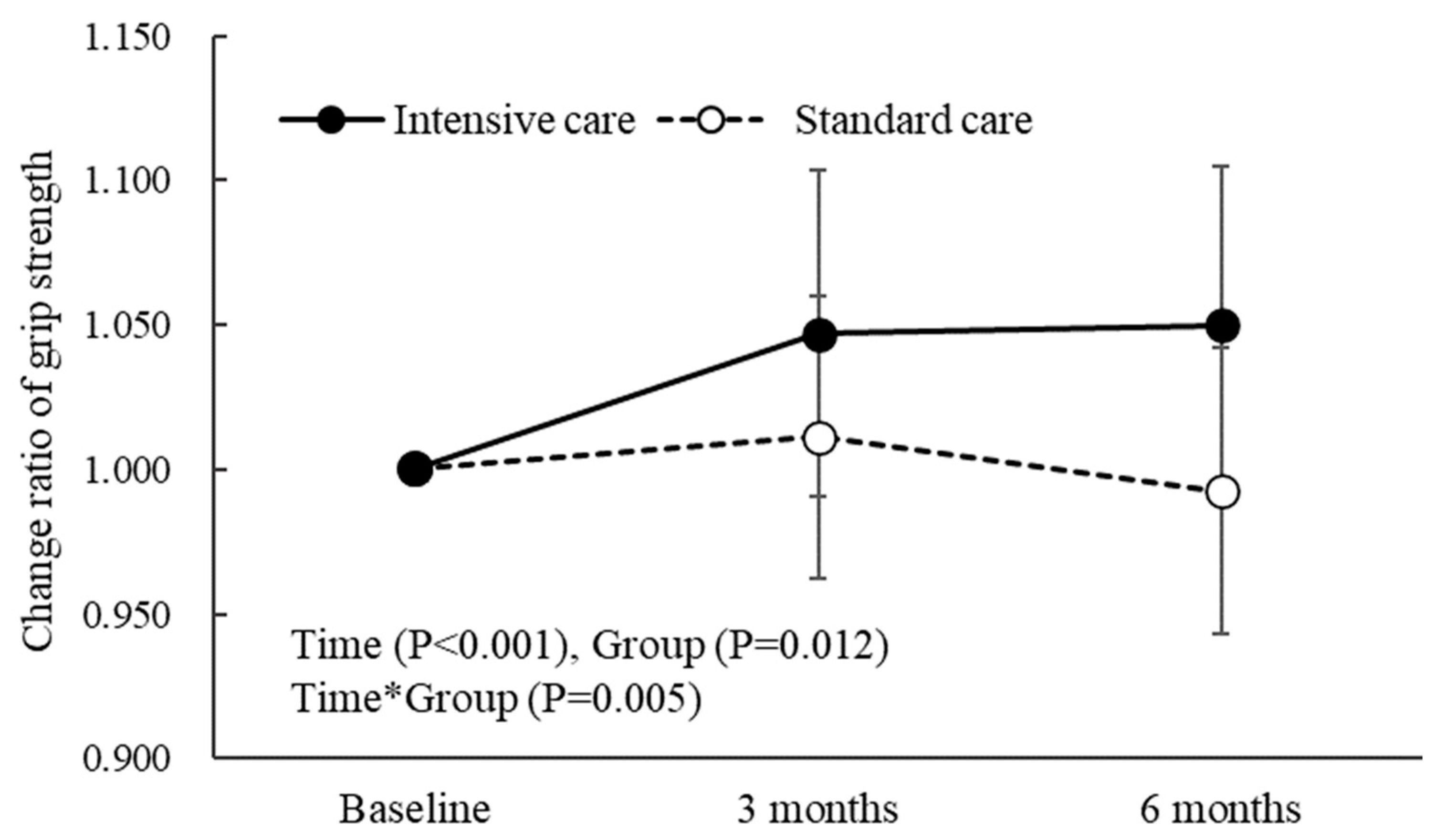
3.2. Muscle Strength, Skeletal Muscle Mass, Physical Function, and Blood Examination
3.3. Nutrient Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerstetter, J.E.; O’Brien, K.O.; Insogna, K.L. Low protein intake: The impact on calcium and bone homeostasis in humans. J. Nutr. 2003, 133, 855s–861s. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Beelen, J.; de Roos, N.M.; de Groot, L. A 12-week intervention with protein-enriched foods and drinks improved protein intake but not physical performance of older patients during the first 6 months after hospital release: A randomised controlled trial. Br. J. Nutr. 2017, 117, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Løvstad, A.T.; Gjevestad, G.O.; Hamarsland, H.; Šaltytė Benth, J.; Andersen, L.F.; Bye, A.; Biong, A.S.; Retterstøl, K.; Iversen, P.O.; et al. Intake of a Protein-Enriched Milk and Effects on Muscle Mass and Strength. A 12-Week Randomized Placebo Controlled Trial among Community-Dwelling Older Adults. J. Nutr. Health Aging 2017, 21, 1160–1169. [Google Scholar] [CrossRef]
- Peng, L.N.; Yu, P.C.; Lee, H.F.; Lin, M.H.; Chen, L.K. Protein-enriched diet improved muscle endurance and marginally reduced intramuscular adiposity: Results from a randomized controlled trial among middle-aged and older adults. Arch. Gerontol. Geriatr. 2021, 96, 104436. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Visser, M.; Jyväkorpi, S.K.; Niskanen, R.T.; Bosmans, J.E.; Jornada Ben, Â.; Brouwer, I.A.; Kuijper, L.D.; Olthof, M.R.; Pitkälä, K.H.; et al. The cost effectiveness of personalized dietary advice to increase protein intake in older adults with lower habitual protein intake: A randomized controlled trial. Eur. J. Nutr. 2022, 61, 505–520. [Google Scholar] [CrossRef]
- Munk, T.; Tolstrup, U.; Beck, A.M.; Holst, M.; Rasmussen, H.H.; Hovhannisyan, K.; Thomsen, T. Individualised dietary counselling for nutritionally at-risk older patients following discharge from acute hospital to home: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2016, 29, 196–208. [Google Scholar] [CrossRef]
- Higashiyama, A.; Watanabe, M.; Kokubo, Y.; Ono, Y.; Okayama, A.; Okamura, T. Relationships between protein intake and renal function in a Japanese general population: NIPPON DATA90. J. Epidemiol. 2010, 20 (Suppl. S3), S537–S543. [Google Scholar] [CrossRef]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef]
- Giordano, M.; Ciarambino, T.; Castellino, P.; Cataliotti, A.; Malatino, L.; Ferrara, N.; Politi, C.; Paolisso, G. Long-term effects of moderate protein diet on renal function and low-grade inflammation in older adults with type 2 diabetes and chronic kidney disease. Nutrition 2014, 30, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Lilamand, M.; Kelaiditi, E.; Demougeot, L.; Rolland, Y.; Vellas, B.; Cesari, M. The Mini Nutritional Assessment-Short Form and mortality in nursing home residents--results from the INCUR study. J. Nutr. Health Aging 2015, 19, 383–388. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshimura, Y.; Kaimoto, T.; Kunii, D.; Komatsu, T.; Yamamoto, S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn. J. Nutr. 2001, 59, 221–232. [Google Scholar] [CrossRef]
- Shen, S.; Abe, T.; Tsuji, T.; Fujii, K.; Ma, J.; Okura, T. The relationship between ground reaction force in sit-to-stand movement and lower extremity function in community-dwelling Japanese older adults using long-term care insurance services. J. Phys. Ther. Sci. 2017, 29, 1561–1566. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Hurt, R.T.; Ebbert, J.O.; Croghan, I.; Nanda, S.; Schroeder, D.R.; Teigen, L.M.; Velapati, S.R.; Mundi, M.S. The Comparison of Segmental Multifrequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Fat Free Mass and Percentage Body Fat in an Ambulatory Population. J. Parenter. Enter. Nutr. 2020, 45, 1231–1238. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Kemmler, W.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: The randomized controlled FranSO study. Clin. Interv. Aging 2017, 12, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Kerr, D.A.; Meng, X.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. Two-Year Whey Protein Supplementation Did Not Enhance Muscle Mass and Physical Function in Well-Nourished Healthy Older Postmenopausal Women. J. Nutr. 2015, 145, 2520–2526. [Google Scholar] [CrossRef]
- Hageman, D.; Fokkenrood, H.J.; Gommans, L.N.; van den Houten, M.M.; Teijink, J.A. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst. Rev. 2018, 4, Cd005263. [Google Scholar] [CrossRef] [PubMed]
- Toots, A.; Littbrand, H.; Lindelöf, N.; Wiklund, R.; Holmberg, H.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J. Am. Geriatr. Soc. 2016, 64, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.F.; Schechtman, K.B.; Ehsani, A.A.; Steger-May, K.; Brown, M.; Sinacore, D.R.; Yarasheski, K.E.; Holloszy, J.O. Effects of exercise training on frailty in community-dwelling older adults: Results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2002, 50, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Incalzi, R.A.; Gemma, A.; Capparella, O.; Cipriani, L.; Landi, F.; Carbonin, P. Energy intake and in-hospital starvation. A clinically relevant relationship. Arch. Intern. Med. 1996, 156, 425–429. [Google Scholar] [CrossRef]
- Sullivan, D.H.; Sun, S.; Walls, R.C. Protein-energy undernutrition among elderly hospitalized patients: A prospective study. JAMA 1999, 281, 2013–2019. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- O’Keefe, P.; Mann, F.D.; Clouston, S.; Voll, S.; Muniz-Terrera, G.; Lewis, N.; Wanström, L.; Hofer, S.M.; Rodgers, J.L. Getting a Grip on Secular Changes: Age-Period-Cohort Modeling of Grip Strength in the English Longitudinal Study of Ageing. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, R.; Watanabe, D.; Ito, K.; Ueda, K.; Nakayama, K.; Sanbongi, C.; Miyachi, M. Dose-response relationship between protein intake and muscle mass increase: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Kabayama, M.; Ryuno, H.; Tanaka, K.; Kiyoshige, E.; Akagi, Y.; Godai, K.; Sugimoto, K.; Akasaka, H.; Takami, Y.; et al. Association between protein intake and changes in renal function among Japanese community-dwelling older people: The SONIC study. Geriatr. Gerontol. Int. 2022, 22, 286–291. [Google Scholar] [CrossRef]
- Bilancio, G.; Cavallo, P.; Ciacci, C.; Cirillo, M. Dietary Protein, Kidney Function and Mortality: Review of the Evidence from Epidemiological Studies. Nutrients 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Alvirdizadeh, S.; Yuzbashian, E.; Mirmiran, P.; Eghtesadi, S.; Azizi, F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. 2020, 21, 489. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
| Intensive Care (n = 44) | Standard Care (n = 49) | |
|---|---|---|
| Age (years) | 79.0 (78.0–84.0) | 79.0 (77.0–84.0) |
| Male | 23 (52.3) | 28 (57.1) |
| Female | 21 (47.7) | 21 (42.9) |
| BMI (kg/m2) | 22.3 (21.1–24.7) | 21.3 (19.8–24.2) |
| Grip strength (kg) | 20.0 (15.0–28.2) | 22.7 (17.1–29.2) |
| SMI (kg/m2) | 6.2 (5.8–7.4) | 6.4 (5.8–7.0) |
| F/w (kgf·kg−1) | 1.19 (1.14–1.27) | 1.23 (1.14–1.29) |
| RFD8.75/w (kgf/s·kg−1) | 7.79 (5.76–9.69) | 7.47 (5.23–9.44) |
| Barthel index (points) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) |
| Lawton score (points) | 8.0 (5.8–8.0) | 7.0 (5.0–8.0) |
| Hb (g/dL) | 11.9 (11.0–13.7) | 12.0 (11.2–13.2) |
| Cre (mg/dL) | 0.76 (0.62–0.96) | 0.77 (0.65–0.97) |
| BUN (mg/dL) | 14.0 (11.0–18.0) | 15.0 (12.0–17.0) |
| eGFR (ml/min/1.73 m2) | 65.9 (55.6–73.0) | 61.8 (53.5–73.8) |
| Albumin (g/dL) | 3.5 (3.3–4.0) | 3.5 (3.1–3.9) |
| CRP (mg/dL) | 0.5 (0.2–1.5) | 0.6 (0.3–2.0) |
| Energy intake (kcal/day) | 1473.0 (1278.3–1621.8) | 1557.7 (1418.5–1705.5) * |
| Energy intake per body weight (kcal/kgw/day) | 24.4 (22.5–28.4) | 29.1 (24.7–33.2) * |
| Protein intake (g/day) | 59.0 (52.2–65.2) | 62.1 (56.3–70.1) |
| Protein intake per bodyweight (g/kgw/day) | 1.0 (0.9–1.2) | 1.2 (1.0–1.4) * |
| Fat intake (g/day) | 40.7 (33.2–45.6) | 42.4 (38.2–49.1) |
| Carbohydrate intake (g/day) | 211.7 (176.1–236.8) | 222.6 (195.3–245.1) * |
| Vitamin D (μg/day) | 6.2 (4.4–7.7) | 5.8 (4.1–8.1) |
| Physical activity (kcal/day) | 108.4 (35.8–196.2) | 39.1 (0.0–228.2) |
| Primary disease on admission | ||
| Cancer | 23 (52.3) | 28 (57.1) |
| Fractures | 8 (18.2) | 7 (14.3) |
| Urinary-tract infection | 1 (2.3) | 1 (2.0) |
| Pneumonia | 12 (27.3) | 13 (26.5) |
| Concomitant disease (%) | ||
| Diabetes | 14 (31.8) | 21 (42.9) |
| Hypertension | 23 (52.3) | 34 (69.4) |
| Dyslipidemia | 15 (34.1) | 20 (40.8) |
| Osteoporosis | 0 (0.0) | 0 (0.0) |
| Cancer | 4 (9.1) | 5 (10.2) |
| Chronic kidney disease | 2 (4.5) | 3 (6.1) |
| Liver dysfunction | 2 (4.5) | 0 (0.0) |
| Intensive Care | Standard Care | Between-Group | |
|---|---|---|---|
| Grip strength (kg) | 1.11 (0.11 to 2.10) * | −0.18 (−1.08 to 0.71) | 1.29 (0.22 to 2.36) * |
| SMI (kg/m2) | 0.07 (0.02 to 0.11) ** | 0.04 (0.01 to 0.07) ** | 0.02 (−0.05 to 0.09) |
| Barthel index (points) | 1.71 (−0.15 to 3.56) | 0.61 (−0.51 to 1.74) | 1.16 (−0.99 to 3.30) |
| Lawton score (points) | 0.30 (−0.17 to 0.76) | 0.10 (−0.46 to 0.66) | 0.41 (−0.19 to 1.02) |
| F/w (kgf·kg−1) | 0.25 (−0.08 to 0.58) | 0.02 (−0.27 to 0.31) | 0.01 (−0.04 to 0.04) |
| RFD8.75/w (kgf/s·kg−1) | 0.90 (0.27 to 1.53) ** | 0.76 (0.01 to 1.51) * | 0.23 (−0.72 to 1.19) |
| BMI (kg/m2) | 0.79 (0.30 to 1.27) ** | 0.36 (−0.25 to 0.75) | 0.73 (−0.46 to 1.93) |
| Hb (g/dL) | 0.31 (−0.28 to 0.90) | 0.80 (0.35 to 1.24) ** | 0.05 (−0.60 to 0.70) |
| Cre (mg/dL) | 0.05 (−0.02 to 0.11) | 0.02 (−0.03 to 0.08) | 0.06 (−0.05 to 0.16) |
| BUN (mg/dL) | 2.28 (0.48 to 4.08) ** | 2.20 (0.37 to 4.04) * | 0.92 (−0.93 to 2.77) |
| eGFR (ml/min/1.73 m2) | −5.40 (−10.94 to 0.15) | −3.26 (−7.93 to 1.41) | −2.04 (−8.70 to 4.61) |
| Albumin (g/dL) | 0.45 (0.27 to 0.63) ** | 0.45 (0.27 to 0.63) ** | 0.03 (−0.12 to 0.17) |
| CRP (mg/dL) | −0.94 (−1.49 to −0.39) ** | −1.20 (−2.09 to −0.32) ** | −0.33 (−0.66 to −0.01) # |
| Intensive Care | Standard Care | |||
|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | |
| Soy products (g) | 31.4 (25.1–42.3) | 70.0 (35.0–90.0) ** | 38.9 (21.3–51.8) | 45.0 (25.0–70.0) |
| Seafood (g) | 63.0 (47.7–82.0) | 73.6 (45.7–92.1) | 58.3 (38.2–89.3) | 71.4 (50.0–100.0) |
| Meat (g) | 55.6 (40.5–76.9) | 74.3 (50.0–98.6) ** | 55.6 (37.5–84.0) | 68.6 (45.7–91.4) |
| Eggs (g) | 27.4 (18.4–37.9) | 46.4 (24.1–50.0) * | 33.3 (23.3–42.1) | 50.0 (21.4–50.0) |
| Dairy products (g) | 118.7 (69.4–148.6) | 149.5 (72.9–232.5) | 98.9 (60.2–122.9) | 159.6 (62.5–232.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyama, S.; Yamada, Y.; Makabe, N.; Fujita, H.; Araki, A.; Suzuki, A.; Seino, Y.; Shide, K.; Kimura, K.; Murotani, K.; et al. Efficacy and Safety of 6-Month High Dietary Protein Intake in Hospitalized Adults Aged 75 or Older at Nutritional Risk: An Exploratory, Randomized, Controlled Study. Nutrients 2023, 15, 2024. https://doi.org/10.3390/nu15092024
Moyama S, Yamada Y, Makabe N, Fujita H, Araki A, Suzuki A, Seino Y, Shide K, Kimura K, Murotani K, et al. Efficacy and Safety of 6-Month High Dietary Protein Intake in Hospitalized Adults Aged 75 or Older at Nutritional Risk: An Exploratory, Randomized, Controlled Study. Nutrients. 2023; 15(9):2024. https://doi.org/10.3390/nu15092024
Chicago/Turabian StyleMoyama, Shota, Yuichiro Yamada, Noboru Makabe, Hiroki Fujita, Atsushi Araki, Atsushi Suzuki, Yusuke Seino, Kenichiro Shide, Kyoko Kimura, Kenta Murotani, and et al. 2023. "Efficacy and Safety of 6-Month High Dietary Protein Intake in Hospitalized Adults Aged 75 or Older at Nutritional Risk: An Exploratory, Randomized, Controlled Study" Nutrients 15, no. 9: 2024. https://doi.org/10.3390/nu15092024
APA StyleMoyama, S., Yamada, Y., Makabe, N., Fujita, H., Araki, A., Suzuki, A., Seino, Y., Shide, K., Kimura, K., Murotani, K., Honda, H., Kobayashi, M., Fujita, S., Yasuda, K., Kuroe, A., Tsukiyama, K., Seino, Y., & Yabe, D. (2023). Efficacy and Safety of 6-Month High Dietary Protein Intake in Hospitalized Adults Aged 75 or Older at Nutritional Risk: An Exploratory, Randomized, Controlled Study. Nutrients, 15(9), 2024. https://doi.org/10.3390/nu15092024






